ABSTRACT
Tularaemia is a zoonotic disease caused by the Gram-negative bacterium, Francisella tularensis. Depending on its entry route into the organism, F. tularensis causes different diseases, ranging from life-threatening pneumonia to less severe ulceroglandular tularaemia. Various strains with different geographical distributions exhibit different levels of virulence. F. tularensis is an intracellular bacterium that replicates primarily in the cytosol of the phagocytes. The main virulence attribute of F. tularensis is the type 6 secretion system (T6SS) and its effectors that promote escape from the phagosome. In addition, F. tularensis has evolved a peculiar envelope that allows it to escape detection by the immune system. In this review, we cover tularaemia, different Francisella strains, and their pathogenicity. We particularly emphasize the intracellular life cycle, associated virulence factors, and metabolic adaptations. Finally, we present how F. tularensis largely escapes immune detection to be one of the most infectious and lethal bacterial pathogens.
Brief history of tularaemia
The history of tularaemia and its causative agent, F. tularensis, dates back to the early 1900s, when Dr. George McCoy investigated a bubonic plague outbreak in California. The outbreak coincided with a massive die-off of ground squirrels in surrounding areas [Citation1]. Between 1908–1910 McCoy’s team autopsied over 105,000 ground squirrels and identified Bacillus pestis (later renamed Yersinia pestis) in the samples, confirming the bubonic plague outbreak. However, microbiological smears from some affected squirrels did not reveal the characteristic bacilli of B. pestis [Citation2]. When the bacterium was finally purified and stained, it was named Bacterium tularense [Citation3], after the county of Tulare in California, one of the areas affected by the epidemic. B. tularense was a very small (0.2–0.7 µm) non-motile oval organism surrounded by a clear area likely corresponding to the capsule. The organisms were present in very high numbers in spleen samples from diseased animals and were often found in leukocytes.
The first human case of tularaemia was confirmed in 1914 [Citation4] in a patient with eye inflammation presenting with ulcers and swelling of the eyelid, an infection that progressed to swollen lymph nodes, abscesses, fever, and overall weakness. Later, Dr. Edward Francis investigated cases of a mysterious febrile disease presumed to be transmitted through deer fly bites [Citation5]. He grew a pathogen that he identified as B. tularense. Between 1917 and 1919, two dozen cases were reported, one of which was fatal [Citation6]. In the years following, Dr. Francis extensively studied the disease he called tularaemia [Citation6] and its agent, B. tularense, gathering data from 14,000 cases by 1944 [Citation7] and himself contracting the disease. He meticulously described each case, cataloguing vectors, routes of infection, symptoms, outcomes, histology, and microbiology of the patient samples [Citation8–10].
Bacteria responsible for tularaemia have been referred to by various names, including Bacterium tularense, Bacillus tularensis, Brucella tularensis, and Pasteurella tularensis. In 1947, Soviet microbiologist Dorofeev proposed the creation of the genus Francisella and the species Francisella tularensis [Citation11], which is now the accepted name for the causative agent of tularaemia.
Although tularaemia is primarily a natural infection, F. tularensis has also been identified as one of the top biowarfare agents. In the 1930s, the Japanese army conducted human tests with F. tularensis [Citation12,Citation13]. Around the same time, the US studied F. tularensis at a bio-defence research laboratory in Fort Detrick, Maryland, to assess its dangers and develop countermeasures against possible bioterrorism weapons. In 1961, vaccinated and non-vaccinated volunteers were inoculated with F. tularensis subcutaneously or through aerosols to test the efficacy of a tularaemia vaccine. The tularaemia respiratory challenge determined that an exposure of less than a minute to 14–15 organisms in 10 litres of air was sufficient to provoke illness in a healthy person [Citation14]. In the 1960s, the US evaluated biological weapon delivery systems and studied the biological decay and dissemination capabilities of F. tularensis and other pathogens in natural environments [Citation15]. Former Soviet scientist Ken Alibek claimed that the USSR, and later Russia, were working on bioweapons, including genetically engineered F. tularensis strains [Citation16]. Currently, F. tularensis is still considered a possible bioterrorism threat in many countries.
Tularaemia in humans; diseases and associated symptoms
Tularemia is a zoonosis with no inter-human transmission. The infection is usually contracted through direct contact with an infected animal (handling, butchering, or being bitten). Hares or small rodents (voles, mice, rats, coypu, squirrels, etc.) are the primary sources of direct contamination, but over 250 animal species have been identified as carrying or being infected with F. tularensis [Citation17]. Most infected animals are accidental hosts, not true reservoirs, but contribute to the pathogen’s life cycle [Citation18].
Tularaemia in humans presents in six clinical forms depending on the point of bacterial entry: ulceroglandular, glandular, oculoglandular, oropharyngeal, pulmonary, and typhoid. At onset, tularaemia symptoms are non-specific and include a flu-like syndrome with fever, headache, muscle and joint aches (myalgia, and/or arthralgia). Later, one or more chronically evolving lymphadenopathies can develop in the lymphatic system draining the site of entry.
Ulceroglandular and glandular forms are the most common, accounting for 60–70% of tularaemia cases. Ulceroglandular forms are characterized by an inoculation ulcer with one or more satellite lymphadenopathies. The glandular forms of the tularaemia do not have an identified ulcer. Despite appropriate treatment, approximately 30% of patients with adenopathy progress to lymph node abscesses requiring surgical removal [Citation19]. Ulceroglandular and glandular forms also occur after bites from haematophagous arthropods (e.g. ticks, mosquitoes, and horse flies) or contact with a contaminated environment during outdoor leisure activities (e.g. gardening, swimming in rivers or lakes, and canyoning) [Citation20,Citation21]. Tick bites are the primary source of tularaemia transmission in the US, and a significant source in Europe [Citation22,Citation23].
The oculoglandular forms are rare and occur through manual transmission of bacteria to the ocular sphere or by contaminated droplets. They cause painful conjunctivitis and swelling around the eye (palpebral oedema) and are associated with enlargement of the lymph nodes localized near the ear or neck (periauricular or cervical lymph nodes, respectively). Oropharyngeal forms result from food contamination by carcasses or animal excrement and present with pharyngitis, digestive disorders, and one or more cervical lymphadenopathy [Citation20].
Pulmonary tularaemia results from airborne infections through infectious aerosols or contaminated dust from various activities (lawn mowing, sweeping a barn, handling hay, etc.) [Citation24]. Symptoms include cough, chest pain, rapid respiration (polypnea), fever, and enlargement of lymph nodes localized in the chest region (mediastinal and/or hilar adenopathy) [Citation20]. The typhoidal or septicaemic form is a systemic form that accounts for approximately 10% of the cases. It has no detected portal of entry or adenopathy, but patients exhibit fever, fatigue, and sometimes neurological and/or digestive disorders (vomiting, diarrhoea, and abdominal pain). Typhoidal tularaemia is usually diagnosed by isolation of the bacteria in blood culture and could represent a bacteraemia phase of tularaemia in some patients before the appearance of adenopathy [Citation20]. The pulmonary and typhoid forms are the most severe forms of tularaemia and are potentially fatal. The fatality rate of tularaemia is less than 1% to 3–5%, in Europe and the US, respectively, but it can be as high as 30% in the pulmonary form [Citation20]. The prognosis of tularaemia depends on several factors, including the clinical form, patient’s condition, time to initiate effective antibiotic therapy, and subspecies or clade infecting the patient (see below).
Francisella genus
The F. tularensis species () includes three subspecies, each distinguished by its metabolic characteristics and virulence: F. tularensis subsp. tularensis, F. tularensis subsp. holarctica and F. tularensis subsp. mediasiatica. Two of these subspecies, tularensis (type A strains) and holarctica (type B strains), are responsible for human cases of tularaemia [Citation25], with the former found only in North America and the latter throughout the Northern Hemisphere. The third subspecies, mediasiatica, is present in Central Asia but has never been isolated from humans. In addition, the environmental species F. novicida sometimes appears as a fourth subspecies (), and the two names F. novicida and F. tularensis subsp. novicida coexist in the literature [Citation31]. F. novicida has lower metabolic requirements than F. tularensis and shares over 97% genome homology with F. tularensis [Citation32] (). F. novicida appears to have only an aquatic reservoir because it has never been isolated from arthropods or small animals. They have a low virulence in humans. Indeed, rare human infections with F. novicida have been reported, mainly in immunocompromised patients, after exposure to contaminated water [Citation33].
Table 1. Reservoir, vectors and pathogenicity for humans of different species of Francisella..
Table 2. Characteristics of Francisella subspecies traditionally used to study virulence.
In this review, the term Francisella refers to both F. tularensis and F. novicida, while F. tularensis and F. holarctica refer to their respective subspecies. Most of the knowledge in the field comes from the study of F. novicida strain U112, F. tularensis strain SCHU S4, and F. holarctica strain LVS (). The latter, which stands for Live Vaccine Strain, is a live attenuated strain obtained through serial passages that demonstrated some immunization efficacy against SCHU S4 challenge in humans. However, to date, neither LVS nor any other strains are currently licenced as vaccine strains for tularaemia [Citation38].
Some other Francisella species can be pathogenic to humans in rare cases (). F. philomiragia is a predominantly waterborne reservoir species with low virulence, but rare cases of human infections linked to this bacterium have been described in immunocompromised patients, notably during chronic septic granulomatosis [Citation39]. F. hispaniensis [Citation40] and F. opportunistica [Citation41] have also been described as responsible for human infections.
Several other species of Francisella exist, but they have not yet been identified as agents of human infection. Some are pathogens of fish or molluscs, such as F. orientalis [Citation42], F. noatunensis [Citation43], F. halioticida [Citation44] and F. marina [Citation45], whereas others are isolated from aquatic environments, such as F. salina [Citation46], F. uliginis [Citation46] and F. salimarina [Citation47].
Infection process-lessons from animal models
Francisella causes different diseases depending on the route of entry. To study these diseases, several mouse models of tularaemia have been developed, including ulceroglandular and pneumonic tularaemia.
Following inhalation, Francisella is found mostly in alveolar macrophages 24 h post-infection. By day three post-infection, 50–80% of the infected cells are neutrophils (for SCHU S4 and F. novicida U112 strains, respectively) [Citation48]. Regardless of the route of infection, Francisella quickly disseminates to various organs, including the spleen and the liver. While phagocytes are thought to be the primary cell type hosting Francisella, Francisella can also replicate in other cell types such as alveolar epithelial cells [Citation49] and hepatocytes [Citation50]. These cells could become important reservoirs at late time points of infection.
In the liver, infection with the LVS strain leads to rapid formation of granulomas that spatially restrict infection [Citation51]. In the murine model, SCHU S4 infection leads to rapid death (within 5 days), making it difficult to study adaptive immune responses unless an appropriate antibiotic regimen is provided [Citation52]. Depending on the route of entry, F. novicida infection can be highly (LD50 ~ 10) or moderately lethal to mice following intranasal or intradermal inoculation, respectively [Citation33]. The difference in LD50 between the intradermal and the intranasal routes is poorly understood. The kinetic of bacterial dissemination to systemic organs is clearly longer upon intradermal than upon intranasal inoculation. The Intradermal injection may promote the establishment of an efficient antibacterial IFN-γ-producing immune response. In contrast, the immune response upon intranasal inoculation appears outcompeted at systemic sites by the rapid bacterial replication, leading to massive inflammation contributing to tissue damage [Citation53].
Intracellular life cycle ()
Entry
As described above, F. tularensis primarily replicates in phagocytes. Hence, bacteria enter cells by phagocytosis to reside in a Francisella-containing phagosome (FCP). The engagement of different phagocytic receptors, such as the mannose receptor, scavenger receptor A, complement receptor CR3, or FcγRs [Citation54–59] depends on the host’s immune status, especially the presence of opsonizing antibodies. Opsonization through either antibody or complement deposition strongly enhances F. tularensis phagocytosis. The route of entry also affects the trafficking of the FCP, the efficiency of phagosome escape, the pro-inflammatory response, and ultimately the infection outcome [Citation54,Citation60]. In particular, IgG-opsonized F. tularensis escapes from the phagosome less frequently than does a non-opsonized bacterium. IgG-opsonized F. tularensis is also highly restricted in its cytosolic replication in a phagosomal NADPH oxidase-dependent manner [Citation54]. Natural IgM recognizing F. tularensis capsule and/or O-antigen can also facilitate F. tularensis entry by initiating the complement cascade and promoting CR3-dependent phagocytosis [Citation61].
Figure 1. Francisella life cycle and the main host and bacterial factors involved at each step.
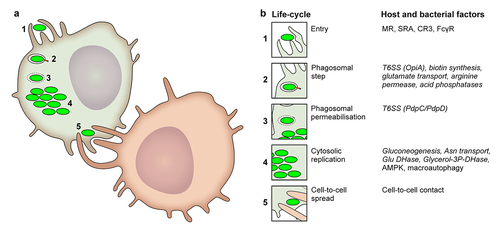
Early trafficking events and phagosomal escape
The kinetics of marker accumulation on the phagosome are consistent with the progressive maturation of the FCP along the endocytic pathway. Early on, within less than 5 min post-entry, FCP accumulates the early endosome marker EEA1. This marker is then lost when the late endosomal/lysosomal marker LAMP-1 is gained [Citation62]. Depending on the studies [Citation62–64], the frequency of LAMP-1+ FCP/intact phagosomes peaks between 20 min and 4 h post-infection, corresponding to the kinetics of Francisella escape into the cytosol. This escape is dependent on several genes clustered on a genomic island known as the Francisella Pathogenicity Island (FPI) [Citation63,Citation65–69]. The FPI, FPI-encoded type VI secretion system (T6SS), its effectors, and their role in FCP maturation and Francisella escape into the host cytosol are described in detail below.
Despite Francisella’s rapid escape from the phagolysosome, several features are important for its short transit through the acidified phagosome and the ensuing bacterial escape. Biotin synthesis is required for phagosomal escape [Citation70] as it is necessary for growth in nutrient-limiting environments. This suggests that nutritional adaption to the phagosome is key to escape. In agreement with this hypothesis, an F. novicida ΔgadC mutant lacking a glutamate transporter is deficient in vacuolar escape [Citation71]. Growth of the ΔgadC mutant is restored in macrophages deficient for NADPH oxidase, suggesting that glutamate and the tricarboxylic acid (TCA) cycle in which glutamate is utilized are important for resisting the transient oxidative burst that occurs in the FCP [Citation71]. Notably, glutamate assimilation is also important for carbon acquisition in the host cell cytosol [Citation72] (see below). Additionally, the arginine permease ArgP [Citation73] (and, to a lesser extent, the isoleucine permease [Citation74] is required for Francisella to escape into the cytosol. In addition, four acid phosphatases (AcpA, AcpB, AcpC, and HapA) have been reported to be required for F. novicida phagosomal escape [Citation75] and to contribute to F. tularensis SCHU S4 escape [Citation76] likely by opposing phagosomal NADPH oxidase assembly and reactive oxygen species production [Citation77]. Interestingly, these studies point towards an important role for NADPH oxidase in blocking phagosomal escape. F. novicida resistance to oxidative stress is also mediated by the lysine decarboxylase LdcF, possibly through the generation of cadaverine which scavenges oxygen radicals [Citation78]. Finally, the generation of ammonia by deaminases buffers the phagosomal pH [Citation79]. Notably, citrulline ureidase, present in type A strains (e.g. SCHU S4) but absent in type B (F. tularensis spp. holarctica), degrades citrulline to ornithine and ammonia and contributes to bacterial growth in IFN-γ-treated macrophages [Citation80]. Alternatively, by diverting citrulline catabolism from L-arginine to ornithine, the citrulline ureidase may reduce L-arginine availability. L-arginine is the substrate of IFN-γ-inducible NO synthase (iNOS), hence the citrulline ureidase may allow F. tularensis type A strain to limit NO production in infected macrophages. Accordingly, the growth of the citrulline ureidase mutant was not reduced compared to that of WT SCHU S4 in iNOSKO macrophages [Citation80].
Cytosolic replication
After escaping phagosomes, Francisella resides in the nutrient-rich cytosol of the host cell, which is equipped with innate immune sensors (see below).
The bacterium adapts its metabolism to the cytosolic environment by relying on gluconeogenesis and amino acid catabolism as carbon and energy sources. In contrast, glycolysis is dispensable [Citation72,Citation81]. Indeed, GlpX, a fructose 1, 6-bisphosphatase enzyme required for gluconeogenesis, is crucial for Francisella growth in the macrophage cytosol, as mutants lacking GlpX do not replicate unless the medium is supplemented with glucose [Citation72,Citation81]. Francisella also utilizes asparagine and glutamate as carbon sources through the asparagine transporter (AnsP) and glutamate dehydrogenase (GdhA), respectively [Citation82]. The catabolism of these two amino acids feeds into the TCA cycle to provide the energy required for replication. In addition to relying on amino acids as a source of carbon and nitrogen, Francisella genomes encode a glycerol uptake and a glycerol-3 phosphate transporter, and thus likely use pyruvate, glycerol, and glycerol-3P as carbon sources to sustain its replication within the host cytosol [Citation83]. Accordingly, glycerol-3P dehydrogenase (GlpA) is required for Francisella replication [Citation72]. Lipolysis (oxidation of fatty acids to generate glycerol) occurs within infected cells and is a process regulated by the activation of the host regulatory kinase AMPK. Inhibition of lipolysis or knockout of AMPK strongly reduces Francisella replication, likely due to the decreased production of glycerol and glycerol-3P [Citation72,Citation84]. Importantly, Francisella spp. are auxotrophs for at least 9 different amino acids [Citation85], and 13 amino acids were found to be essential to sustain Francisella growth in a chemically defined growth medium [Citation86]. These observations indicate that importing amino acids from the host cytosol is key to sustaining virulence. The assimilation of one amino acid, cysteine, has been particularly studied and shown to rely on the import of host glutathione (GSH, a tripeptide γ-L-glutamyl-L-cysteinyl-glycine) [Citation87–89].
These metabolic requirements clearly indicate that Francisella needs to scavenge a large quantity of nutrients to sustain its intracellular replication. Macro-autophagy, a pathway leading to the degradation of organelles and cytoplasmic content, promotes Francisella intracytosolic replication [Citation90]. Inhibition of autophagy impairs Francisella replication unless the cell culture medium is supplemented with amino acids or pyruvate. Thus, Francisella utilizes macroautophagy as a process to generate sufficient essential carbon and energy sources from the macrophage cytosol. Interestingly, this process is independent of the canonical autophagy protein Atg5 [Citation90]. Atg5-dependent xenophagy (a specific macro-autophagy process targeting intracellular pathogens) is usually an antibacterial mechanism that leads to the capture and degradation of cytosolic bacteria [Citation91]. However, wild-type Francisella strains can escape atg5-dependent xenophagy [Citation92,Citation93]. In contrast, mutants of genes implicated in LPS O-antigen synthesis or strains unable to replicate within the host cytosol (e.g. purine auxotrophs) are targeted by atg5-dependent xenophagy process [Citation92]. Remarkably, myeloid-specific atg5KO mice are more resistant to LVS infection than WT mice, emphasizing that WT Francisella benefits from autophagy responses [Citation94].
Another layer of complexity in the interactions between Francisella and autophagy lies in the formation of Francisella-containing vacuoles (FCVs). FCVs are observed at late time points of infection in primary murine bone marrow-derived macrophages but are absent in infected human monocyte-derived macrophages [Citation62]. These large vacuoles, whose formation is blocked by 3-methyladenine (3-MA, an autophagy inhibitor), require cytosolic replication of Francisella, contain a large number of bacteria, are positive for both autophagy markers (e.g. LC3), and interact with late endocytic/lysosomal compartments. The outcome of Francisella enclosed within these FCP remains unknown, although bacteria appear intact [Citation62].
Cell to cell spread
In contrast to most cytosolic pathogens [Citation95], Francisella is devoid of actin-based motility, which is one of the mechanisms used by other pathogens to disseminate from cell to cell. Francisella may escape from infected cells after cell death, which often occurs when the cells are filled with bacteria. However, at least in the case of pyroptosis, cell death might trap bacteria inside host cell remnants, leading to their elimination by efferocytosis (removal of dead cells) [Citation96]. Another mechanism of cell-to-cell transfer, termed merocytophagy (“mero,” Greek for partial; “cytophagy” for cell eating), has been observed during cell-to-cell contact [Citation97]. During this process, uninfected macrophages come in contact with infected cells and phagocytose portions (membrane and cytosolic materials) of neighbouring cells. Whenever these phagocytosed bits include cytosolic Francisella, the “eating” macrophages become infected. Importantly, in this merocytophagy process, the engulfed Francisella is found in a double-membrane vacuole and require T6SS and its effectors to escape into the cytosol of the newly infected cell [Citation97]. Compared with the uptake of extracellular bacteria, this mode of cell-to-cell transfer appears to enhance transmission, at least in vitro.
Francisella virulence factors
The central virulence device: F. tularensis T6SS
In 2002, transposon mutagenesis was used in F. novicida to identify two ORFs in a four-gene operon as being required for intracellular replication [Citation69]. The four genes were named IglA-D for “intracellular growth locus.” This locus was later found to belong to a larger locus of ~ 30 kb containing 16–19 ORFs, with G+C content deviating from the rest of the genome, and hence named FPI for “Francisella Pathogenicity Island” [Citation98]. The genes within the FPI were named iglA-J (cf above) and pdpA-E as “pathogenicity-determinant proteins.” IglC, previously found to be one of the main bacterial proteins induced during host cell infection [Citation99], was identified to be required for Francisella escape from the phagosome in 2004 [Citation66]. Early studies [Citation67,Citation69,Citation98,Citation100] suggested that FPI may encode a secretion system, and in 2009, FPI was demonstrated to encode a Type VI Secretion System (T6SS) [Citation63]. F. tularensis and F. holarctica contain two almost identical copies of FPI. These two copies may be functionally redundant, as deletion of only one FPI copy does not affect the virulence of F. tularensis, in stark contrast to the removal of both FPI loci [Citation101]. Interestingly, F. novicida contains only a single copy of FPI, but another genomic island termed “Francisella novicida Island” (FNI) [Citation32,Citation102,Citation103] is present in the F. novicida genome. FNI presents some similarity with FPI, suggesting that it might encode another T6SS [Citation104]. FNI is not involved in F. novicida virulence in a mouse model of tularaemia [Citation102].
T6SSs are widespread among Gram-negative bacteria. They belong to the family of contractile injection systems (CISs), a needle-like structure that shares 13 or 14 core components, and is implicated in the secretion of effectors from the bacterial cytosol into target cells. Evolutionarily divergences in terms of structure, function, or gene content allow the classification of four subtypes [Citation105]. Francisella T6SSs are the only representatives of the second subtype of T6SS, T6SSii, and show structural and functional homologies to canonical T6SS (T6SSi) components [Citation106].
Francisella T6SS is required for phagosomal escape, intracytoplasmic replication in host cells, and virulence in animals [Citation107]. Deletion of FPI fully abolishes Francisella escape into the host cytosol and its virulence in mice and in Galleria mellonella larvae [Citation65,Citation103,Citation108].
Six proteins have been characterized in F. novicida as T6SS effectors based on an in vitro secretion assay: PdpC, PdpD, OpiA, OpiB1, OpiB2, OpiB3 [Citation36]. PdpD is absent in F. holarctica [Citation98]. Furthermore, in both F. holarctica and F. tularensis, OpiA is split into two ORFs, and a single OpiB paralog, devoid of the ankyrin repeats found in F. novicida OpiB1–3 [Citation36,Citation37], is present. PdpD and PdpC are the two key effectors required for phagosomal escape of bacteria into the host cytosol. While deletion of either pdpC or pdpD has only a minor impact on phagosomal escape, an F. novicida double ΔpdpCΔpdpD mutant is fully deficient in its ability to reach the cytosol [Citation109]. Surprisingly, in LVS (which is naturally deficient in PdpD), a ΔpdpC mutant, which is unable to reach the host cytosol, lies in damaged phagosomes, suggesting that other effector(s) might impact phagosomal membrane integrity [Citation110]. The molecular modes of action of PdpC and PdpD remain unknown.
OpiA, OpiB1, OpiB2, and OpiB3 are encoded outside the FPI (hence their names Opi standing for “Outside Pathogenicity Island”). OpiA is a phosphatidylinositol 3-kinase that phosphorylates phosphatidylinositol to generate the glycerophospholipid phosphatidylinositol(3)-phosphate (PI(3)P) on the external leaflet of the FCP. The presence of PI(3)P on the FCP delays its maturation along the endosomal pathway and enhances the intracellular survival of Francisella by providing sufficient time for other Francisella effectors (e.g. PdpC and PdpD) to act and promote escape into the host cytosol [Citation37]. The contribution of OpiA to the overall virulence of Francisella appears to be minor, and has only been demonstrated in a ΔpdpC background. No virulence defects have been observed in a ΔopiB1–3 mutant, and the function of OpiB effectors remains to be discovered [Citation36].
Several studies using various techniques, such as microinjections [Citation111], functional transcomplementation [Citation112] and inducible complementation [Citation97] indicate that T6SS is not required for replication in the host cytosol, suggesting that its primary function is to promote phagosomal escape.
The structure-function of the Francisella T6SS machinery involved in effector secretion is starting to be well understood () following a series of very elegant studies [Citation36,Citation104,Citation109,Citation113–115] and following the overall advances in the T6SS field [Citation116]. Whenever relevant, we will extrapolate the results from T6SSi studies to propose hypotheses regarding Francisella T6SS structure and function.
Figure 2. Francisella pathogenicity island, its transcriptomic regulation and its encoded T6SS.
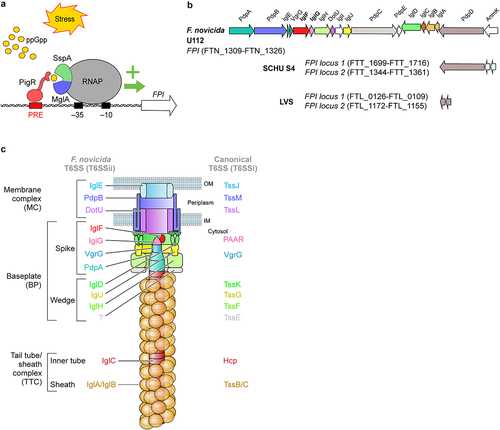
The T6SS machine can be subdivided into i) a membrane complex that anchors the T6SS to the cell envelope, ii) a baseplate composed of a wedge attached to a spike, which is the central organizer of the T6SS, and iii) a tail tube/sheath complex that includes a contractile sheath and an inner tube. The inner tube is capped by the spike complex, and sheath contraction promotes expulsion of this tube/spike complex through the transenvelope membrane complex and into the target cell.
The membrane complex of Francisella T6SS is thought to be composed of three components: IglE, PdpB, and DotU. IglE is an outer membrane lipoprotein that shares structural homology with TssJ [Citation117–119] (The Tss nomenclature refers to T6SSi machinery core proteins [Citation120]). The periplasmic domain of IglE interacts with inner membrane PdpB, which shares homology with TssM. In addition, PdpB interacts with the inner membrane protein, DotU/TssL [Citation118,Citation121,Citation122]. IglE, PdpB and DotU are required for Francisella T6SS function [Citation109] and although the structure of the IglE/PdpB/DotU complex remains to be determined, we can hypothesize that it is closely similar to the TssJML membrane complex. In Escherichia coli, the assembly of the membrane complex occurs first and is independent of other components. TssJLM complex forms a double-ring, allowing the passage of the T6SS inner tube [Citation123].
In E. coli T6SS, parallel to the assembly of the membrane complex, a baseplate is assembled in the cytosol and acts as a scaffold onto which the whole T6SS is assembled. The baseplate is composed of TssE, TssF, TssG, and TssK proteins forming the wedge complex, and of the proteins composing the spike (VgrG and PAAR proteins in E.coli). Francisella wedge complex is not well described, but IglH, IglJ, and IglD are likely homologues of TssF, TssG, and TssK, respectively [Citation113]. Notably, IglD has a similar 3D structure to that of TssK [Citation113]. No TssE homologs have been identified in Francisella. In T6SSi, the spike complex includes a trimer of valine-glycine protein G (VgrG) proteins that are closely related to the T4 contractile bacteriophage tail spike proteins gp27-gp5 and, when present, a PAAR protein that caps and sharpens the spike complex [Citation124]. Francisella VgrG is much smaller than its T6SSi homologues and encodes only the gp5-like domain. VgrG interacts with PdpA and PdpA structure is partially homologous to gp27. The (VgrG-PdpA)3 complex forms the central spike of Francisella T6SS and demonstrates unique features, including the presence of a basal lid of unknown function and a cavity that might accommodate a small effector [Citation36,Citation115]. IglG was identified by homology modelling as a PAAR-like protein [Citation104] and is now classified as belonging to the PAAR-H subtype [Citation125]. Although IglG is thought to cap Francisella T6SS, an interaction between IglG and VgrG has not been demonstrated. IglG interacts with another FPI protein of unknown function, IglF. In T6SSi, one of the main mechanisms of effector secretion is associated with spike proteins. The mechanism underlying effector secretion in Francisella remains unexplored.
The baseplate interacts with the membrane complex, and the other side acts as a scaffold to polymerize the tail tube/sheath complex. Indeed, PdpA interacts with IglC, an Hcp homologue [Citation68]. Hcp proteins homopolymerize to form hollow hexameric rings that are stacked on each other to form an inner tube. Some effectors are present within the Hcp tube and secreted together with the tube. However, this mechanism has not yet been described in Francisella. The Hcp tube promotes the assembly of the contractile sheath, which is composed of a helix of the TssB/C heterodimer. F. novicida T6SS sheath was purified and visualized by cryoelectron microscopy and was demonstrated to be composed of polymerized IglA/B heterodimers [Citation114].
The contraction of the sheath results in secretion of the inner tube capped by the spike complex and loaded with effectors [Citation116]. Accordingly, in an in vitro secretion assay, IglC, PdpA, VgrG, and the six effectors described above were identified in the supernatant [Citation36]. It remains unclear whether IglG and IglF were not secreted or detected, and whether additional effectors remain to be identified.
Recycling of the contracted TSS6i sheath is mediated by unfoldase ClpV, which is missing in Francisella and is functionally replaced by ClpB. Indeed, ClpB is required for the disassembly of the IglA/B contracted sheath and likely promotes the recycling of T6SSii components to promote multiple firing events [Citation109,Citation126]. Interestingly, the function of ClpB as a heat shock response protein can be dissociated from its function as a T6SSii unfoldase, indicating that in Francisella ClpB is a moonlighting protein [Citation127].
Regulation of T6SS gene transcription and T6SS assembly
FPI gene expression is induced during macrophage infection. In Francisella tularensis SCHU S4, FPI gene expression reaches its highest expression 12-16 h post-infection, corresponding to the intra-macrophage replication phase of the bacterium [Citation128]. The activation of the FPI cluster is controlled by a complex transcription regulatory system that includes stringent starvation protein A (SspA) and macrophage growth locus protein A (MglA) [Citation129–131]. The heterodimer SspA/MglA is constitutively associated with RNA polymerase (RNAP) and stabilizes the σ [Citation79] subunit of RNAP [Citation132]. However, to induce FPI gene expression, two other players are needed: the pathogenicity island regulator (PigR [Citation133] also termed FevR [Citation134]) and the alarmone (p)ppGpp, which is produced upon stringent stress response (a stress response triggered by several cues including amino acid starvation). (p)ppGpp binds to the SspA-MglA complex and promotes the high affinity binding of PigR to SspA-MglA complex [Citation132,Citation135,Citation136]. PigR recognizes a 7bp motif named PigR response element (PRE) located in promoters, including those of iglA and pdpA [Citation137]. In the presence of (p)ppGpp, PigR via its tight association with SspA-MglA recruits the SspA-MglA-RNAP complex to PRE-containing promoters to drive transcription initiation and ultimately FPI genes transcription. PigR transcription is itself positively regulated by MglA [Citation134], and by the histone-like HU protein [Citation138], which among numerous targets binds the pigR promoter [Citation139].
Factors other than stringent stress induce T6SS expression. Indeed, IglC is induced during iron restriction, a condition that is associated with the intracellular environment [Citation140].
Finally, another layer of regulation controls the T6SS assembly. Indeed, specific cues, such as the presence of high potassium concentration (as revealed by culture in medium containing 5% KCl) or specific oxygen tension (which is modified when F. novicida is grown on an agarose pad covered with a coverglass) are needed to induce T6SS assembly [Citation109,Citation114]. The mechanism underlying this regulation of T6SS assembly and whether T6SS sheath contraction is itself under specific regulation remains to be discovered, but may implicate post-translational modifications [Citation141].
Francisella envelope and innate immune responses
As an intracellular pathogen, Francisella is exposed to several innate immune sensors and immune effector mechanisms. Francisella is considered a stealth pathogen [Citation142–144] largely because of the properties of its envelope, including the peculiar LPS.
Francisella LPS
Francisella LPS structure is largely conserved between F. tularensis and F. novicida (although small differences exist in the O-chain moiety) [Citation145]. Thus, it is expected that most studies performed on one (sub)species will prove to be true for other (sub)species of Francisella.
While the prototypical LPS from enterobacteria contains a Lipid A part with six acyl chains (12–14 carbon atoms per chain), Francisella Lipid A is tetra-acylated, possesses long acyl chains (16–18 carbons), and is hypophosphorylated. These characteristics are key to evading the innate immune response. Indeed, Francisella Lipid A is recognized neither by Toll-Like Receptor 4 [Citation146] nor by the murine intracytosolic LPS sensor, Caspase-11 and only poorly by its human counterpart Caspase-4 [Citation147].
The lack of TLR4 activation results from multiple layers of evasion. Indeed, Francisella LPS evades detection by CD14, the most upstream event in the CD14/MD2/TLR4 signalling cascade. In addition, hexacylation is required to trigger dimerization of the TLR4/MD-2 complex and downstream signalling, suggesting that Francisella LPS would similarly not trigger this event [Citation148]. Accordingly, innate immune responses against Francisella are mainly driven by TLR2 [Citation149–153] which recognizes lipoproteins [Citation154]. Francisella has evolved strategies to downregulate lipoprotein expression and TLR2-mediated responses [Citation155,Citation156]. Indeed, F. novicida uses the CRISPR/cas9 system to regulate lipoprotein expression (FTN_1103) and evades TLR2 recognition. Interestingly, Cas9 and the associated tracrRNA and scaRNA are induced by a 100 fold during infection, which correlates with the downregulation of FTN_1103 [Citation156]. Surprisingly, in contrast to F. novicida, F. tularensis has a deficient CRISPR/Cas9 system and is still able to limit TLR activation. This observation suggests that highly virulent Francisella species have evolved different mechanisms to downregulate bacterial lipoprotein expression/recognition.
Francisella Lipid A largely evades recognition by caspase-11 and non-canonical inflammasomes in murine macrophages. Caspase-11 is a cytosolic Lipid A receptor that dimerizes and is activated by Lipid A binding. Active caspase-11 cleaves Gasdermin D, releasing its N-terminal pore-forming domain that oligomerizes into the plasma membrane and triggers rapid inflammatory cell death, termed pyroptosis (). Evasion of caspase-11 recognition is due to its low acylation level. Indeed, Lipid A purified from the Francisella LpxF mutant is pentacylated and activates caspase-11 [Citation157]. Caspase-4 is more reactive to Francisella LPS (although ∽ 10 times less than to E. coli LPS) and under-acylated lipid A [Citation147] than its murine homologue caspase-11. Accordingly, the caspase-4 inflammasome detects F. novicida in human macrophages [Citation147].
Figure 3. Overview of the innate immune responses upon macrophage infection.
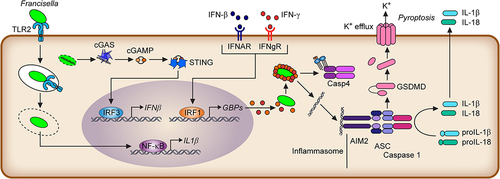
The inner core of Francisella LPS also displays immunoevasive properties. Indeed, while most Gram-negative bacteria contain heptose residues in the LPS inner core, Francisella LPS core is relatively small and consists mainly of two mannose residues [Citation158]. Importantly, ADP-heptose is an intermediate metabolite in the LPS biosynthetic pathway of bacteria containing heptose residues in their core but is also a potent activator of NF-kB. Indeed, the host cytosolic receptor ALPK1 binds ADP-heptose, leading to the activation of its kinase activity and triggering TIFA-TRAF6-dependent activation of NF-kB. Although not experimentally proven, owing to its atypical LPS core, this innate immune pathway should not detect Francisella infection [Citation159].
In addition, Francisella LPS has other atypical features, including a single 2-keto-3-deoxy-D-manno-octulosonic acid (Kdo) residue in its inner core (whereas LPS from most Gram-negative bacteria displays two or three Kdo residues) [Citation158]. Deletion of the two-component Kdo hydrolase (KdhAB) responsible for this unique feature leads to severe attenuation of the corresponding Francisella mutant [Citation160]. Furthermore, over 70% of its lipid A is present as “free lipid A,” which is not attached to the classical core and O-antigen sugar residues [Citation161]. Finally, Francisella Lipid A is hypophosphorylated due to the action of the two enzymes LpxE [Citation162]and LpxF [Citation163] that hydrolyse lipid A phosphate residues. The lpxF mutant is highly attenuated for virulence [Citation164,Citation165] suggesting that hypophosphorylation is important for virulence. In agreement with the reduction in negative charge associated with the lack of a phosphate group on Lipid A, WT Francisella is highly resistant to cationic antimicrobial peptides, a resistance that is lost in the lpxF mutant [Citation165]. Notably, since LPS from the lpxF mutant displays penta-acylated Lipid A, it remains difficult to conclude specifically on the role of hypophosphorylation in virulence. Similarly, the NaxD enzyme neutralizes the negative charge of a phosphate group present on free lipid A by the addition of galactosamine, which also contributes to cationic antimicrobial peptide resistance [Citation166].
Capsule
A capsule-like structure was observed around F. tularensis in 1977 [Citation167,Citation168] and later characterized as a capsule of polysaccharide identical to the O-antigen subunit of LPS [Citation169]. The purified capsule lacked other LPS components, suggesting that it is a different entity. Unfortunately, to date, there is no convincing genetic way to invalidate the capsule without affecting LPS O-antigen, rendering the characterization of the capsule and its roles in virulence highly challenging [Citation167].
Membrane phospholipids
The anti-inflammatory properties of highly virulent F. tularensis have been associated with membrane lipids enriched in this specific strain but absent in LVS [Citation170]. An atypical phosphatidylethanolamine with a very long-chain fatty acid (C24) and a 10 carbon chain was identified as a candidate lipid, although such atypical phosphatidylethanolamine is also present in the more inflammatory subspecies F. novicida [Citation171]. As phosphatidylethanolamine is the main component of the inner membrane, this anti-inflammatory lipid may quantitatively contribute to the active inhibition of innate immune responses.
Other virulence factors
While the T6SS is considered the most important secretion system for virulence, other secretion systems are present in Francisella genomes, including the TolC-dependent efflux system and type IV-pilus (Tfp), which acts as a T2-like secretion system [Citation172].
T1SS are tripartite complexes, including the prototypical outer membrane channel of the TolC protein. TolC proteins are also outer membrane components of multidrug exporters. Francisella genomes contain three TolC homologues (TolC, FtlC, and SilC) that are involved in multidrug export, while the role of TolC as a T1SS component remains speculative [Citation173,Citation174]. TolC mutants are highly attenuated in virulence, although it is difficult to ascribe this attenuation phenotype to a deficit in multidrug efflux, a potential role in T1SS, or even a role in bacterial envelope structure [Citation175].
T2SS relies on the Sec or Tat export system for translocation into the periplasm and allows export of proteins from the periplasm to the extracellular medium. In F. novicida, the closely related type IV pilus (tfp) secretion machinery acts as a T2SS-like secretion system and is involved in the secretion of several proteins including three glycosidases and a protease (PepO). PepO is absent in F. tularensis strains, and its role in F. novicida virulence is controversial [Citation176,Citation177]. Importantly, tfp formation can be genetically distinguished from tfp-mediated secretion. The latter appears to be more important for virulence than the tfp appendage. The tfp-exported substrates that are important for virulence remain to be identified. Although tfp-mediated secretion has only been demonstrated in F. novicida, the SCHU S4 strain appears to possess all the genes required for this secretion [Citation178].
In addition, Francisella virulence depends on a tight control of its gene expression program, that relies on master virulence regulators including the above described MglA/SspA/PigR complex, on several transcription factors (recently reviewed [Citation179] and two component systems (e.g. KdpD/E) [Citation180,Citation181] sensing various signals (oxidative stress, iron starvation, K+ concentration …).
Finally, numerous proteins allowing the adaptation to immune response-mediated stress are considered as virulence factors and include enzymes and proteins fighting oxidative stress (e.g. KatG [Citation182], SodB [Citation183], SodC [Citation184], thioredoxinA1 [Citation185], AhpC [Citation184]), chaperone proteins (DsbA [Citation186], Hfq [Citation187]), and efflux pumps (e.g. the AcrAB system conferring resistance to antimicrobial peptides [Citation188]).
Immune responses
Innate immune responses
Innate immune responses to Francisella have been reviewed elsewhere [Citation142,Citation189] and are only briefly presented (). As described above, owing to its particular envelope, Francisella is largely resistant to cationic antimicrobial peptides and to the bactericidal activity of serum components [Citation189]. Following the first encounter with host cells, Francisella fully escapes TLR4 recognition and is recognized by TLR2, although, as described above, F. novicida limits the expression of TLR2-recognized lipoproteins. However, F. tularensis is much less pro-inflammatory than F. novicida and appears to evade TLR2 responses [Citation33].
Following escape into the host cytosol, Francisella is recognized by the so-called cytosolic responses and as a first line by the cGAS/STING pathway, leading to type I IFN production [Citation190,Citation191]. cGAS is a cytosolic DNA sensor, suggesting that following Francisella escape into the host cytosol, Francisella genomic DNA can be detected in the cytosol, leading to IRF3 activation and type I IFN production [Citation191]. Mice deficient in type I IFN receptor, STING, or IRF3 are more resistant to F. novicida infection [Citation191,Citation192]. Type I IFNs [Citation193] control numerous genes during infection, and the deleterious role of type I IFN signalling for the host is likely multifactorial [Citation194] but at least partly due to the anti-inflammatory actions of type I IFNs. Type I IFNs suppress IL-12 production by human dendritic cells following F. tularensis infection [Citation195] and IL-17 production by gamma delta T cells in murine models of tularaemia .
However, type I IFN can also induce pro-inflammatory responses. Notably, type I IFN (or IFN-γ) activates IRF1 to induce Guanylate binding proteins (GBPs). GBPs target cytosolic F. novicida [Citation147,Citation196–199] to promote bacteriolysis, release bacterial DNA into the host cytosol, and activate the cytosolic DNA sensor Aim2 (Absent in Melanoma 2) in murine macrophages [Citation190,Citation200,Citation201]. Aim2 is an inflammasome sensor that leads to the activation of caspase-1, release of the pro-inflammatory cytokines IL-1β and IL-18, and rapid cell death termed pyroptosis. IRF1, GBP-deficient-, and Aim2-deficient mice are highly susceptible to F. novicida infection [Citation190,Citation197–199]. The NLRP3 inflammasome is also important for inflammasome responses during LVS and SCHU S4 infections, although the nature of the detected stimulus remains unknown [Citation202].
In human macrophages, GBPs are key to promoting the activation of the non-canonical caspase-4 inflammasome [Citation147] (see above), although F. novicida appears to partially escape GBP targeting [Citation203]. SCHU S4 also partially escapes GBP-mediated growth restriction and inflammasome activation [Citation196,Citation200,Citation204]. IFN-γ is one of the key cytokines controlling Francisella-immune responses [Citation205]. While GBPs appear to be the main effectors against F. novicida [Citation200], inducible NO synthase and the produced reactive nitrogen species are key effectors against LVS [Citation206–208] and, to a lesser extent, SCHU S4 [Citation209,Citation210]. In addition, IFN-γ rewires macrophage metabolism by inducing IRG-1 and producing itaconate to control intracellular replication [Citation211].
While these pathways have mostly been described in infected macrophages, Francisella also infects neutrophils [Citation48]. Neutrophils may be important cells in fighting infection [Citation205,Citation212]. However, they clearly contribute to host tissue damage and tularaemia pathogenesis [Citation213]. The activation and antimicrobial activities of neutrophils are ablated by Francisella infection. Notably, neutrophils fail to assemble NADPH oxidase on FCP, and infected neutrophils even fail to produce reactive oxygen species in response to strong stimuli such as PMA (Phorbol 12-myristate 13-acetate), indicating that Francisella infection imposes a general block on neutrophil functions. The bacterial factors responsible for this impairment of neutrophil function remain unclear, although Francisella strains mutated in the master regulator, fevR/pigR or mglA genes are unable to block PMA-induced ROS production [Citation213].
Despite the stealth nature of Francisella and its ability to dampen immune responses, the late stages of lethal infections are characterized by an unleashed immune response leading to severe sepsis. This delayed deregulated host response likely represents the cause of mortality in infected animals [Citation214].
Adaptive immune responses
Adaptive immune responses to Francisella spp. have recently been reviewed [Citation215]. Surprisingly, for intracellular pathogens, antibodies have been found to be protective against tularaemia [Citation216]. Notably, anti-LPS IgMs generated in a timely manner by B1a B cells in the peritoneal cavity are protective in passive immunization experiments [Citation217]. Both CD4 and CD8 T cells are important factors in the Francisella immune response, notably through the production of IFN-γ and TNF [Citation218]. While IFN-γ is highly bactericidal in macrophages infected with LVS and F. novicida [Citation200,Citation219], F. tularensis appears to escape at least partially IFN-γ-mediated restriction, and macrophages require both TNF and IFN-γ to control infection [Citation210,Citation220].
Conclusions
Tularemia is currently a rare disease. Yet, it remains a concern in many countries because of its poorly understood ecological dynamics and high infectivity of F. tularensis through aerosol transmission. Although Francisella is a zoonotic pathogen and may not have evolved with the human host, it evades detection by the innate immune system. This stealth ability appears to be related to its lipopolysaccharide, envelope, and capsule, which are poorly immune-activating. Recently, the study of Francisella has shed new light on its main virulence factors, the T6SS, and its effectors, although the central mechanism of phagosomal rupture is still not well understood. Additionally, Francisella appears to hijack host cell metabolism to promote massive replication within the host cytosol. Undoubtedly, future research on Francisella will continue to reveal new and original bacterial mechanisms for replicating within host cells and causing diseases.
Acknowledgements
This historical perspective was adapted from the SV PhD manuscript. We apologize for all colleagues that we could not cite in this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability statement
The data, materials and original svg figures that support the results or analyses presented in this paper are freely available upon request.
Additional information
Funding
References
- McCoy GW. Plague among ground squirrels in America. J Hyg (Lond). 1910;10(4):589–21. doi: 10.1017/S002217240004314X
- Public Health Bulletin. U.S. Government printing office. 1911.
- McCoy GW, Chapin CW. Further observations on a plague-like disease of rodents with a preliminary note on the causative agent, bacterium tularense. J Infect Dis. 1912;10(1):61–72. doi: 10.1093/infdis/10.1.61
- Wherry WB, Lamb BH. Infection of man with bacterium tularense. J Infect Dis. 1914;15(2):331–340. doi: 10.1093/infdis/15.2.331
- Francis E. Weekly reports for SEPTEMBER 12, 1919. Public Health Rep. 1919;34(37):2061–2103. doi: 10.2307/4575306
- Francis E, Mayne B, Lake GC. Tularæmia Francis 1921. Public Health Rep. 1921;36(30):1731–1792. doi: 10.2307/4576069
- Jellison WL. Tularemia: Dr. Edward Francis and his first 23 isolates of Francisella tularensis. Bull Hist Med. 1972;46(5):477–485.
- Francis E. A SUMMARY OF PRESENT KNOWLEDGE OF TULARAEMIA1. Medicine. 1928;7(4):411–432. doi: 10.1097/00005792-192812000-00002
- FRANCIS FE. SYMPTOMS, DIAGNOSIS and PATHOLOGY of TULAREMIA. JAMA. 1928;91(16):1155. doi: 10.1001/jama.1928.02700160007002
- Vonderlehr RA. Weekly reports for JANUARY 22, 1937. Public Health Rep. 1937;52(4):95–123. doi: 10.2307/4582066
- Philip CB, Owen CR. Comments on the nomenclature of the causative agent of tularemia. Int Bull Bacteriol Nomencl Taxon. 1961;11(3):67–72. doi: 10.1099/0096266X-11-3-67
- Rider DR. Japan’s biological and chemical weapons programs. War Crimes And Atrocities: who’s Who, What’s What. And Where’s Where – 1928-1945. 2014 . 761. http://www.mansell.com/Resources/Rider_Whos_Who_in_Japanese_BW_2018-10-09_IN_PROCESS--SEEK-PERMISSION-TO-USE.pdf
- Harris S. Japanese biological warfare research on humans: a case study of Microbiology and ethics. Ann N Y Acad Sci. 1992;666(1):21–52. doi: 10.1111/j.1749-6632.1992.tb38021.x
- Saslaw S. Tularemia Vaccine Study: II. Respiratory Challenge. Arch Intern Med. 1961;107(5):702. doi: 10.1001/archinte.1961.03620050068007
- Office of the Assistant Secretary of Defense (Health Affairs). Desert Test Center [Internet]. 2009 [cited 2022 Jan 26]; Available from: https://web.archive.org/web/20090309172516/http://fhp.osd.mil/CBexposures/pdfs/red_cloud.pdf
- Engineering Bio-Terror Agents: Lessons from the Offensive U.S. and Russian Biological Weapons Programs [Internet]. [cited 2022 Jan 26]; Available from: https://irp.fas.org/congress/2005_hr/bioterror.html
- Keim PS, Johansson A, Wagner DM. Molecular Epidemiology, Evolution, and Ecology of Francisella. Ann N Y Acad Sci. 2007. [Internet]: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17435120
- Telford SR, Goethert HK. Ecology of Francisella tularensis. Annu Rev Entomol. 2020;65:351–372. doi: 10.1146/annurev-ento-011019-025134
- Maurin M, Pelloux I, Brion JP, et al. Human tularemia in France, 2006-2010. Clin Infect Dis. 2011;53(10):e133–41. doi: 10.1093/cid/cir612
- Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis. 2016;16(1):113–124. doi: 10.1016/S1473-3099(15)00355-2
- Hestvik G, Warns-Petit E, Smith LA, et al. The status of tularemia in Europe in a one-health context: a review. Epidemiol Infect. 2015;143(10):2137–2160. doi: 10.1017/S0950268814002398
- Ellis J, Oyston PCF, Green M, et al. Tularemia. Clin Microbiol Rev. 2002;15(4):631–646. doi: 10.1128/CMR.15.4.631-646.2002
- Gehringer H, Schacht E, Maylaender N, et al. Presence of an emerging subclone of Francisella tularensis holarctica in Ixodes ricinus ticks from south-western Germany. Ticks Tick Borne Dis. 2013;4(1–2):93–100. doi: 10.1016/j.ttbdis.2012.09.001
- Hauri AM, Hofstetter I, Seibold E, et al. Investigating an airborne tularemia outbreak, Germany. Emerg Infect Dis. 2010;16(2):238–243. doi: 10.3201/eid1602.081727
- Sjostedt A. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann N Y Acad Sci. 2007;1105(1):1–29. doi: 10.1196/annals.1409.009
- Larson MA, Nalbantoglu U, Sayood K, et al. Reclassification of Wolbachia persica as Francisella persica comb. nov. And emended description of the family francisellaceae. Int J Syst Evol Microbiol. 2016;66(3):1200–1205. doi: 10.1099/ijsem.0.000855
- Wagner DM, Birdsell DN, McDonough RF, et al. Genomic characterization of Francisella tularensis and other diverse Francisella species from complex samples. PLoS One. 2022;17(10):e0273273. doi: 10.1371/journal.pone.0273273
- Timofeev V, Titareva G, Bahtejeva I, et al. The Comparative virulence of Francisella tularensis Subsp. mediasiatica for vaccinated laboratory animals. Microorganisms. 2020;8(9):8. doi: 10.3390/microorganisms8091403
- Hennebique A, Peyroux J, Brunet C, et al. Amoebae can promote the survival of Francisella species in the aquatic environment. Emerg Microbes Infect. 2021;10(1):277–290. doi: 10.1080/22221751.2021.1885999
- Respicio-Kingry LB, Byrd L, Allison A, et al. Cutaneous infection caused by a novel Francisella sp. J Clin Microbiol. 2013;51(10):3456–3460. doi: 10.1128/JCM.01105-13
- Johansson A, Celli J, Conlan W, et al. Objections to the transfer of Francisella novicida to the subspecies rank of Francisella tularensis. Int J Syst Evol Microbiol. 2010;60(8):1717–1718. author reply 1718-1720. doi: 10.1099/ijs.0.022830-0
- Larsson P, Elfsmark D, Svensson K, et al. Molecular evolutionary consequences of niche restriction in Francisella tularensis, a facultative intracellular pathogen. PLOS Pathog. 2009;5(6):e1000472. doi: 10.1371/journal.ppat.1000472
- Kingry LC, Petersen JM. Comparative review of Francisella tularensis and Francisella novicida. Front Cell Infect Microbiol. 2014;4:35. doi: 10.3389/fcimb.2014.00035
- Rohmer L, Fong C, Abmayr S, et al. Comparison of Francisella tularensis genomes reveals evolutionary events associated with the emergence of human pathogenic strains. Genome Biol. 2007;8(6):R102. doi: 10.1186/gb-2007-8-6-r102
- Clemens DL, Lee B-Y, Horwitz MA. The Francisella type VI secretion system. Front Cell Infect Microbiol. 2018;8:121. doi: 10.3389/fcimb.2018.00121
- Eshraghi A, Kim J, Walls AC, et al. Secreted effectors encoded within and outside of the Francisella pathogenicity island promote intramacrophage growth. Cell Host Microbe. 2016;20(5):573–583. doi: 10.1016/j.chom.2016.10.008
- Ledvina HE, Kelly KA, Eshraghi A, et al. A phosphatidylinositol 3-kinase effector alters phagosomal maturation to promote intracellular growth of Francisella. Cell Host Microbe. 2018;24(2):285–295.e8. doi: 10.1016/j.chom.2018.07.003
- Conlan JW. Tularemia vaccines: recent developments and remaining hurdles. Future Microbiol. 2011;6(4):391–405. doi: 10.2217/fmb.11.22
- Kreitmann L, Terriou L, Launay D, et al. Disseminated infection caused by Francisella philomiragia, France, 2014. Emerg Infect Dis. 2015;21(12):2260–2261. doi: 10.3201/eid2112.150615
- Zhou H, Yang Q, Shen L, et al. Seawater-associated highly pathogenic Francisella hispaniensis infections causing multiple organ failure. Emerg Infect Dis. 2020;26(10):2424–2428. doi: 10.3201/eid2610.190844
- Dietrich EA, Kingry LC, Kugeler KJ, et al. Francisella opportunistica sp. nov., isolated from human blood and cerebrospinal fluid. Int J Syst Evol Microbiol. 2020;70(2):1145–1151. doi: 10.1099/ijsem.0.003891
- Sridhar S, Sharma A, Kongshaug H, et al. Whole genome sequencing of the fish pathogen Francisella noatunensis subsp. orientalis Toba04 gives novel insights into Francisella evolution and pathogenecity. BMC Genomics. 2012;13(1):598. doi: 10.1186/1471-2164-13-598
- Mikalsen J, Olsen AB, Tengs T, et al. Francisella philomiragia subsp. noatunensis subsp. nov., isolated from farmed Atlantic cod (Gadus morhua L.). Int J Syst Evol Microbiol. 2007;57(9):1960–1965. doi: 10.1099/ijs.0.64765-0
- Brevik OJ, Ottem KF, Kamaishi T, et al. Francisella halioticida sp. nov., a pathogen of farmed giant abalone (Haliotis gigantea) in Japan. J Appl Microbiol. 2011;111(5):1044–1056. doi: 10.1111/j.1365-2672.2011.05133.x
- Soto E, Griffin MJ, Morales JA, et al. Francisella marina sp. nov., etiologic agent of systemic disease in cultured spotted rose snapper (Lutjanus guttatus) in central America. Appl Environ Microbiol. 2018;84(16):84. doi: 10.1128/AEM.00144-18
- Challacombe JF, Petersen JM, Gallegos-Graves LV, et al. Correction for challacombe et al., whole-genome relationships among Francisella bacteria of diverse origins define new species and provide specific regions for detection. Appl Environ Microbiol. 2017;83(6). doi: 10.1128/AEM.00174-17
- Li L-H, Luo H-M, Feng J-H, et al. Francisella salimarina sp. nov., isolated from coastal seawater. Int J Syst Evol Microbiol. 2020;70(5):3264–3272. doi: 10.1099/ijsem.0.004164
- Hall JD, Woolard MD, Gunn BM, et al. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun. 2008;76(12):5843–5852. doi: 10.1128/IAI.01176-08
- Hall JD, Craven RR, Fuller JR, et al. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect Immun. 2006;75(2):1034–1039. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17088343
- Law HT, Lin A-J, Kim Y, et al. Francisella tularensis uses cholesterol and clathrin-based endocytic mechanisms to invade hepatocytes. Sci Rep. 2011;1(1):192. doi: 10.1038/srep00192
- Bokhari SM, Kim K-J, Pinson DM, et al. NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect Immun. 2008;76(4):1379–1389. doi: 10.1128/IAI.00745-07
- Crane DD, Scott DP, Bosio CM, et al. Generation of a convalescent model of virulent Francisella tularensis infection for Assessment of host requirements for survival of tularemia. PLoS One. 2012;7(3):e33349. doi: 10.1371/journal.pone.0033349
- Nicol MJ, Williamson DR, Place DE, et al. Differential immune response following intranasal and intradermal infection with Francisella tularensis: implications for vaccine development. Microorganisms. 2021;9(5):973. doi: 10.3390/microorganisms9050973
- Geier H, Celli J, Morrison RP. Phagocytic receptors dictate phagosomal escape and intracellular proliferation of Francisella tularensis. Infect Immun. 2011;79(6):2204–2214. doi: 10.1128/IAI.01382-10
- Balagopal A, MacFarlane AS, Mohapatra N, et al. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74(9):5114–5125. doi: 10.1128/IAI.00795-06
- Ben Nasr A, Haithcoat J, Masterson JE, et al. Critical role for serum opsonins and complement receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) in phagocytosis of Francisella tularensis by human dendritic cells (DC): uptake of Francisella leads to activation of immature DC and intracellular survival of the bacteria. J Leukocyte Biol. 2006;80(4):774–786. doi: 10.1189/jlb.1205755
- Clemens DL, Lee BY, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73(9):5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005
- Pierini LM. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class a scavenger receptors. Cell Microbiol. 2006;8(8):1361–1370. doi: 10.1111/j.1462-5822.2006.00719.x
- Schulert GS, Allen L-A. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukocyte Biol. 2006;80(3):563–571. doi: 10.1189/jlb.0306219
- Dai S, Rajaram MVS, Curry HM, et al. Fine tuning inflammation at the Front door: macrophage complement receptor 3-mediates phagocytosis and immune suppression for Francisella tularensis. PLOS Pathogens. 2013;9(1):e1003114. doi: 10.1371/journal.ppat.1003114
- Schwartz JT, Barker JH, Long ME, et al. Natural IgM mediates complement-dependent uptake of Francisella tularensis by human neutrophils via complement receptors 1 and 3 in nonimmune serum. J Immunol. 2012;189(6):3064–3077. doi: 10.4049/jimmunol.1200816
- Checroun C, Wehrly TD, Fischer ER, et al. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103(39):14578–14583. doi: 10.1073/pnas.0601838103
- Barker JR, Chong A, Wehrly TD, et al. The Francisella tularensis pathogenicity island encodes a secretion system that is required for phagosome escape and virulence. Mol Microbiol. 2009;74(6):1459–1470. doi: 10.1111/j.1365-2958.2009.06947.x
- Golovliov I, Baranov V, Krocova Z, et al. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect Immun. 2003;71(10):5940–5950. doi: 10.1128/IAI.71.10.5940-5950.2003
- Nano FE, Schmerk C. The Francisella pathogenicity island. Ann N Y Acad Sci. 2007;1105(1):122–137. doi: 10.1196/annals.1409.000
- Lindgren H, Golovliov I, Baranov V, et al. Factors affecting the escape of Francisella tularensis from the phagolysosome. J Med Microbiol. 2004;53(10):953–958. doi: 10.1099/jmm.0.45685-0
- Ludu JS, de Bruin OM, Duplantis BN, et al. The Francisella pathogenicity island protein PdpD is required for full virulence and associates with homologues of the type VI secretion system. J Bacteriol. 2008;190:4584–4595. doi: 10.1128/JB.00198-08
- de Bruin OM, Duplantis BN, Ludu JS, et al. The biochemical properties of the Francisella pathogenicity island (FPI)-encoded proteins IglA, IglB, IglC, PdpB and DotU suggest roles in type VI secretion. Microbiology. 2011;157(12):3483–3491. doi: 10.1099/mic.0.052308-0
- Gray CG, Cowley SC, Cheung KK, et al. The identification of five genetic loci of Francisella novicida associated with intracellular growth. FEMS Microbiol Lett. 2002;215(1):53–56. doi: 10.1111/j.1574-6968.2002.tb11369.x
- Napier BA, Meyer L, Bina JE, et al. Link between intraphagosomal biotin and rapid phagosomal escape in Francisella. Proc Natl Acad Sci U S A. 2012;109(44):18084–18089. doi: 10.1073/pnas.1206411109
- Ramond E, Gesbert G, Rigard M, et al. Glutamate utilization couples oxidative stress defense and the tricarboxylic acid cycle in Francisella phagosomal escape. PLOS Pathog. 2014;10(1):e1003893. doi: 10.1371/journal.ppat.1003893
- Radlinski LC, Brunton J, Steele S, et al. Defining the metabolic pathways and host-derived carbon substrates required for Francisella tularensis intracellular growth. MBio. 2018;9(6). doi: 10.1128/mBio.01471-18
- Ramond E, Gesbert G, Guerrera IC, et al. Importance of host cell arginine uptake in Francisella phagosomal escape and ribosomal protein amounts. Mol & Cell Proteomics. 2015;14(4):870–881. doi: 10.1074/mcp.M114.044552
- Gesbert G, Ramond E, Tros F, et al. Importance of branched-chain amino acid utilization in Francisella intracellular adaptation. Infect Immun. 2015;83(1):173–183. doi: 10.1128/IAI.02579-14
- Mohapatra NP, Soni S, Reilly TJ, et al. Combined Deletion of Four Francisella novicida Acid Phosphatases Attenuates Virulence and Macrophage Vacuolar Escape. Infect Immun. 2008;76(8):3690–3699. doi: 10.1128/IAI.00262-08
- Mohapatra NP, Soni S, Rajaram MVS, et al. Type A Francisella tularensis Acid Phosphatases Contribute to Pathogenesis. PLoS One. 2013;8(2):e56834. doi: 10.1371/journal.pone.0056834
- Mohapatra NP, Soni S, Rajaram MVS, et al. Francisella acid phosphatases inactivate the NADPH oxidase in human phagocytes. J Immunol. 2010;184(9):5141–5150. doi: 10.4049/jimmunol.0903413
- Felix J, Siebert C, Ducassou JN, et al. Structural and functional analysis of the Francisella lysine decarboxylase as a key actor in oxidative stress resistance. Sci Rep. 2021;11(1):972. doi: 10.1038/s41598-020-79611-5
- Larsson P, Oyston PC, Chain P, et al. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet. 2005;37(2):153–159. doi: 10.1038/ng1499
- Mahawar M, Kirimanjeswara GS, Metzger DW, et al. Contribution of citrulline ureidase to Francisella tularensis strain Schu S4 pathogenesis. J Bacteriol. 2009;191(15):4798–4806. doi: 10.1128/JB.00212-09
- Brissac T, Ziveri J, Ramond E, et al. Gluconeogenesis, an essential metabolic pathway for pathogenic Francisella. Mol Microbiol. 2015;98(3):518–534. doi: 10.1111/mmi.13139
- Gesbert G, Ramond E, Rigard M, et al. Asparagine assimilation is critical for intracellular replication and dissemination of Francisella. Cell Microbiol. 2014;16(3):434–449. doi: 10.1111/cmi.12227
- Ziveri J, Barel M, Charbit A. Importance of metabolic adaptations in Francisella pathogenesis. Front Cell Infect Microbiol. 2017;7:96. doi: 10.3389/fcimb.2017.00096
- Dominguez SR, Whiles S, Deobald KN, et al. Francisella tularensis exploits AMPK activation to harvest host-derived nutrients liberated from host Lipolysis. Infect Immun. 2022;90(8):e0015522. doi: 10.1128/iai.00155-22
- Meibom KL, Charbit A. Francisella tularensis metabolism and its relation to virulence. Front Microbiol. 2010;1:140. doi: 10.3389/fmicb.2010.00140
- Traub A, Mager J, Grossowicz N. STUDIES on the NUTRITION of PASTEURELLA TULARENSIS. J Bacteriol. 1955;70(1):60–69. doi: 10.1128/jb.70.1.60-69.1955
- Wang Y, Ledvina HE, Tower CA et al. Discovery of a unique pathway for glutathione utilization in Francisella. Cell Host Microbe. 2023 Aug 9;31(8):1359-1370.e7. doi:10.1016/j.chom.2023.06.010.
- Ramsey KM, Ledvina HE, Tresko TM, et al. Tn-Seq reveals hidden complexity in the utilization of host-derived glutathione in Francisella tularensis. PLOS Pathog. 2020;16(6):e1008566. doi: 10.1371/journal.ppat.1008566
- Alkhuder K, Meibom KL, Dubail I, et al. Glutathione provides a source of cysteine essential for intracellular multiplication of Francisella tularensis. PLOS Pathog. 2009;5(1):e1000284. doi: 10.1371/journal.ppat.1000284
- Steele S, Brunton J, Ziehr B, et al. Francisella tularensis harvests nutrients derived via ATG5-independent autophagy to support intracellular growth. PLOS Pathog. 2013;9(8):e1003562. doi: 10.1371/journal.ppat.1003562
- Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7(10):767–777. doi: 10.1038/nri2161
- Case EDR, Chong A, Wehrly TD, et al. The Francisella O-antigen mediates survival in the macrophage cytosol via autophagy avoidance. Cell Microbiol. 2014;16(6):862–877. doi: 10.1111/cmi.12246
- Chong A, Wehrly TD, Child R, et al. Cytosolic clearance of replication-deficient mutants reveals Francisella tularensis interactions with the autophagic pathway. Autophagy. 2012;8(9):1342–1356. doi: 10.4161/auto.20808
- Kelava I, Mihelčić M, Ožanič M, et al. Atg5-deficient mice infected with Francisella tularensis LVS demonstrate increased survival and less severe pathology in internal organs. Microorganisms. 2020;8(10):8. doi: 10.3390/microorganisms8101531
- Ray K, Marteyn B, Sansonetti PJ, et al. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7(5):333–340. doi: 10.1038/nrmicro2112
- Jorgensen I, Zhang Y, Krantz BA, et al. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J Exp Med. 2016;213(10):2113–2128. doi: 10.1084/jem.20151613
- Steele SP, Chamberlain Z, Park J, et al. Francisella tularensis enters a double membraned compartment following cell-cell transfer. Elife. 2019;8. doi: 10.7554/eLife.45252
- Nano FE, Zhang N, Cowley SC, et al. A Francisella tularensis pathogenicity island required for intramacrophage growth. J Bacteriol. 2004;186(19):6430–6436. doi: 10.1128/JB.186.19.6430-6436.2004
- Golovliov I, Ericsson M, Sandstrom G, et al. Identification of proteins of Francisella tularensis induced during growth in macrophages and cloning of the gene encoding a prominently induced 23-kilodalton protein. Infect Immun. 1997;65(6):2183–2189. doi: 10.1128/iai.65.6.2183-2189.1997
- de Bruin OM, Ludu JS, Nano FE. The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 2007;7(1):1. doi: 10.1186/1471-2180-7-1
- Bröms JE, Sjöstedt A, Lavander M. The role of the Francisella Tularensis pathogenicity island in type VI secretion, intracellular survival, and modulation of host cell signaling. Front Microbiol [Internet]. 2010 [[cited 2021 May 21]];1. doi: 10.3389/fmicb.2010.00136
- Rigard M, Broms JE, Mosnier A, et al. Francisella tularensis IglG belongs to a novel family of PAAR-Like T6SS proteins and harbors a unique N-terminal extension required for virulence. PLOS Pathog. 2016;12(9):e1005821. doi: 10.1371/journal.ppat.1005821
- Broms JE, Sjostedt A, Lavander M. The role of the Francisella Tularensis pathogenicity island in type VI secretion, intracellular survival, and modulation of host cell signaling. Front Microbiol. 2010;1:1. doi: 10.3389/fmicb.2010.00136
- Rigard M, Bröms JE, Mosnier A, et al. Francisella tularensis IglG belongs to a novel family of PAAR-Like T6SS proteins and harbors a unique N-terminal extension required for virulence. PLOS Pathogens [Internet]. 2016 [[cited 2023 Apr 3]];12(9):e1005821. doi: 10.1371/journal.ppat.1005821
- Rapisarda C, Cherrak Y, Kooger R, et al. In situ and high-resolution cryo-EM structure of a bacterial type VI secretion system membrane complex. EMBO J. 2019;38(10). doi: 10.15252/embj.2018100886
- Russell AB, Wexler AG, Harding BN, et al. A type VI secretion-related pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe. 2014;16(2):227–236. doi: 10.1016/j.chom.2014.07.007
- Tian D, Uda A, Ami Y, et al. Protective effects of the Francisella tularensis ΔpdpC mutant against its virulent parental strain SCHU P9 in cynomolgus macaques. Sci Rep. 2019;9(1):9193. doi: 10.1038/s41598-019-45412-8
- Brodmann M, Schnider ST, Basler M, et al. Type VI secretion system and its effectors PdpC, PdpD, and OpiA contribute to Francisella virulence in Galleria mellonella larvae. Infect Immun. 2021;89(7):e0057920. doi: 10.1128/IAI.00579-20
- Brodmann M, Dreier RF, Broz P, et al. Francisella requires dynamic type VI secretion system and ClpB to deliver effectors for phagosomal escape. Nat Commun. 2017;8(1):15853. doi: 10.1038/ncomms15853
- Lindgren M, Broms JE, Meyer L, et al. The Francisella tularensis LVS ΔpdpCmutant exhibits a unique phenotype during intracellular infection. BMC Microbiol. 2013;13(1):20. doi: 10.1186/1471-2180-13-20
- Meyer L, Broms JE, Liu X, et al. Microinjection of Francisella tularensis and listeria monocytogenes reveals the importance of bacterial and host factors for successful replication. Infect Immun. 2015;83(8):3233–3242. doi: 10.1128/IAI.00416-15
- Steele S, Taft-Benz S, Kawula T, et al. A method for functional trans-complementation of intracellular Francisella tularensis. PLoS One. 2014;9(2):e88194. doi: 10.1371/journal.pone.0088194
- Liu X, Clemens DL, Lee B-Y, et al. Atomic structure of IglD demonstrates its role as a component of the baseplate complex of the Francisella type VI secretion system.mBio [Internet]. 2022 [[cited 2023 Apr 17]];13(5):e01277–22. doi: 10.1128/mbio.01277-22
- Clemens DL, Ge P, Lee B-Y, et al. Atomic structure of T6SS reveals interlaced array essential to function. Cell. 2015;160(5):940–951. doi: 10.1016/j.cell.2015.02.005
- Yang X, Clemens DL, Lee B-Y, et al. Atomic structure of the Francisella T6SS central spike reveals a unique α-helical lid and a putative cargo. Structure. 2019;27(12):1811–1819.e6. doi: 10.1016/j.str.2019.10.007
- Cherrak Y, Flaugnatti N, Durand E, et al. Structure and activity of the type VI secretion system. Microbiol Spectr [Internet]. 2019 [cited 2023 Mar 31];7(4):7.4.11. doi: 10.1128/microbiolspec.PSIB-0031-2019
- Robb CS, Nano FE, Boraston AB. Cloning, expression, purification, crystallization and preliminary X-ray diffraction analysis of intracellular growth locus E (IglE) protein from Francisella tularensis subsp. novicida. Acta Crystallogr Sect F. 2010;66(12):1596–1598. doi: 10.1107/S1744309110034378
- Nguyen JQ, Gilley RP, Zogaj X, et al. Lipidation of the FPI protein IglE contributes to Francisella tularensis ssp. novicida intramacrophage replication and virulence.Pathog Dis [Internet]. 2014 [[cited 2023 Apr 6]];72(1):10–18. doi: 10.1111/2049-632X.12167
- Broms JE, Meyer L, Sjostedt A. A mutagenesis-based approach identifies amino acids in the N-terminal part of Francisella tularensis IglE that critically control type VI system-mediated secretion. Virulence. 2017;8(6):821–847. doi: 10.1080/21505594.2016.1258507
- Shalom G, Shaw JG, Thomas MS. In vivo expression technology identifies a type VI secretion system locus in Burkholderia pseudomallei that is induced upon invasion of macrophages. Microbiology. 2007;153(8):2689–2699. doi: 10.1099/mic.0.2007/006585-0
- Durand E, Zoued A, Spinelli S, et al. Structural characterization and oligomerization of the TssL protein, a component shared by bacterial type VI and type IVb secretion systems *. J Biol Chem. 2012;287(17):14157–14168. doi: 10.1074/jbc.M111.338731
- Robb CS, Nano FE, Boraston AB. The structure of the conserved type six secretion protein TssL (DotU) from Francisella novicida.J Mol Biol [Internet]. 2012 [[cited 2023 Apr 6]];419(5):277–283. doi: 10.1016/j.jmb.2012.04.003
- Durand E, Nguyen VS, Zoued A, et al. Biogenesis and structure of a type VI secretion membrane core complex. Nat. 2015 [[cited 2023 Apr 6]];523(7562):555–560. doi: 10.1038/nature14667
- Shneider MM, Buth SA, Ho BT, et al. PAAR-repeat proteins sharpen and diversify the type VI secretion system spike. Nature. 2013;500(7462):350–353. doi: 10.1038/nature12453
- Zhang Z, Liu Y, Zhang P, et al., . PAAR Proteins Are Versatile Clips That Enrich the Antimicrobial Weapon Arsenals of Prokaryotes. mSystems. 2021;6(6):e0095321. doi: 10.1128/mSystems.00953-21
- Alam A, Golovliov I, Javed E, et al. ClpB mutants of Francisella tularensis subspecies holarctica and tularensis are defective for type VI secretion and intracellular replication. Sci Rep. 2018;8(1):11324. doi: 10.1038/s41598-018-29745-4
- Alam A, Golovliov I, Javed E, et al. Dissociation between the critical role of ClpB of Francisella tularensis for the heat shock response and the DnaK interaction and its important role for efficient type VI secretion and bacterial virulence. PLOS Pathog. 2020;16(4):e1008466. doi: 10.1371/journal.ppat.1008466
- Wehrly TD, Chong A, Virtaneva K, et al. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol [Internet]. 2009 [[cited 2023 Apr 26]];11(7):1128–1150. doi: 10.1111/j.1462-5822.2009.01316.x
- Brotcke A, Weiss DS, Kim CC, et al. Identification of MglA-Regulated genes reveals novel virulence factors in Francisella tularensis. Infect Immun. 2006;74(12):6642–6655. doi: 10.1128/IAI.01250-06
- Baron GS, Nano FE. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol Microbiol. 1998;29(1):247–259. doi: 10.1046/j.1365-2958.1998.00926.x
- Lauriano CM, Barker JR, Yoon S-S, et al. MglA regulates transcription of virulence factors necessary for Francisella tularensis intraamoebae and intramacrophage survival. Proc Natl Acad Sci U S A. 2004;101(12):4246–4249. doi: 10.1073/pnas.0307690101
- Travis BA, Ramsey KM, Prezioso SM, et al. Structural Basis for Virulence Activation of Francisella tularensis. Mol Cell[internet]. 2021 [[cited 2023 Apr 27]];81(1):139–152.e10. doi: 10.1016/j.molcel.2020.10.035
- Charity JC, Blalock LT, Costante-Hamm MM, et al. Small molecule control of virulence gene expression in Francisella tularensis. PLOS Pathog. 2009;5(10):e1000641. doi: 10.1371/journal.ppat.1000641
- Brotcke A, Monack DM. Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida. Infect Immun. 2008;76(8):3473–3480. doi: 10.1128/IAI.00430-08
- Cuthbert BJ, Ross W, Rohlfing AE, et al. Dissection of the molecular circuitry controlling virulence in Francisella tularensis. Genes Dev. 2017;31(15):1549–1560. doi: 10.1101/gad.303701.117
- Ramsey KM, Osborne ML, Vvedenskaya IO, et al. Ubiquitous promoter-localization of essential virulence regulators in Francisella tularensis. PLOS Pathog. 2015;11(4):e1004793. doi: 10.1371/journal.ppat.1004793
- Ramsey KM, Osborne ML, Vvedenskaya IO, et al. Ubiquitous Promoter-Localization of Essential Virulence Regulators in Francisella tularensis.PLOS Pathogens [Internet]. 2015 [[cited 2023 Apr 27]];11(4):e1004793. doi: 10.1371/journal.ppat.1004793
- Stojkova P, Spidlova P, Lenco J, et al. HU protein is involved in intracellular growth and full virulence of Francisella tularensis. Virulence. 2018;9(1):754–770. doi: 10.1080/21505594.2018.1441588
- Pavlik P, Spidlova P. Arginine 58 is indispensable for proper function of the Francisella tularensis subsp. holarctica FSC200 HU protein, and its substitution alters virulence and mediates immunity against wild-type strain. Virulence. 2022;13(1):1790–1809. doi: 10.1080/21505594.2022.2132729
- Deng K, Blick RJ, Liu W, et al. Identification of Francisella tularensis genes affected by iron limitation.Infect Immun [Internet]. 2006 [[cited 2023 Apr 26]];74(7):4224–4236. doi: 10.1128/IAI.01975-05
- Ziveri J, Chhuon C, Jamet A, et al. Critical role of a sheath phosphorylation site on the assembly and function of an atypical type VI secretion system. Mol & Cell Proteomics. 2019;18(12):2418–2432. doi: 10.1074/mcp.RA119.001532
- Wallet P, Lagrange B, Henry T. Francisella Inflammasomes: Integrated responses to a cytosolic stealth bacterium. Curr Top Microbiol Immunol. 2016;397:229–256.
- Jones B, Faron M, Rasmussen J, et al. Uncovering the components of the Francisella tularensis virulence stealth strategy. Front Cell Infect Microbiol. 2014;4. doi: 10.3389/fcimb.2014.00032
- Sjöstedt A. Intracellular survival mechanisms of Francisella tularensis, a stealth pathogen. Microbes Infect. 2006;8(2):561–567. doi: 10.1016/j.micinf.2005.08.001
- Okan NA, Kasper DL. The atypical lipopolysaccharide of Francisella. Carbohydr Res. 2013;378:79–83. doi: 10.1016/j.carres.2013.06.015
- Hajjar AM, Harvey MD, Shaffer SA, et al. Lack of in vitro and in vivo recognition of Francisella tularensis subspecies lipopolysaccharide by Toll-like receptors. Infect Immun. 2006;74(12):6730–6738. doi: 10.1128/IAI.00934-06
- Lagrange B, Benaoudia S, Wallet P, et al. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat Commun. 2018;9(1):242. doi: 10.1038/s41467-017-02682-y
- Tan Y, Zanoni I, Cullen TW, et al. Mechanisms of Toll-like receptor 4 endocytosis reveal a common immune-evasion strategy used by pathogenic and commensal bacteria. Immunity. 2015;43(5):909–922. doi: 10.1016/j.immuni.2015.10.008
- Hong K-J, Wickstrum JR, Yeh H-W, et al. Toll-like receptor 2 controls the gamma interferon response to Francisella tularensis by mouse liver lymphocytes. Infect Immun. 2007;75(11):5338–5345. doi: 10.1128/IAI.00561-07
- Jones CL, Weiss DS, Moreno E. TLR2 signaling contributes to rapid inflammasome activation during F. novicida infection. PLoS One. 2011;6(6):e20609. doi: 10.1371/journal.pone.0020609
- Katz J, Zhang P, Martin M, et al. Toll-like receptor 2 is required for inflammatory responses to Francisella tularensis LVS. Infect Immun. 2006;74(5):2809–2816. doi: 10.1128/IAI.74.5.2809-2816.2006
- Malik M, Bakshi CS, Sahay B, et al. Toll-like receptor 2 is required for control of pulmonary infection with Francisella tularensis. Infect Immun. 2006;74(6):3657–3662. doi: 10.1128/IAI.02030-05
- Li H, Nookala S, Bina XR, et al. Innate immune response to Francisella tularensis is mediated by TLR2 and caspase-1 activation. J Leukocyte Biol. 2006;80(4):766–773. doi: 10.1189/jlb.0406294
- Thakran S, Li H, Lavine CL, et al. Identification of Francisella tularensis lipoproteins that stimulate the toll-like receptor (TLR) 2/TLR1 heterodimer. J Biol Chem. 2008;283(7):3751–3760. doi: 10.1074/jbc.M706854200
- Jones CL, Sampson TR, Nakaya HI, et al. Repression of bacterial lipoprotein production by Francisella novicida facilitates evasion of innate immune recognition. Cell Microbiol [Internet]. 2012;14(10):1531–1543. http://www.ncbi.nlm.nih.gov/pubmed/22632124
- Sampson TR, Saroj SD, Llewellyn AC, et al. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013;497(7448):254–257. doi: 10.1038/nature12048
- Hagar JA, Powell DA, Aachoui Y, et al. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science. 2013;341(6151):1250–1253. doi: 10.1126/science.1240988
- Vinogradov E, Perry MB, Conlan JW. Structural analysis of Francisella tularensis lipopolysaccharide. Eur J Biochem [Internet]. 2002;269(24):6112–6118. doi: 10.1046/j.1432-1033.2002.03321.x
- García-Weber D, Arrieumerlou C. ADP-heptose: a bacterial PAMP detected by the host sensor ALPK1. Cell Mol Life Sci. 2021;78(1):17–29. doi: 10.1007/s00018-020-03577-w
- Okan NA, Chalabaev S, Kim T-H, et al. Kdo hydrolase is required for Francisella tularensis virulence and evasion of TLR2-mediated innate immunity. MBio. 2013;4(1):e00638–00612. doi: 10.1128/mBio.00638-12
- Wang X, Ribeiro AA, Guan Z, et al. Structure and biosynthesis of free lipid a molecules that replace lipopolysaccharide in Francisella tularensis subsp. novicida. Biochemistry. 2006;45(48):14427–14440. doi: 10.1021/bi061767s
- Wang X, Karbarz MJ, McGrath SC, et al. MsbA transporter-dependent lipid a 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of Francisella novicida LpxE expressed in Escherichia coli. J Biol Chem. 2004;279(47):49470–49478. doi: 10.1074/jbc.M409078200
- Kanistanon D, Hajjar AM, Pelletier MR, et al. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLOS Pathog. 2008;4(2):e24. doi: 10.1371/journal.ppat.0040024
- Kanistanon D, Powell DA, Hajjar AM, et al. Role of Francisella lipid A phosphate modification in virulence and long-term protective immune responses. Infect Immun. 2012;80(3):943–951. doi: 10.1128/IAI.06109-11
- Wang X, Ribeiro AA, Guan Z, et al. Attenuated virulence of a Francisella mutant lacking the lipid A 4′-phosphatase. Proc Natl Acad Sci U S A. 2007;104(10):4136–4141. doi: 10.1073/pnas.0611606104
- Llewellyn AC, Zhao J, Song F, et al. NaxD is a deacetylase required for lipid a modification and Francisella pathogenesis. Mol Microbiol. 2012;86(3):611–627. doi: 10.1111/mmi.12004
- Rowe HM, Huntley JF. From the outside-in: the Francisella tularensis envelope and virulence. Front Cell Infect Microbiol. 2015;5:94. doi: 10.3389/fcimb.2015.00094
- Hood AM. Virulence factors of Francisella tularensis. J Hyg (Lond). 1977;79(1):47–60. doi: 10.1017/S0022172400052840
- Apicella MA, Post DMB, Fowler AC, et al. Identification, characterization and immunogenicity of an O-antigen capsular polysaccharide of Francisella tularensis. PLoS One. 2010;5(7):e11060. doi: 10.1371/journal.pone.0011060
- Ireland R, Wang R, Alinger JB, et al. Francisella tularensis SchuS4 and SchuS4 lipids inhibit IL-12p40 in primary human dendritic cells by inhibition of IRF1 and IRF8. J Immunol. 2013;191(3):1276–1286. doi: 10.4049/jimmunol.1300867
- Ireland R, Schwarz B, Nardone G, et al. Unique Francisella phosphatidylethanolamine acts as a potent anti-inflammatory lipid. J Innate Immun. 2018;10(4):291–305. doi: 10.1159/000489504
- Forsberg AKE, Guina T. Type II secretion and type IV pili of Francisella. Ann NY Acad Sci %R. 2007;101196(annals14090161105):187–201. doi: 10.1196/annals.1409.016
- Gil H, Platz GJ, Forestal CA, et al. Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proc Natl Acad Sci, USA. 2006;103(34):12897–12902. Internet. doi: 10.1073/pnas.0602582103
- Kopping EJ, Doyle CR, Sampath V, et al. Contributions of TolC orthologs to Francisella tularensis Schu S4 multidrug resistance, modulation of host cell responses, and virulence. Infect Immun. 2019;87(4). doi: 10.1128/IAI.00823-18
- Peng K, Broz P, Jones J, et al. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell Microbiol. 2011;13(10):1586–1600. doi: 10.1111/j.1462-5822.2011.01643.x
- Zogaj X, Chakraborty S, Liu J, et al. Characterization of the Francisella tularensis subsp. novicida type IV pilus. Microbiology. 2008;154(7):2139–2150. doi: 10.1099/mic.0.2008/018077-0
- Hager AJ, Bolton DL, Pelletier MR, et al. Type IV pili-mediated secretion modulates Francisella virulence. Mol Microbiol. 2006;62(1):227–237. doi: 10.1111/j.1365-2958.2006.05365.x
- Ark NM, Mann BJ. Impact of Francisella tularensis pilin homologs on pilus formation and virulence. Microb Pathog. 2011;51(3):110–120. doi: 10.1016/j.micpath.2011.05.001
- Spidlova P, Stojkova P, Sjöstedt A, et al. Control of Francisella tularensis virulence at Gene Level: network of transcription factors. Microorganisms. 2020;8(10):8. doi: 10.3390/microorganisms8101622
- Moule MG, Monack DM, Schneider DS, et al. Reciprocal analysis of Francisella novicida infections of a drosophila melanogaster model reveal host-pathogen conflicts mediated by reactive oxygen and imd-regulated innate immune response. PLOS Pathog. 2010;6(8):e1001065. doi: 10.1371/journal.ppat.1001065
- Dean SN, Milton ME, Cavanagh J, et al. Francisella novicida two-component system response regulator BfpR modulates iglC gene expression, antimicrobial peptide resistance, and biofilm production. Front Cell Infect Microbiol. 2020;10:82. doi: 10.3389/fcimb.2020.00082
- Honn M, Lindgren H, Bharath GK, et al. Lack of OxyR and KatG results in extreme susceptibility of Francisella tularensis LVS to oxidative stress and marked attenuation in vivo. Front Cell Infect Microbiol. 2017;7:14. doi: 10.3389/fcimb.2017.00014
- Bakshi CS, Malik M, Regan K, et al. Superoxide dismutase B gene (sodB)-deficient mutants of Francisella tularensis demonstrate hypersensitivity to oxidative stress and attenuated virulence. J Bacteriol. 2006;188(17):6443–6448. doi: 10.1128/JB.00266-06
- Alharbi A, Rabadi SM, Alqahtani M, et al. Role of peroxiredoxin of the AhpC/TSA family in antioxidant defense mechanisms of Francisella tularensis. PLoS One. 2019;14(3):e0213699. doi: 10.1371/journal.pone.0213699
- Ma Z, Higgs M, Alqahtani M, et al. ThioredoxinA1 controls the oxidative stress response of Francisella tularensis live vaccine strain (LVS). J Bacteriol. 2022;204(5):e0008222. doi: 10.1128/jb.00082-22
- Straskova A, Pavkova I, Link M, et al. Proteome analysis of an attenuated Francisella tularensis dsbA mutant: identification of potential DsbA substrate proteins. J Proteome Res. 2009;8(11):5336–5346. doi: 10.1021/pr900570b
- Chambers JR, Bender KS, Bereswill S. The RNA chaperone hfq is important for growth and stress tolerance in Francisella novicida. PLoS One. 2011;6(5):e19797. doi: 10.1371/journal.pone.0019797
- Bina XR, Lavine CL, Miller MA, et al. The AcrAB RND efflux system from the live vaccine strain of Francisella tularensis is a multiple drug efflux system that is required for virulence in mice. FEMS Microbiol Lett [Internet]. 2008;279(2):226–233. doi: 10.1111/j.1574-6968.2007.01033.x
- Bosio CM. The subversion of the immune system by Francisella tularensis. Front Microbiol. 2011;2:9. doi: 10.3389/fmicb.2011.00009
- Jones JW, Kayagaki N, Broz P, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107(21):9771–9776. doi: 10.1073/pnas.1003738107
- Storek KM, Gertsvolf NA, Ohlson MB, et al. cGAS and Ifi204 cooperate to produce type I IFNs in response to Francisella infection. J Immunol. 2015;194(7):3236–3245. doi: 10.4049/jimmunol.1402764
- Henry T, Brotcke A, Weiss DS, et al. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204(5):987–994. doi: 10.1084/jem.20062665
- Henry T, Kirimanjeswara GS, Ruby T, et al. Type I IFN signaling constrains IL-17A/F secretion by γδ T cells during bacterial infections. J Immunol. 2010;184(7):3755–3767. doi: 10.4049/jimmunol.0902065
- Peignier A, Parker D. Impact of type I interferons on susceptibility to bacterial pathogens. Trends Microbiol. 2021;29(9):823–835. doi: 10.1016/j.tim.2021.01.007
- Bauler TJ, Chase JC, Bosio CM. IFN-β mediates suppression of IL-12p40 in human dendritic cells following infection with virulent Francisella tularensis. J Immunol. 2011;187(4):1845–1855. doi: 10.4049/jimmunol.1100377
- Mohammadi N, Lindgren H, Yamamoto M, et al. Macrophages demonstrate guanylate-binding protein-dependent and bacterial strain-dependent responses to Francisella tularensis. Front Cell Infect Microbiol. 2021;11:784101. doi: 10.3389/fcimb.2021.784101
- Meunier E, Wallet P, Dreier RF, et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol. 2015;16(5):476–484. doi: 10.1038/ni.3119
- Feng S, Enosi Tuipulotu D, Pandey A, et al. Pathogen-selective killing by guanylate-binding proteins as a molecular mechanism leading to inflammasome signaling. Nat Commun. 2022;13(1):4395. doi: 10.1038/s41467-022-32127-0
- Man SM, Karki R, Malireddi RKS, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol. 2015;16(5):467–475. doi: 10.1038/ni.3118
- Wallet P, Benaoudia S, Mosnier A, et al. IFN-γ extends the immune functions of guanylate binding proteins to inflammasome-independent antibacterial activities during Francisella novicida infection. PLOS Pathog. 2017;13(10):e1006630. doi: 10.1371/journal.ppat.1006630
- Fernandes-Alnemri T, Yu JW, Juliana C, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11(5):385–393. doi: 10.1038/ni.1859
- Periasamy S, Le HT, Duffy EB, et al. Inflammasome-independent NLRP3 restriction of a protective Early neutrophil response to pulmonary tularemia. PLOS Pathog. 2016;12(12):e1006059. doi: 10.1371/journal.ppat.1006059
- Valeva SV, Degabriel M, Michal F, et al. Comparative study of GBP recruitment on two cytosol-dwelling pathogens, Francisella novicida and shigella flexneri highlights differences in GBP repertoire and in GBP1 motif requirements. Pathog Dis. 2023;81:ftad005. doi: 10.1093/femspd/ftad005
- Mohammadi N, Lindgren H, Golovliov I, et al. Guanylate-binding proteins are critical for effective control of Francisella tularensis strains in a mouse co-culture system of Adaptive immunity. Front Cell Infect Microbiol. 2020;10:594063. doi: 10.3389/fcimb.2020.594063
- Elkins KL, Rhinehart-Jones TR, Culkin SJ, et al. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect Immun. 1996;64(8):3288–3293. doi: 10.1128/iai.64.8.3288-3293.1996
- Anthony LS, Morrissey PJ, Nano FE. Growth inhibition of Francisella tularensis live vaccine strain by IFN-gamma-activated macrophages is mediated by reactive nitrogen intermediates derived from L-arginine metabolism. J Immunol. 1992;148(6):1829–1834. doi: 10.4049/jimmunol.148.6.1829
- Lindgren H, Stenman L, Tarnvik A, et al. The contribution of reactive nitrogen and oxygen species to the killing of Francisella tularensis LVS by murine macrophages. Microbes Infect. 2005;7(3):467–475. doi: 10.1016/j.micinf.2004.11.020
- Lindgren H, Stenmark S, Chen W, et al. Distinct roles of reactive nitrogen and oxygen species to control infection with the facultative intracellular bacterium Francisella tularensis. Infect Immun. 2004;72(12):7172–7182. doi: 10.1128/IAI.72.12.7172-7182.2004
- Golovliov I, Lindgren H, Eneslätt K, et al. An In Vitro Co-culture Mouse Model Demonstrates Efficient Vaccine-Mediated Control of Francisella tularensis SCHU S4 and Identifies Nitric Oxide as a Predictor of Efficacy. Front Cell Infect Microbiol. 2016;6:152. doi: 10.3389/fcimb.2016.00152
- Edwards JA, Rockx-Brouwer D, Nair V, et al. Restricted cytosolic growth of Francisella tularensis subsp. tularensis by IFN-γ activation of macrophages. Microbiol [Internet]. 2009;156(2):327–339. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19926654
- Jessop F, Buntyn R, Schwarz B, et al. Interferon gamma reprograms host mitochondrial metabolism through inhibition of complex II to control intracellular bacterial replication. Infect Immun. 2020;88(2):88. doi: 10.1128/IAI.00744-19
- Sarria JC, Vidal AM, Kimbrough RC, et al. Fatal infection caused by Francisella tularensis in a neutropenic bone marrow transplant recipient. Ann Hematol. 2003;82(1):41–43. doi: 10.1007/s00277-002-0570-4
- Kinkead LC, Allen L-A. Multifaceted effects of Francisella tularensis on human neutrophil function and lifespan. Immunol Rev. 2016;273(1):266–281. doi: 10.1111/imr.12445
- Sharma J, Mares CA, Li Q, et al. Features of sepsis caused by pulmonary infection with Francisella tularensis type a strain. Microb Pathog. 2011;51(1–2):39–47. doi: 10.1016/j.micpath.2011.03.007
- Roberts LM, Powell DA, Frelinger JA. Adaptive immunity to Francisella tularensis and considerations for vaccine development. Front Cell Infect Microbiol. 2018;8:115. doi: 10.3389/fcimb.2018.00115
- Kubelkova K, Macela A. Francisella and Antibodies. Microorganisms. 2021;9(10):2136. doi: 10.3390/microorganisms9102136
- Del Barrio L, Sahoo M, Lantier L, et al. Production of anti-LPS IgM by B1a B cells depends on IL-1β and is protective against lung infection with Francisella tularensis LVS. PLOS Pathog. 2015;11(3):e1004706. doi: 10.1371/journal.ppat.1004706
- Elkins KL, Cowley SC, Bosio CM. Innate and Adaptive immunity to Francisella. Ann NY Acad Sci %R. 2007;101196(annals14090141105):284–324. doi: 10.1196/annals.1409.014
- Fortier AH, Polsinelli T, Green SJ, et al. Activation of macrophages for destruction of Francisella tularensis: identification of cytokines, effector cells, and effector molecules. Infect Immun. 1992;60(3):817–825. doi: 10.1128/iai.60.3.817-825.1992
- Sjostedt A, North RJ, Conlan JW. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology. 1996;142(Pt 6):1369–1374. doi: 10.1099/13500872-142-6-1369
