ABSTRACT
Chlamydia infection is an important cause of public health diseases, and no effective vaccine is currently available. Owing to its unique intracellular lifestyle, Chlamydia requires a variety of nutrients and substrates from host cells, particularly sphingomyelin, cholesterol, iron, amino acids, and the mannose-6-phosphate receptor, which are essential for inclusion development. Here, we summarize the recent advances in Chlamydia nutrient acquisition mechanism by hijacking host cell vesicular transport, which plays an important role in chlamydial growth and development. Chlamydia obtains the components necessary to complete its intracellular developmental cycle by recruiting Rab proteins (major vesicular trafficking regulators) and Rab effector proteins to the inclusion, interfering with Rab-mediated multivesicular trafficking, reorienting the nutrition of host cells, and reconstructing the intracellular niche environment. Consequently, exploring the role of vesicular transport in nutrient acquisition offers a novel perspective on new approaches for preventing and treating Chlamydia infection.
Introduction
Chlamydia is obligate intracellular parasitic pathogens with a unique developmental cycle that is completely dependent on host cell nutrients. Chlamydia spp. comprise multiple species, such as Chlamydia psittaci, Chlamydia suis, Chlamydia abortus, Chlamydia caviae, Chlamydia pecorum, Chlamydia felis, Chlamydia avium, Chlamydia gallinacea, Chlamydia muridarum, Chlamydia pneumoniae, and Chlamydia trachomatis. Among these, C. trachomatis and C. pneumoniae are the major species that threaten human health. C. pneumoniae causes respiratory tract infections, and is associated with various chronic diseases [Citation1]. C. trachomatis is responsible for several acute and chronic infectious diseases, whichle not only cause infertility, ectopic pregnancy, blindness, and pelvic inflammatory disease [Citation2–5], but also contribute to the spread and increased incidence of HIV [Citation6]. Currently, C. trachomatis infections are a global epidemic, with > 130 million people being infected each year worldwide [Citation7].
All Chlamydiae undergo a unique two-phase developmental cycle, alternating between the infectious but non-reproductive form, which is also called the elementary body (EB), and the replicative but non-infectious form, also called the reticulate body (RB) [Citation8]. During the infection process, Chlamydia accomplishes biosynthesis, replication, and differentiation within inclusions, a membrane-bundled parasitophorous vacuole that not only protects Chlamydia from host cell immune attack, but also provides nutrients for chlamydial replication [Citation9,Citation10]. After EBs are internalized into inclusions, chlamydial organisms interact with host cells by secreting effector proteins to hijack host cell vesicular transport, thereby obtaining nutrients necessary for growth and interfering with various host cell functions, creating a safe environment conducive to chlamydial growth and replication.
Rab GTPases, which belong to the largest family of the Ras-like small GTPase superfamily, play an important role in vesicle production, transport, docking, fusion, and various other membrane transport functions through specifically recruiting effector molecules [Citation11]. Previous studies have shown that many intracellular bacterial pathogens, including Mycobacterium tuberculosis [Citation12], Salmonella typhimurium [Citation13], and Legionella pneumophila [Citation14], interact with Rab GTPases to regulate the biogenesis of the phagosomes in which they reside and ensure their intracellular survival. Rab GTPases are associated with a set of effector proteins that regulate distinct aspects of membrane dynamics, thereby regulating vesicular transport. Interactions between active GTP-bound Rab GTPases and these effector proteins promote vesicle budding, division, transport, membrane fusion, and tethering to their target sites, a collaborative system that ensures precise and efficient intracellular cargo delivery [Citation15,Citation16]. Rabs and their effectors are targets for pathogens that manipulate them to evade host defences and accomplish intracellular replication [Citation15].
Attributed to an evolutionary reduced genome [Citation17,Citation18], Chlamydia spp. lack several biosynthetic pathways for growth and development and has evolved various unique strategies to supplement lipids [Citation19,Citation20], amino acids [Citation21], irons [Citation20,Citation22], and other nutrients from the host cells. Overcoming nutritional limitations by interfering with host cell processes and metabolism is becoming a hallmark of the pathogenesis of this intracellular pathogen, ensuring access to sufficient nutrients for stable replication within host cells. Therefore, nutrient acquisition is an important aspect of Chlamydia pathogenesis. However, the molecular mechanisms by which Chlamydia obtains nutrients from the host cells have not yet been elucidated.
Here, we discuss novel strategies used by Chlamydia, an obligate intracellular parasite, to manipulate subcellular trafficking pathways for growth. In particular, we focused on the extensive interactions between Chlamydia and Rab GTPase families to disrupt host vesicular transport pathways, thereby highlighting the mechanisms by which Chlamydia obtains nutrients for their intracellular replication and growth.
Biological characteristics of chlamydial developmental cycle
Chlamydia is a class of gram-negative obligate intracellular bacteria with a unique two-phase developmental cycle. Chlamydial intracellular infections begin when the EB enters the cell by swallowing and quickly differentiates into RBs [Citation23,Citation24]. After intracellular RB matures, it re-differentiates into progeny EBs, is released from the infected host cell, and infects new target cells to initiate a new developmental cycle. Label-free quantitative proteomic analysis confirmed that the EB contains multiple proteins involved in central and glucose metabolism, which supports the metabolic requirements for EB invasion into host cells and subsequent differentiation into RB, thereby triggering metabolic activities. In contrast, the RBs are enriched in proteins related to protein synthesis, ATP production, and nutrient transport, providing the energy and nutrients necessary for chlamydial replication from host cells [Citation23].
Chlamydia completes the entire developmental cycle of the eukaryotic cells. As a strict intracellular parasite, it interacts with host cells and regulates key signalling pathways in host cells to establish a relatively stable subcellular environment and escape host immune defence mechanisms, thereby preventing the elimination of pathogen by host immune responses [Citation25,Citation26]. Various host cell-manipulating strategies have been developed to create replication-conducive environments.
Chlamydia interacts with host cells to acquire nutrients
Chlamydia cannot synthesize the high-energy compounds ATP and GTP, and its entire growth and developmental cycle relies on host metabolites to survive and reproduce. To maintain normal growth and nutrient uptake in the host cell, Chlamydia interacts with the host cell to modify the internal environment and facilitate its developmental process [Citation25]. Previous studies have verified that Chlamydia widely alters host protein expression, thus changing the biological behaviour of the host cell to complete its normal growth and development [Citation27]. Phosphorylation proteomic and transcriptomic analysis confirmed that Chlamydia regulates host protein phosphorylation and participates in epithelial – mesenchymal transition (EMT), providing new evidence for the carcinogenic potential of Chlamydia [Citation28]. Importantly, this study highlights that the interaction between Chlamydia and host cells goes beyond altering host protein expression. It also changes the modification of host proteins, which ultimately impacts host cell biological functions. These dynamic alterations in protein expression and modifications represent essential mechanisms that contribute to the Chlamydia-initiated pathogenic processes.
The Chlamydia genome contains approximately 900 open reading frames (ORFs) [Citation29], which encode several effector proteins that are transported to the inclusion membrane and cytoplasm through specific secretion systems and then interact with host cells to manipulate host functions [Citation30,Citation31]. Some effector proteins are transported to the chlamydial surface by the Type V secretion system (T5SS), while others are translocated to the inclusion lumen via the Type II secretion system (T2SS) and a subset (over 100 effector proteins) is delivered to the host cell cytoplasm or inclusion membrane through the Type III secretion system (T3SS) [Citation8,Citation32], which is a key device in Chlamydia virulence.
Chlamydia interaction with host cells is mediated through effector protein secretion, alteration of host cell gene expression and protein stability, and regulation of host cell signal pathway transduction, thereby establishing a suitable subcellular environment for survival and complete growth and development [Citation27,Citation33,Citation34]. The T3SS primarily transports pathogen effector proteins to the host cell cytoplasm through contact with the host cell membrane [Citation35], which is the key to Chlamydia pathogenicity [Citation36,Citation37]. Inclusion membrane proteins (Incs), a large subset of effectors, are translocated by the T3SS and inserted into the inclusion membrane [Citation38], modulating the host – bacterium interaction interface.
Incs act as an interface that mediates the interaction between Chlamydia and host cells. Through this interfacial interaction, Chlamydia interferes with cell trafficking pathways and regulates host cell metabolism to relocate host metabolites, sequentially obtaining key nutritional substrates from cells, which is extremely important for intracellular growth and development. Till date, the identified interactions between Inc proteins and host cells suggest that Incs are involved in various cellular activities, including host cellular cytoskeleton modulation [Citation39], membrane trafficking modulation [Citation39], centrosome positioning [Citation40,Citation41], Golgi distribution [Citation40], lipid procurement [Citation42], and programmed cell death pathway evasion [Citation43]. As important T3SS effector proteins of Chlamydia, Incs interact with host cells to hijack vesicular and non-vesicular host lipid transport to meet the nutritional needs of inclusion. A key mechanism for delivering nutrients to Chlamydia involves membrane fusion between host-derived vesicles and inclusion [Citation44,Citation45]. Several studies have noted that soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) [Citation46], ADP-ribosylating factors (ARFs) [Citation47], and Rab GTPases are closely related to the regulation of membrane-bound vesicle fusion and vesicular trafficking [Citation11] (). A family of Incs and T3SS effector proteins facilitate the interaction between vesicular transport regulators (Rabs and ARFs) and the chlamydial inclusion membrane for nutrient acquisition (). Previous studies have shown that SNAREs play a critical role in the development and pathogenicity of pathogens by mediating cell membrane fusion and vesicular transport [Citation66–68]. The biological activity of a protein is closely related to its structure. Interestingly, Inc proteins are partially homologous with the coiled-coil or SNARE-like domain [Citation69–71], which implies that chlamydial Inc proteins interact with host cells to regulate eukaryotic intracellular transport and membrane fusion. These findings provide evidence that Chlamydia acquires crucial nutrients through interactions between Incs or T3SS effector proteins and pivotal host cell membrane trafficking and intracellular membrane fusion regulators.
Table 1. The role of host vesicular and non-vesicular transport pathway regulators in the nutrient acquisition process of Chlamydia..
Interception of host vesicular and non-vesicular trafficking pathways by Chlamydia for nutrient acquisition
Chlamydia potentially performs the uptake of essential nutrients and biosynthetic precursors from the host by intercepting the vesicular transport pathway, and it contributes to the development of inclusion bodies and survival mechanisms. By intercepting these pathways, Chlamydia gains access to critical resources essential for growth and survival within the host cell. This strategy may represent a key mechanism driving the pathogenic processes of Chlamydia by allowing the bacterium to co-opt the host intracellular transport mechanisms. Although the molecular mechanisms underlying this phenomenon remain unclear, recent evidence suggests that a subset of Rab GTPases and SNARE proteins that play vital roles in regulating membrane trafficking are recruited to the inclusion membrane. This recruitment likely facilitates the diversion of vesicles and other membrane-bound structures towards inclusions, allowing Chlamydia to acquire nutrients and other critical resources necessary for growth and survival within the host cell. Therefore, vesicular transport interception may represent a fundamental mechanism driving Chlamydia pathogenesis [Citation20,Citation60]. Chlamydia also interferes with vesicular and non-vesicular transport pathways to acquire sphingomyelin from host cells by recruiting specific Rab proteins and the lipid transfer protein ceramide-transfer protein (CERT), which is crucial for infection [Citation42]. CERT is a cytoplasmic protein that transports ceramide from the ER to the Golgi, which is essential for sphingomyelin biosynthesis, and may be recruited for inclusion through interactions with the inclusion membrane protein IncD [Citation65,Citation72]. This is also the mechanism by which Chlamydia hijacks host cells via non-vesicular trafficking pathways. Moreover, CERT depletion significantly reduces infectious Chlamydia progeny generation [Citation59,Citation65]. Collectively, these studies demonstrate that Chlamydia effectively acquires essential nutrients from the host cell by hijacking both vesicular and non-vesicular transport mechanisms, ensuring inclusion growth and bacterial development ().
Figure 1. Chlamydia hijacks host vesicular and non-vesicular trafficking pathways to acquire nutrients during infection. Inclusion serves as the interface that mediates the interaction between chlamydia and host cells. A series of effector proteins, including inclusion membrane proteins (incs) and T3SS effectors, interact with soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE), ADP-ribosylating factors (ARFs), and Rab GTPases, relocating from fragmented golgi mini-stacks, multivesicular bodies, endoplasmic reticulum, and endosome to acquire nutrients such as sphingomyelin, cholesterol, iron, cation-independent mannose 6-phosphate receptors (CI-M6PR), and transferrin. In addition, chlamydia hijacks host cell non-vesicular transport through incs–CERT interaction to obtain ceramide. Some elements in the figure have been sourced from the SMART database (https://smart.servier.com).
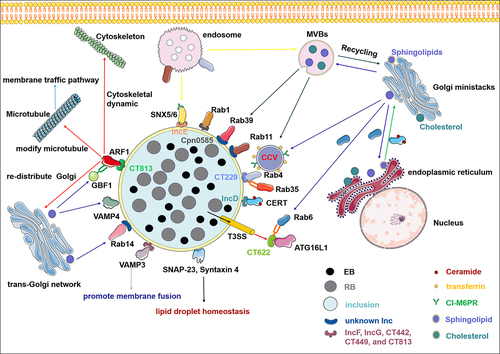
Hijacking the host vesicular trafficking pathways to acquire nutrients is essential for the development of chlamydial inclusion
Owing to its metabolically inert nature, Chlamydia need to scavenge essential nutrients, such as lipids, iron, amino acids, and nucleotides, from the host cell to meet their metabolic requirements [Citation73,Citation74]. Although Chlamydia biosynthesizes most phospholipids, sphingomyelin, cholesterol, and phosphatidylcholine must be obtained from host cells [Citation42], which are important chlamydial inclusion membrane components. Interactions between inclusions and vesicles containing sphingomyelin were found during C. trachomatis and C. pneumoniae developmental cycle [Citation59,Citation75,Citation76], indicating that all Chlamydiae species may share this feature. Like many other intracellular pathogens, C. trachomatis hijacks the sphingolipids and cholesterol produced by the endoplasmic reticulum and Golgi apparatus to meet the lipid requirements necessary for inclusion development [Citation77]. As a membrane-bound intracellular vacuole, the inclusion acquires lipids, particularly sphingomyelin, through intercepting vesicular transport and multivesicular bodies (MVBs) derived from the trans-Golgi, and is re-routed to the inclusion to ensure chlamydial replication and survival [Citation44,Citation78,Citation79].
It has become increasingly perspicuous that Chlamydia has developed various strategies to subvert vesicular trafficking of host cell lipids by targeting SNARE, ARFs, and the Rab protein family that tightly regulate intracellular membrane fusion, Golgi complex dynamics and structure, and vesicular trafficking. The inclusion is decorated with multiple Inc proteins, which serve as an interface for Chlamydia to interact with host nutrients and proteins [Citation20,Citation60]. IncA shares a motif structure and function with the SNARE protein of eukaryotic cells and mediates membrane fusion of inclusions during Chlamydia infection, which is essential for enhancing Chlamydia pathogenicity [Citation70]. Recent studies have demonstrated that multiple host SNARE proteins, such as SNAP-23 [Citation62], Syntaxin 4 [Citation62], Syntaxin 6 [Citation80], vesicle-associated membrane protein (VAMP) 3 [Citation60], VAMP 4 [Citation61], and Syntaxin 10 [Citation81] are recruited to chlamydial inclusions, which play a critical role in Chlamydia development and infectious progeny generation. Among these proteins, SNAP-23 and Syntaxin 4 are involved in regulating lipid droplet homoeostasis during Chlamydia infection [Citation62], knocking down Syntaxin 10 leads to defects in chlamydial inclusion maturation and disturbs or interrupts the progression of RB – EB differentiation [Citation81]. As an eukaryotic SNARE protein, VAMP 3 is recruited for inclusion, interacts with IncF, IncG, CT442, CT449, and CT813, and promotes membrane fusion to facilitate chlamydial inclusion growth [Citation60]. Moreover, another trans-Golgi SNARE and Syntaxin 6-binding partner, VAMP 4 mediates sphingomyelin transport to inclusions during Chlamydia infection, which is important for supporting chlamydial lipid acquisition and regulation during Chlamydia development [Citation61].
As key vesicular transport regulators in all eukaryotes, ARFs transport cargo proteins to subcellular sites and regulate Golgi dynamics [Citation82,Citation83] to manipulate cellular physiological processes such as lipid metabolism, mitochondrial architecture, and microtubule regulation [Citation84]. To facilitate intracellular development, Chlamydia effectively rearranges the microtubule network through effector protein secretion [Citation85]. The chlamydial inclusion protein CT813/CTL0184/InaC recruits and activates ARF1 and ARF4 to modify microtubules and redistribute the Golgi complex around inclusions during infection [Citation58]. In addition, Golgi reorganization around inclusions delivers sphingolipids to chlamydial inclusions. Although CT813-mediated Golgi redistribution is not required for chlamydial replication, sphingolipid acquisition, and inclusion development, microtubule modification inhibition impairs infectious progeny generation [Citation86]. ARF1-dependent vesiclular trafficking pathway is regulated by the Golgi-specific BFA resistance guanine nucleotide exchange factor 1 (GBF1). Chlamydia selectively co-opts GBF1 to acquire sphingomyelin, indicating that the ARF1/GBF1-dependent sphingomyelin acquisition pathway is indispensable for inclusion membrane development [Citation59]. In addition to microtubule modification, ARF1 plays an important role in the regulation of cytoskeletal dynamics [Citation87]. Dynamic cytoskeletal networks play critical roles in chlamydial invasion [Citation36]. These results suggest that Chlamydia regulates Golgi apparatus and microtubule structure and function through effector proteins, which may serve as a strategy to hijack vesicle delivery to the chlamydial inclusion membrane.
Similar to other intracellular bacteria, Chlamydia has evolved various strategies to acquire the necessary nutrients, including the hijacking of plasma membrane-derived sphingomyelin and cholesterol-containing Golgi-vesicular traffic, to survive within its intracellular niche. Together, the sophisticated mechanisms of intracellular chlamydial replication and growth involve Inc secretion to intercept intracellular trafficking by mimicking SNAREs, recruiting ARFs, modifying microtubules around the inclusion, and repositioning the Golgi complex. These findings provide further insights into the mechanism of Chlamydia targeting vesicular lipid-containing trafficking for inclusion development.
Chlamydia manipulates host vesicular transport for nutrient acquisition through its effector proteins interacting with Rab GTPases
Chlamydia exploits Rab GTPases for nutrient acquisition and intracellular replication
Eukaryotic cells contain various membranous organelles with specific lipid and protein components that regulate material transport between the membrane organelles by exploiting vesicular transport, transporting proteins and lipids to precise locations, and performing their functions. The intracellular membrane transport system plays a key role in extracellular nutrient uptake by eukaryotic cells during endocytosis [Citation88]. GTP-binding proteins are important vesicular transport regulators. Rab proteins represent the largest branch of the Ras-like small GTPase superfamily, alternating between the GTP- and GDP-bound states, and function as molecular switches in intracellular membrane trafficking regulation in all eukaryotic cells [Citation88–90]. The Rab protein family, which controls various cellular processes, such as membrane identity, vesicle budding, uncoating, motility, and fusion, consists of over 60 members within the human genome that recruit effector molecules such as sorting adaptors, tethering factors, kinases, phosphatases, and motors to execute their functions [Citation89].
Since Rab proteins are key intracellular vesicular transport regulators, several Rabs are required for chlamydial replication and development [Citation20,Citation91,Citation92]. However, the function of the Rab-mediated vesicular transport during Chlamydia infection and development remains unclear.
Rab GTPases are recruited to chlamydial inclusions through both species- and species-independent mechanisms [Citation93]. This dual strategy indicates that Chlamydia manipulates host vesicular transport by specifically targeting Rab-dependent trafficking processes to facilitate survival and replication within the host cell. A large and diverse Rab protein family that controls the transport of host vesicles, including Rab1, Rab4, Rab6, Rab11, Rab14, and Rab39, is recruited to the chlamydial inclusion membrane to provide nutrition and key substrates necessary for intracellular growth and to escape the degradative phagocytic pathway (). Chlamydial infection forms Golgi ministacks necessary lipid uptake from host cells [Citation77]. Rab6 and Rab11 are key regulators of the stability of the Golgi apparatus and recruited to inclusions. The nutrients carried by Rab6- and Rab11-positive vesicles are transported from the Golgi apparatus to inclusions during Chlamydia infection [Citation52]. Chlamydia recruits Rab39a and Rab39b to the periphery of inclusions, thereby intercepting multivesicular body-derived lipids (sphingomyelin and phospholipid) and endoplasmic reticulum – Golgi biosynthesized sphingolipids to chlamydial inclusions [Citation57]. Rab4 and Rab11 regulate the transferrin circulation pathway, which is affected by inclusion. C. trachomatis growth and development depend on the iron supply from the host [Citation94], and Rab4 and Rab11 depletion deposits transferrin around inclusions, inhibiting C. trachomatis growth [Citation95]. The T3SS effector protein, CT622/Taip, which is crucial for C. trachomatis growth and infection, interacts with Rab6 and ATG16L1 to divert Rab6- and ATG16L1-dependent vesicular trafficking to inclusions [Citation51]. However, the inclusion size in Chlamydia decreased after Rab6 silencing, suggesting that chlamydial inclusion growth depends on the membrane supply of Rab6 [Citation51]. CT622 interacts with ATG16L1, disrupting the normal interaction of ATG16L1 with TMEM59 [Citation51] (a transmembrane protein localized in the Golgi and endosomes [Citation96,Citation97], which may eliminate the restriction of bacterial access to host vesicular trafficking.
Chlamydia relocates the host trafficking pathway for nutrient acquisition through the interaction between Incs and Rab GTPases
Chlamydia can exploit the host cell processes to establish safe replication environments. C. pneumoniae interacts with various Rab GTPases, including Rab1, Rab10, and Rab11, through Cpn0585, an inclusion membrane protein that regulates intracellular chlamydial replication [Citation48].
As the Inc protein of C. trachomatis, CT229 also recruits Rab4 and Rab35 to the inclusion membrane through its coiled-coil SNARE-like domain, thereby targeting the clathrin-dependent vesicular trafficking pathway and modulating transferrin and cation-independent mannose 6-phosphate receptor (CI-M6PR) transport to acquire substrates essential for chlamydial development and infection, such as iron and lipids [Citation20]. Furthermore, CI-M6PR association with the retromer complex (a protein complex that facilitates cargo transport from endosomes to the trans-Golgi network [Citation98,Citation99] occurs specifically through interactions with sorting nexin 5 (SNX5) and SNX6. This interaction plays a crucial role in controlling the transmembrane cargo trafficking pathway. By regulating the transport of these proteins from endosomes to the trans-Golgi network (TGN), the retromer complex ensures proper localization and function of these important cellular components. This process highlights the intricate and coordinated mechanisms governing intracellular protein trafficking and compartmentalization. Rab GTPases mediate cargo transport between the TGN and endosomes [Citation100]. C. trachomatis exploits CT229 as an interactive interface to recruit Rab GTPases, modulates clathrin-dependent transport between the TGN and endosomes, and redirects M6PR-containing vesicles to inclusion periphery [Citation20]. Strikingly, IncE/CT116, another C. trachomatis Inc protein, relocalizes the retromer components SNX5/6 (a key endosomal trafficking regulator) and disrupts SNX5:CI-M6PR interaction by binding its C-terminal to SNX5, specifically the PX domain, remodelling retromer-dependent CI-M6PR trafficking [Citation63,Citation64], which is crucial for progeny reproduction and infection [Citation101]. The SNX5/6-IncE interaction illustrates the complex competition between the host and Chlamydia in controlling cell trafficking [Citation102]. Accordingly, the interaction between Incs and Rab GTPases may be a key event controlling the vesicular interactions of chlamydial inclusions; therefore, it may eventually relocate the host trafficking pathway to facilitate intracellular growth. Understanding the molecular mechanisms underlying Chlamydia–host interactions will provide a new perspective on nutrient acquisition by Chlamydia ().
Rab effector proteins and cascading signalling pathways are associated with chlamydial replication and development
The crucial role of Rab GTPases and effector proteins in intracellular transport pathways
As a molecular “switch” that regulates the transport between various organelles, Rab proteins precisely regulate transport vesicle formation, transport, connection, and fusion, and recruit specific effector proteins to play key functions in their respective vesicular transport pathways. Most intracellular transport pathways in eukaryotic cells depend on interactions between Rabs and specific Rab effector proteins. Activated Rab GTPases target specific organelles or membrane compartments and interact with a series of specific effector proteins to facilitate membrane transport or fusion. To execute the functions of Rab GTPase and its effector proteins, Rab effector proteins interact with activated Rab GTPase, the GTP-binding form, and activate related signalling pathways to regulate the molecular mechanism of cargo delivery, including budding and scission of vesicles, formation, transport, membrane fusion, and tethering [Citation15].
The significant role of Rab effector proteins in chlamydial replication and nutrient acquisition
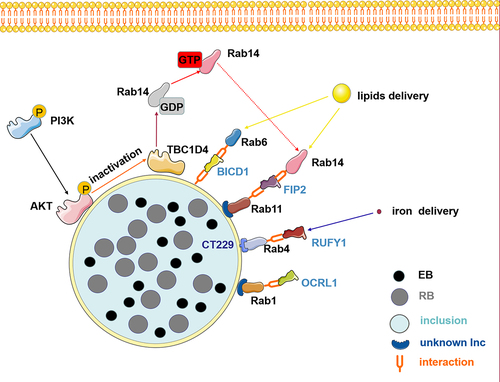
Owing to the relationship between vesicular transport and Chlamydia, the function of Rabs and Rab-related effector proteins in chlamydial replication has received extensive attention. Interference with the function of host cell Rabs not only inhibits inclusion growth and development, but also hinders chlamydial progeny generation. Moreover, inhibiting the function of host Rab6, Rab11, and Rab14 can reduce C. trachomatis offspring production [Citation52,Citation92,Citation103]. Furthermore, the depletion of Rab effector proteins, such as Rab-associated proteins OCRL1 (Rab1 [Citation49], Rab5 [Citation49], and Rab6 [Citation49] interacting protein) and FIP2 (a member of the Rab11-family of interacting proteins, also called Rab11/Rab14 adaptor protein) can achieve the same effect [Citation54,Citation56].
FIP2 specifically interacts with the chlamydial inclusion membrane through its Rab11-binding domain, leading to Rab14 recruitment [Citation54]. This interaction facilitates the redirection of host vesicular transport and resources for inclusion. Additionally, FIP2 promotes Rab14 recruitment to redirect Rab14-labelled vesicles full of host cell lipids towards the periphery of the inclusion [Citation54]. This provides a clear understanding of the complex molecular mechanisms by which Chlamydia acquires host nutrients. Intriguingly, the adaptor protein Fip2 recruits the actin protein myosin VB to early inclusions and regulates the intracellular positioning of the inclusion to construct a suitable intracellular niche necessary for its internalization and intracellular growth [Citation56]. In addition, the Rab4 effector protein RUFY1, which is essential for transferrin recycling, is recruited to the periphery of inclusions in a CT229-dependent manner [Citation20]. Similarly, Chlamydia recruits Rab6 to the inclusion by mimicking the Rab6 effector BICD1 or directly interacting with BICD1 [Citation53], whereas Rab6 and Rab11 gene knockdown in host cells inhibit ceramide transport from the Golgi to the chlamydial inclusion, accompanied by a decrease in the number of progeny [Citation52], which further supports the notion that Chlamydia could hijack Rab-mediated nutrient relocation by interacting with Rab effectors to maintain offspring development and reproduction. When one or more of these proteins are inhibited simultaneously, C. trachomatis infection decreased, suggesting that Rab proteins and effector proteins have partially overlapping functions that are crucial for C. trachomatis survival and proliferation.
The role of Rab cascade signaling pathways in disrupting vesicular transport during Chlamydia infection
Another event in Chlamydia is intracellular vesicular trafficking interruption, which subverts host signalling processes during infection. Recent studies have shown that fragmentation of the Golgi apparatus and post-translationally modified microtubules promotes chlamydial infection and propagation [Citation104,Citation105]. Furthermore, most membrane trafficking pathways depend on microtubules. Rab proteins and effectors act as bridges between membrane traffic and microtubules. Phosphatidylinositol-3-kinase (PI3K)/AKT/TBC1D4 signalling pathway plays a significant role in regulating Rab GTP/GDP cycling [Citation55,Citation106]. AKT phosphorylation affects Chlamydia-induced Golgi fragmentation in infected cells, thereby influencing the intracellular development of Chlamydia and persistent chlamydial infection [Citation107]. During Chlamydia infection, AKT is phosphorylated and recruited for inclusion, followed by AKT substrate AS160 (also named TBC1D4) inactivation. Inactivated AS160 slows GTP hydrolysis and promotes the binding of Golgi-associated Rab14 to GTP; consequently, Rab14-marked vesicles with sphingolipids are relocated to the inclusion [Citation55]. Taken together, the bacterium interferes with the AKT signalling pathway, which may be another strategy for hijacking vesicular trafficking to obtain key substrates for intracellular growth and development ().
Figure 2. The role of Rab effector proteins and cascade signaling pathways is central to disrupting vesicular transport during chlamydia infection. chlamydia interacts with Rab effectors such as OCRL1, RUFY1, BICD1, and FIP2 to hijack Rab-mediated nutrient transport, redirecting lipid and iron delivery, which are crucial for the development and reproduction of its offspring. Furthermore, chlamydia infection induces AKT phosphorylation and recruits phosphorylated AKT to the inclusion membrane, thus phosphorylating and inactivating TBC1D4. Subsequently, the inactivated TBC1D4 activates Rab14 in a membrane-associated GTP-bound state. Therefore, vesicles marked by Rab14 that contain sphingolipids are redirected toward the inclusion. Elements in the figure are sourced from the SMART database (https://smart.servier.com).
Concluding remarks and future perspectives
Through its interactions with Rab proteins, the primary vesicular trafficking and Rab effector protein regulators, Chlamydia manipulates multivesicular trafficking, redirects host cell nutrition, and reshapes the intracellular niche environment to obtain the necessary components for successful intracellular developmental cycle. This interplay between Chlamydia and host vesicular transport system opens new avenues for Chlamydia infection prevention and treatment. The current findings highlight that specific Rab GTPases and their effector proteins are key factors in Chlamydia hijacking host vesicle transport. Therefore, future development of targeted therapeutic interventions could include small molecule inhibitors, peptidomimetics, or monoclonal antibodies designed to disrupt these interactions without affecting host cell function. These interventions can specifically block interaction sites or regulate the activity of these GTPases, thereby inhibiting Chlamydia’s access to essential nutrients and its ability to establish replication niches within host cells.
Understanding the role of vesicular transport in nutrient acquisition in Chlamydia offers new perspectives for developing therapeutic strategies. Targeting this specific mechanism presents several challenges: (i) The vesicular transport system is complex and involved in numerous cellular processes beyond its interaction with Chlamydia. Ensuring that potential therapeutics disrupt only the interactions key to chlamydial lifecycle while preserving normal cellular processes is a major challenge. (ii) The need for high specificity in therapeutic targeting is crucial to avoid off-target effects that could impair normal host cellular functions. Identifying targets that are either unique to the interaction between Chlamydia and its host, or are minimally involved in essential host pathways, represents a significant challenge.
Continued research to further elucidate the molecular details of the Chlamydia-host vesicular transport interaction will facilitate the identification of highly specific targets. High throughput screening of small molecules and biologics could then be utilized to identify candidates that selectively disrupt the nutrient acquisition of Chlamydia. Applying structural biology and bioinformatics tools can help to design molecules that precisely interfere with the interaction between Chlamydia and host vesicular transport while minimizing impact on the transport system itself.
Future research should focus on addressing these challenges by conducting a detailed molecular analysis of the interaction between the host and Chlamydia, and by identifying the most potent targets for therapeutic intervention.
Targeting the interactions between Chlamydia and host vesicular transport machinery may provide opportunities to disrupt essential nutrient acquisition, thereby impeding the intracellular growth and replication of Chlamydia. In addition, deciphering the complex network of molecular interactions involved in nutrient acquisition by Chlamydia may identify novel drug targets for developing effective anti-chlamydial therapies. As we focus on the role of Rab proteins, Rab effectors, and the cascading signalling pathways involved in Chlamydia trafficking and nutrient acquisition, several potential targets should be highlighted.
Rab Proteins: Chlamydia manipulates specific Rab GTPases to facilitate the maintenance of the inclusion membrane. Rab proteins such as Rab4 and Rab11, which are involved in recycling endosomes, could serve as potential targets for therapy.
Rab Effectors: The identification of specific Rab effector proteins that interact with chlamydial inclusion membrane could provide highly targeted intervention points.
Signalling Pathways: Chlamydia may modulate signalling pathways that regulate the vesicular transport machinery. Targeting these pathways could be effective in preventing nutrient acquisition by the pathogen.
To validate these potential candidate targets in future research, we propose the following approaches: (i) The use of RNA interference (RNAi) and CRISPR-Cas9 gene editing can help to dissect the role of specific candidate targets in vitro. Loss-of-function studies allow us to observe the effects of silencing or knocking out these genes on chlamydial life cycle. (ii) Small molecule inhibitors targeting specific Rab GTPases or signaling pathways can be tested for their ability to disrupt Chlamydia nutrient acquisition and growth. (iii) Understanding the three-dimensional structure of target proteins and their complexes with Chlamydia factors is critically important for targeted drug design.
By focusing on these prospective targets, future research is expected to move towards intervention strategies that could specifically disrupt Chlamydia nutrient acquisition processes without negatively affecting the host cell.
Further studies should focus on unravelling the precise mechanisms by which Chlamydia interacts with specific vesicular transport system components. Based on the synthesis of the current literature, certain components may be particularly promising candidates for further study due to their regulatory roles in key processes of chlamydial development, such as Rab4, Rab11, and Rab35. These Rab proteins regulate the transport of transferrin and mannose-6-phosphate receptor (M6PR), both of which are essential for the normal development of Chlamydia.
Additionally, studying the interplay between Chlamydia and host immune responses during vesicular transport-mediated nutrient acquisition may shed light on developing host-directed therapies. Understanding the manipulation of host cellular processes by Chlamydia, specifically through the recruitment of Rab GTPases and Rab effector proteins to the inclusion, is indeed crucial for developing targeted interventions. This complex interaction not only disrupts Rab-mediated multivesicular transport but also acquires host cell nutrition, reshaping the intracellular niche to favour chlamydial growth. The recruitment of host Rab GTPases and their effectors by Chlamydia is central to its strategy for redirecting host cell nutrients. Investigating the precise molecular mechanisms behind this recruitment of Rab could reveal potential targets for interventions aimed at preventing Chlamydia’s subversion of host cellular processes. Chlamydia-induced redirection of host cell metabolism and nutrient acquisition is a critical aspect of its developmental cycle. Unraveling the specific pathways and host cell metabolites targeted by Chlamydia can identify host-directed interventions that inhibit the pathogen’s access to essential resources. With a better understanding of the specific host-pathogen interactions, novel drug targets can be identified. This includes inhibitors that specifically block the interaction between Chlamydia and the host vesicular transport. The use of CRISPR/Cas9 and other gene-editing technologies to disrupt the genes responsible for the critical interactions between Chlamydia and host proteins presents another frontier for therapy development. This approach could allow for the precise targeting of the molecular mechanisms essential for chlamydial intracellular survival and growth.
We summarize the mechanisms by which Chlamydia manipulates host vesicular transport pathways for nutrient acquisition, providing novel targets for therapeutic intervention. Future research could focus on silencing or knocking out these target genes in the host through RNA interference (RNAi) and CRISPR-Cas9 gene editing, as well as developing small molecule inhibitors targeting specific Rab GTPases or signalling pathways that disrupt Chlamydia’s ability to acquire nutrients. Designing interventions that interrupt the manipulation of host cellular processes by Chlamydia presents a major challenge, particularly in maintaining the fundamental roles of Rab proteins that are essential for normal cell physiology. Moreover, achieving therapeutic concentrations of small molecule inhibitors at the site of infection without inducing toxicity remains a challenge. Additionally, the application of gene editing in human therapy not only raises substantial ethical issues but also requires rigorous regulatory scrutiny.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
Data availability was not applicable to this study. No new datasets were generated in this study and the data cited in this review were obtained from published articles.
Additional information
Funding
References
- Grayston JT, Campbell LA, Kuo CC, et al. A new respiratory tract pathogen. Chlamydia pneumoniae strain TWAR J Infect Dis. 1990;161(4):618–14. doi: 10.1093/infdis/161.4.618
- Taylor HR, Burton MJ, Haddad D, et al. Trachoma. Lancet. 2014;384(9960):2142–2152. doi: 10.1016/S0140-6736(13)62182-0
- Oakeshott P, Kerry S, Aghaizu A, et al. Randomised controlled trial of screening for chlamydia trachomatis to prevent pelvic inflammatory disease: the POPI (prevention of pelvic infection) trial. BMJ. 2010;340(apr08 1):c1642–c1642. doi: 10.1136/bmj.c1642
- Price MJ, Ades AE, Welton NJ, et al. How much tubal factor infertility is caused by Chlamydia? Estimates based on serological evidence corrected for sensitivity and specificity. Sex Transm Dis. 2012;39(8):608–613. doi: 10.1097/OLQ.0b013e3182572475
- Malhotra M, Sood S, Mukherjee A, et al. Genital chlamydia trachomatis: an update. Indian J Med Res. 2013;138(3):303–316.
- Buckner LR, Amedee AM, Albritton HL, et al. Chlamydia trachomatis infection of endocervical epithelial cells enhances early HIV transmission events. PLoS One. 2016;11(1):e0146663. doi: 10.1371/journal.pone.0146663
- Rother M, Gonzalez E, Teixeira da Costa AR, et al. Combined human genome-wide RNAi and metabolite analyses identify IMPDH as a host- directed target against chlamydia infection. Cell Host Microbe. 2018;23(5):661–671.e8. doi: 10.1016/j.chom.2018.04.002
- Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14(6):385–400. doi: 10.1038/nrmicro.2016.30
- Dong F, Su H, Huang Y, et al. Cleavage of host keratin 8 by a Chlamydia-secreted protease. Infect Immun. 2004;72(7):3863–3868. doi: 10.1128/IAI.72.7.3863-3868.2004
- Li Z, Chen D, Zhong Y, et al. The chlamydial plasmid-encoded protein pgp3 is secreted into the cytosol of Chlamydia-infected cells. Infect Immun. 2008;76(8):3415–3428. doi: 10.1128/IAI.01377-07
- Prasai B, Haber GJ, Strub MP, et al. The nanoscale molecular morphology of docked exocytic dense-core vesicles in neuroendocrine cells. Nat Commun. 2021;12(1):3970. doi: 10.1038/s41467-021-24167-9
- Schnettger L, Rodgers A, Repnik U, et al. A Rab20- dependent membrane trafficking pathway controls M. tuberculosis replication by regulating phagosome spaciousness and integrity. Cell Host Microbe. 2017;21(5):619–628.e5. doi: 10.1016/j.chom.2017.04.004
- Marsman M, Jordens I, Kuijl C, et al. Dynein-mediated vesicle transport controls intracellular salmonella replication. Mol Biol Cell. 2004;15(6):2954–2964. doi: 10.1091/mbc.e03-08-0614
- Allgood SC, Romero DueñDueñAs BP, Noll RR, et al. Legionella effector AnkX disrupts host cell endocytic recycling in a phosphocholination-dependent manner. Front Cell Infect Microbiol. 2017;7:397. doi: 10.3389/fcimb.2017.00397
- Hutagalung AH, Novick PJ. Role of rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–149. doi: 10.1152/physrev.00059.2009
- Rai A, Goody RS, Müller MP. Multivalency in Rab effector interactions. Small GTPases. 2019;10(1):40–46. doi: 10.1080/21541248.2016.1265700
- Stephens RS, Kalman S, Lammel C, et al. Genome sequence of an obligate intracellular pathogen of humans: chlamydia trachomatis. Science. 1998;282(5389):754–759. doi: 10.1126/science.282.5389.754
- Moran NA. Microbial minimalism: genome reduction in bacterial pathogens. Cell. 2002;108(5):583–586. doi: 10.1016/S0092-8674(02)00665-7
- Chatterjee R, Chowdhury AR, Mukherjee D, et al. Lipid larceny: channelizing host lipids for establishing successful pathogenesis by bacteria. Virulence. 2021;12(1):195–216. doi: 10.1080/21505594.2020.1869441
- Faris R, Merling M, Andersen SE, et al. Chlamydia trachomatis CT229 subverts Rab GTPase-dependent CCV trafficking pathways to promote chlamydial infection. Cell Rep. 2019;26(12):3380–3390.e5. doi: 10.1016/j.celrep.2019.02.079
- Hatch ND, Ouellette SP, Roy CR. Inhibition of tRNA synthetases induces persistence in Chlamydia. Infect Immun. 2020;88(4):e00943–19. doi: 10.1128/IAI.00943-19
- Pokorzynski ND, Thompson CC, Carabeo RA. Ironing out the unconventional mechanisms of iron acquisition and gene regulation in chlamydia. Front Cell Infect Microbiol. 2017;7:394. doi: 10.3389/fcimb.2017.00394
- Saka HA, Thompson JW, Chen YS, et al. Quantitative proteomics reveals metabolic and pathogenic properties of chlamydia trachomatis developmental forms. Mol Microbiol. 2011;82(5):1185–1203. doi: 10.1111/j.1365-2958.2011.07877.x
- Rajeeve K, Vollmuth N, Janaki-Raman S, et al. Reprogramming of host glutamine metabolism during chlamydia trachomatis infection and its key role in peptidoglycan synthesis. Nat Microbiol. 2020;5(11):1390–1402. doi: 10.1038/s41564-020-0762-5
- Fischer A, Rudel T. Subversion of cell-autonomous host defense by Chlamydia infection. Curr Top Microbiol Immunol. 2018;412:81–106.
- Bastidas RJ, Elwell CA, Engel JN, et al. Chlamydial intracellular survival strategies. Cold Spring Harb Perspect Med. 2013;3(5):a010256. doi: 10.1101/cshperspect.a010256
- Olive AJ, Haff MG, Emanuele MJ, et al. Chlamydia trachomatis-induced alterations in the host cell proteome are required for intracellular growth. Cell Host Microbe. 2014;15(1):113–124. doi: 10.1016/j.chom.2013.12.009
- Zadora PK, Chumduri C, Imami K, et al. Integrated phosphoproteome and transcriptome analysis reveals Chlamydia-induced epithelial-to-mesenchymal transition in Host cells. Cell Rep. 2019;26(5):1286–1302.e8. doi: 10.1016/j.celrep.2019.01.006
- Jensen KT, Petersen L, Falk S, et al. Novel overlapping coding sequences in chlamydia trachomatis. FEMS Microbiol Lett. 2006;265(1):106–117. doi: 10.1111/j.1574-6968.2006.00480.x
- Lei L, Yang C, Patton MJ, et al. A chlamydial plasmid-dependent secretion system for the delivery of virulence factors to the host cytosol. MBio. 2021;12(3):e0117921. doi: 10.1128/mBio.01179-21
- Rucks EA. Type III secretion in Chlamydia. Microbiol Mol Biol Rev. 2023;87(3):e0003423. doi: 10.1128/mmbr.00034-23
- Betts-Hampikian HJ, Fields KA. The chlamydial type III secretion mechanism: revealing cracks in a tough nut. Front Microbiol. 2010;1:114. doi: 10.3389/fmicb.2010.00114
- Zhong G. Chlamydia trachomatis secretion of proteases for manipulating host signaling pathways. Front Microbiol. 2011;2:14. doi: 10.3389/fmicb.2011.00014
- Siegl C, Prusty BK, Karunakaran K, et al. Tumor suppressor p53 alters host cell metabolism to limit chlamydia trachomatis infection. Cell Rep. 2014;9(3):918–929. doi: 10.1016/j.celrep.2014.10.004
- Nans A, Kudryashev M, Saibil HR, et al. Structure of a bacterial type III secretion system in contact with a host membrane in situ. Nat Commun. 2015;6(1):10114. doi: 10.1038/ncomms10114
- Faris R, McCullough A, Andersen SE, et al. The chlamydia trachomatis secreted effector TmeA hijacks the N-WASP-ARP2/3 actin remodeling axis to facilitate cellular invasion. PLOS Pathog. 2020;16(9):e1008878. doi: 10.1371/journal.ppat.1008878
- George Z, Omosun Y, Azenabor AA, et al. The molecular mechanism of induction of unfolded protein response by chlamydia. Biochem Biophys Res Commun. 2019;508(2):421–429. doi: 10.1016/j.bbrc.2018.11.034
- Moore ER, Ouellette SP. Reconceptualizing the chlamydial inclusion as a pathogen-specified parasitic organelle: an expanded role for Inc proteins. Front Cell Infect Microbiol. 2014;4:157. doi: 10.3389/fcimb.2014.00157
- Meier K, Jachmann LH, Türköz G, et al. The chlamydia effector CpoS modulates the inclusion microenvironment and restricts the interferon response by acting on Rab35. MBio. 2023;14(4):e0319022. doi: 10.1128/mbio.03190-22
- Luís MP, Pereira IS, Bugalhão JN, et al. The chlamydia trachomatis IncM protein interferes with host cell cytokinesis, centrosome positioning, and Golgi distribution and contributes to the stability of the pathogen-containing vacuole. Infect Immun. 2023;91(4):e0040522. doi: 10.1128/iai.00405-22
- Almeida F, Luís MP, Pereira IS, et al. The human centrosomal protein CCDC146 binds chlamydia trachomatis inclusion membrane protein CT288 and is recruited to the periphery of the chlamydia-containing vacuole. Front Cell Infect Microbiol. 2018;8:254. doi: 10.3389/fcimb.2018.00254
- Banhart S, Schafer EK, Gensch JM, et al. Sphingolipid metabolism and transport in chlamydia trachomatis and chlamydia psittaci infections. Front Cell Dev Biol. 2019;7:223. doi: 10.3389/fcell.2019.00223
- Tang T, Wu H, Chen X, et al. The hypothetical inclusion membrane protein CPSIT_0846 regulates mitochondrial-mediated host cell apoptosis via the ERK/JNK signaling pathway. Front Cell Infect Microbiol. 2021;11:607422. doi: 10.3389/fcimb.2021.607422
- Beatty WL. Trafficking from CD63-positive late endocytic multivesicular bodies is essential for intracellular development of chlamydia trachomatis. J Cell Sci. 2006;119(Pt 2):350–359. doi: 10.1242/jcs.02733
- Beatty WL. Late endocytic multivesicular bodies intersect the chlamydial inclusion in the absence of CD63. Infect Immun. 2008;76(7):2872–2881. doi: 10.1128/IAI.00129-08
- González-Méndez L, Gradilla AC, Sánchez-Hernández D, et al. Polarized sorting of patched enables cytoneme-mediated hedgehog reception in the drosophila wing disc. Embo J. 2020;39(11):e103629. doi: 10.15252/embj.2019103629
- Ismail SA, Vetter IR, Sot B, et al. The structure of an arf-ArfGAP complex reveals a Ca2+ regulatory mechanism. Cell. 2010;141(5):812–821. doi: 10.1016/j.cell.2010.03.051
- Cortes C, Rzomp KA, Tvinnereim A, et al. Chlamydia pneumoniae inclusion membrane protein Cpn0585 interacts with multiple Rab GTPases. Infect Immun. 2007;75(12):5586–5596. doi: 10.1128/IAI.01020-07
- Hyvola N, Diao A, McKenzie E, et al. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. Embo J. 2006;25(16):3750–3761. doi: 10.1038/sj.emboj.7601274
- Yamamoto H, Koga H, Katoh Y, et al. Functional cross-talk between Rab14 and Rab4 through a dual effector, RUFY1/Rabip4. Mol Biol Cell. 2010;21(15):2746–2755. doi: 10.1091/mbc.e10-01-0074
- Hamaoui D, Cosse MM, Mohan J, et al. The Chlamydia effector CT622/TaiP targets a nonautophagy related function of ATG16L1. Proc Natl Acad Sci U S A. 2020;117(43):26784–26794. doi: 10.1073/pnas.2005389117
- Rejman Lipinski A, Heymann J, Meissner C, et al. Rab6 and Rab11 regulate Chlamydia trachomatis development and golgin-84-dependent golgi fragmentation. PLOS Pathog. 2009;5(10):e1000615. doi: 10.1371/journal.ppat.1000615
- Moorhead AR, Rzomp KA, Scidmore MA. The Rab6 effector bicaudal D1 associates with Chlamydia trachomatis inclusions in a biovar-specific manner. Infect Immun. 2007;75(2):781–791. doi: 10.1128/IAI.01447-06
- Leiva N, Capmany A, Damiani MT. Rab11-family of interacting protein 2 associates with chlamydial inclusions through its Rab-binding domain and promotes bacterial multiplication. Cell Microbiol. 2013;15(1):114–129. doi: 10.1111/cmi.12035
- Capmany A, Gambarte Tudela J, Alonso Bivou M, et al. Akt/AS160 signaling pathway inhibition impairs infection by decreasing Rab14-controlled sphingolipids delivery to chlamydial inclusions. Front Microbiol. 2019;10:666. doi: 10.3389/fmicb.2019.00666
- Molleken K, Hegemann JH, Coombes BK. Acquisition of Rab11 and Rab11-Fip2 – A novel strategy for chlamydia pneumoniae early survival. PLOS Pathog. 2017;13(8):e1006556. doi: 10.1371/journal.ppat.1006556
- Gambarte Tudela J, Buonfigli J, Lujan A, et al. Rab39a and Rab39b display different intracellular distribution and function in sphingolipids and phospholipids transport. Int J Mol Sci. 2019;20(7):1688. doi: 10.3390/ijms20071688
- Wesolowski J, Weber MM, Nawrotek A, et al. Chlamydia hijacks ARF GTPases to coordinate microtubule posttranslational modifications and Golgi complex positioning. MBio. 2017;8(3):e02280–16. doi: 10.1128/mBio.02280-16
- Elwell CA, Jiang S, Kim JH, et al. Chlamydia trachomatis co-opts GBF1 and CERT to acquire host sphingomyelin for distinct roles during intracellular development. PLOS Pathog. 2011;7(9):e1002198. doi: 10.1371/journal.ppat.1002198
- Bui DC, Jorgenson LM, Ouellette SP, et al. Eukaryotic SNARE VAMP3 dynamically interacts with multiple chlamydial inclusion membrane proteins. Infect Immun. 2021;89(2):e00409–20. doi: 10.1128/IAI.00409-20
- Kabeiseman EJ, Cichos K, Hackstadt T, et al. Vesicle-associated membrane protein 4 and syntaxin 6 interactions at the chlamydial inclusion. Infect Immun. 2013;81(9):3326–3337. doi: 10.1128/IAI.00584-13
- Monteiro-Bras T, Wesolowski J, Paumet F. Depletion of SNAP-23 and Syntaxin 4 alters lipid droplet homeostasis during chlamydia infection. Microb Cell. 2019;7(2):46–58. doi: 10.15698/mic2020.02.707
- Paul B, Kim HS, Kerr MC, et al. Structural basis for the hijacking of endosomal sorting nexin proteins by chlamydia trachomatis. Elife. 2017;6:e22311. doi: 10.7554/eLife.22311
- Elwell CA, Czudnochowski N, von Dollen J, et al. Chlamydia interfere with an interaction between the mannose-6-phosphate receptor and sorting nexins to counteract host restriction. Elife. 2017;6:e22709. doi: 10.7554/eLife.22709
- Derre I, Swiss R, Agaisse H, et al. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLOS Pathog. 2011;7(6):e1002092. doi: 10.1371/journal.ppat.1002092
- Li B, Dong X, Zhao R, et al. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in fusarium graminearum. PLOS Pathog. 2019;15(5):e1007754. doi: 10.1371/journal.ppat.1007754
- Bisio H, Chaabene RB, Sabitzki R, et al. The ZIP code of vesicle trafficking in apicomplexa: SEC1/Munc18 and SNARE proteins. MBio. 2020;11(5):e02092–20. doi: 10.1128/mBio.02092-20
- Li B, Gao Y, Mao HY, et al. The SNARE protein FolVam7 mediates intracellular trafficking to regulate conidiogenesis and pathogenicity in Fusarium oxysporum. lycopersici Environ Microbiol. 2019;21(8):2696–2706. doi: 10.1111/1462-2920.14585
- Weber MM, Noriea NF, Bauler LD, et al. A functional core of IncA is required for chlamydia trachomatis inclusion fusion. J Bacteriol. 2016;198(8):1347–1355. doi: 10.1128/JB.00933-15
- Cingolani G, McCauley M, Lobley A, et al. Structural basis for the homotypic fusion of chlamydial inclusions by the SNARE-like protein IncA. Nat Commun. 2019;10(1):2747. doi: 10.1038/s41467-019-10806-9
- Ronzone E, Wesolowski J, Bauler LD, et al. An alpha-helical core encodes the dual functions of the chlamydial protein IncA. J Biol Chem. 2014;289(48):33469–33480. doi: 10.1074/jbc.M114.592063
- Hanada K. Intracellular trafficking of ceramide by ceramide transfer protein. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(4):426–437. doi: 10.2183/pjab.86.426
- Mehlitz A, Eylert E, Huber C, et al. Metabolic adaptation of chlamydia trachomatis to mammalian host cells. Mol Microbiol. 2017;103(6):1004–1019. doi: 10.1111/mmi.13603
- Saka HA, Valdivia RH. Acquisition of nutrients by Chlamydiae: unique challenges of living in an intracellular compartment. Curr Opin Microbiol. 2010;13(1):4–10. doi: 10.1016/j.mib.2009.11.002
- Wolf K, Hackstadt T. Sphingomyelin trafficking in chlamydia pneumoniae-infected cells. Cell Microbiol. 2001;3(3):145–152. doi: 10.1046/j.1462-5822.2001.00098.x
- Moore ER, Fischer ER, Mead DJ, et al. The chlamydial inclusion preferentially intercepts basolaterally directed sphingomyelin-containing exocytic vacuoles. Traffic. 2008;9(12):2130–2140. doi: 10.1111/j.1600-0854.2008.00828.x
- Heuer D, Rejman Lipinski A, Machuy N, et al. Chlamydia causes fragmentation of the golgi compartment to ensure reproduction. Nature. 2009;457(7230):731–735. doi: 10.1038/nature07578
- Carabeo RA, Mead DJ, Hackstadt T. Golgi-dependent transport of cholesterol to the chlamydia trachomatis inclusion. Proc Natl Acad Sci USA. 2003;100(11):6771–6776. doi: 10.1073/pnas.1131289100
- Hackstadt T, Rockey DD, Heinzen RA, et al. Chlamydia trachomatis interrupts an exocytic pathway to acquire endogenously synthesized sphingomyelin in transit from the golgi apparatus to the plasma membrane. Embo J. 1996;15(5):964–977. doi: 10.1002/j.1460-2075.1996.tb00433.x
- Moore ER, Mead DJ, Dooley CA, et al. The trans-Golgi SNARE syntaxin 6 is recruited to the chlamydial inclusion membrane. Microbiology (Reading). 2011;157(Pt 3):830–838. doi: 10.1099/mic.0.045856-0
- Lucas AL, Ouellette SP, Kabeiseman EJ, et al. The trans-Golgi SNARE syntaxin 10 is required for optimal development of chlamydia trachomatis. Front Cell Infect Microbiol. 2015;5:68. doi: 10.3389/fcimb.2015.00068
- Brumm S, Singh MK, Nielsen ME, et al. Coordinated activation of ARF1 GTPases by ARF-GEF GNOM dimers is essential for vesicle trafficking in arabidopsis. Plant Cell. 2020;32(8):2491–2507. doi: 10.1105/tpc.20.00240
- Xue S, Zou J, Liu Y, et al. Involvement of BIG5 and BIG3 in BRI1 trafficking reveals diverse functions of BIG-subfamily ARF-GEFs in plant growth and gravitropism. Int J Mol Sci. 2019;20(9):2339. doi: 10.3390/ijms20092339
- Fisher S, Kuna D, Caspary T, et al. ARF family GTPases with links to cilia. Am J Physiol Cell Physiol. 2020;319(2):C404–C418. doi: 10.1152/ajpcell.00188.2020
- Dumoux M, Menny A, Delacour D, et al. A chlamydia effector recruits CEP170 to reprogram host microtubule organization. J Cell Sci. 2015;128(18):3420–3434. doi: 10.1242/jcs.169318
- Kokes M, Dunn JD, Granek JA, et al. Integrating chemical mutagenesis and whole-genome sequencing as a platform for forward and reverse genetic analysis of chlamydia. Cell Host Microbe. 2015;17(5):716–725. doi: 10.1016/j.chom.2015.03.014
- Haines A, Wesolowski J, Ryan NM, et al. Cross talk between ARF1 and RhoA coordinates the formation of cytoskeletal scaffolds during chlamydia infection. MBio. 2021;12(6):e0239721. doi: 10.1128/mBio.02397-21
- Li G, Marlin MC. Rab family of GTPases. Methods Mol Biol. 2015;1298:1–15.
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728
- Pfeffer SR, Kellogg D. Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol Biol Cell. 2017;28(6):712–715. doi: 10.1091/mbc.e16-10-0737
- Gambarte Tudela J, Capmany A, Romao M, et al. The late endocytic Rab39a GTPase regulates the interaction between multivesicular bodies and chlamydial inclusions. J Cell Sci. 2015;128(16):3068–3081. doi: 10.1242/jcs.170092
- Capmany A, Damiani MT, Valdivia RH. Chlamydia trachomatis intercepts Golgi-derived sphingolipids through a Rab14-mediated transport required for bacterial development and replication. PLoS One. 2010;5(11):e14084. doi: 10.1371/journal.pone.0014084
- Rzomp KA, Scholtes LD, Briggs BJ, et al. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun. 2003;71(10):5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003
- Pokorzynski ND, Brinkworth AJ, Carabeo R. A bipartite iron-dependent transcriptional regulation of the tryptophan salvage pathway in chlamydia trachomatis. Elife. 2019;8:e42295. doi: 10.7554/eLife.42295
- Ouellette SP, Carabeo RA. A functional slow recycling pathway of transferrin is required for growth of chlamydia. Front Microbiol. 2010;1:112. doi: 10.3389/fmicb.2010.00112
- Boada-Romero E, Letek M, Fleischer A, et al. TMEM59 defines a novel ATG16L1-binding motif that promotes local activation of LC3. Embo J. 2013;32(4):566–582. doi: 10.1038/emboj.2013.8
- Zheng Q, Zheng X, Zhang L, et al. The neuron-specific protein TMEM59L mediates oxidative stress-induced cell death. Mol Neurobiol. 2017;54(6):4189–4200. doi: 10.1007/s12035-016-9997-9
- Bonifacino JS, Hurley JH. Retromer. Curr Opin Cell Biol. 2008;20(4):427–436. doi: 10.1016/j.ceb.2008.03.009
- Li C, Shah SZ, Zhao D, et al. Role of the retromer complex in neurodegenerative diseases. Front Aging Neurosci. 2016;8:42. doi: 10.3389/fnagi.2016.00042
- Progida C, Bakke O. Bidirectional traffic between the Golgi and the endosomes – machineries and regulation. J Cell Sci. 2016;129(21):3971–3982. doi: 10.1242/jcs.185702
- Mirrashidi KM, Elwell CA, Verschueren E, et al. Global mapping of the Inc-human interactome reveals that retromer restricts Chlamydia infection. Cell Host Microbe. 2015;18(1):109–121. doi: 10.1016/j.chom.2015.06.004
- Sun Q, Yong X, Sun X, et al. Structural and functional insights into sorting nexin 5/6 interaction with bacterial effector IncE. Signal Transduct Target Ther. 2017;2(1):17030. doi: 10.1038/sigtrans.2017.30
- Capmany A, Leiva N, Damiani MT. Golgi-associated Rab14, a new regulator for chlamydia trachomatis infection outcome. Commun Integr Biol. 2011;4(5):590–593. doi: 10.4161/cib.16594
- Pruneda JN, Bastidas RJ, Bertsoulaki E, et al. A Chlamydia effector combining deubiquitination and acetylation activities induces golgi fragmentation. Nat Microbiol. 2018;3(12):1377–1384. doi: 10.1038/s41564-018-0271-y
- Campanacci V, Urvoas A, Cantos-Fernandes S, et al. Insight into microtubule nucleation from tubulin-capping proteins. Proc Natl Acad Sci USA. 2019;116(20):9859–9864. doi: 10.1073/pnas.1813559116
- Miinea CP, Sano H, Kane S, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional rab GTPase-activating protein domain. Biochem J. 2005;391(Pt 1):87–93. doi: 10.1042/BJ20050887
- Huang X, Tan J, Chen X, et al. Akt phosphorylation influences persistent chlamydial infection and Chlamydia-induced Golgi fragmentation without involving Rab14. Front Cell Infect Microbiol. 2021;11:675890. doi: 10.3389/fcimb.2021.675890
