ABSTRACT
The incidence rate of pyogenic liver abscess caused by multidrug-resistant bacteria has increased in recent years. This study aimed to identify the clinical characteristics and risk factors for pyogenic liver abscess caused by multidrug-resistant bacteria. We conducted a retrospective analysis of the clinical features, laboratory test results, and causes of pyogenic liver abscesses in 239 patients admitted to a tertiary hospital. Multivariable logistic regression was used to identify risk factors for multidrug resistance. Among patients with pyogenic liver abscesses, the rate of infection caused by multidrug-resistant organisms was observed to be 23.0% (55/239), with a polymicrobial infection rate of 14.6% (35/239). Additionally, 71 cases (29.7%) were associated with biliary tract disease. Patients with pyogenic liver abscesses caused by multidrug-resistant organisms had a significantly higher likelihood of polymicrobial infection and increased mortality (7/44 [15.9%] vs. 3/131 [2.3%]; p = .003). The Charlson Comorbidity Index (adjusted odds ratio [aOR]: 1.32, 95% confidence interval [CI]: 1.06–1.68), hospitalization (aOR: 10.34, 95% CI: 1.86–60.3) or an invasive procedure (aOR: 9.62; 95% CI: 1.66–71.7) within the past 6 months, and gas in the liver on imaging (aOR: 26.0; 95% CI: 3.29–261.3) were independent risk factors for pyogenic liver abscess caused by multidrug-resistant bacteria. A nomogram was constructed based on the risk factors identified. The nomogram showed high diagnostic accuracy (specificity, 0.878; sensitivity 0.940). Multidrug-resistant organisms causing pyogenic liver abscesses have specific characteristics. Early identification of patients at high risk of infection with multidrug-resistant organisms could help improve their management and enable personalized treatment.
Introduction
Pyogenic liver abscess (PLA) is a life-threatening disease with a mortality rate of 2–13%. It is caused by the invasion of the liver by various types of microbes through several routes, such as the bloodstream and biliary system [Citation1–3]. The clinical manifestations and prognosis of PLA vary depending on the causative organism and the patient’s basic condition.
Escherichia coli used to be the most common organism isolated from patients with PLA. Over the past three decades, since liver abscesses caused by Klebsiella pneumoniae were first described in the 1980s in Taiwan [Citation4,Citation5], K. pneumoniae has become more common than E. coli as the predominant cause of PLA [Citation6]. An invasive syndrome with extrahepatic complications resulting from haematogenous dissemination, including endophthalmitis, meningitis, necrotizing fasciitis, and other illnesses, has been widely reported [Citation5,Citation7]. Typically, hypervirulent K. pneumoniae remains susceptible to most antimicrobial agents. However, the incidence of PLA caused by antimicrobial-resistant bacteria has increased in recent years, leading to increased difficulty with treatment, and increased mortality [Citation8].
Multidrug-resistant organisms (MDRO) are defined as microorganisms that are resistant to one or more classes of antimicrobial agents [Citation9]. The main types include vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, such as E. coli and K. pneumoniae, carbapenem-resistant Enterobacteriaceae, multidrug-resistant Pseudomonas aeruginosa, and multidrug-resistant Acinetobacter baumannii. Little is known about the bacterial spectrum and antibacterial sensitivity of PLA caused by MDRO. The aim of this study was to identify the risk factors for infections caused by MDRO in patients with PLA. Understanding the risk factors could enable clinicians to initiate timely and appropriate empiric antimicrobial therapy.
Patients and methods
Ethical approval of the study protocol
This study was a retrospective review of medical records. The study protocol was approved by the Ethics Committee of Fudan University Huashan Hospital (2022–1038). It was carried out by the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The requirement for patient consent was waived because of the retrospective study design confirmed by Ethics Committee of Fudan University Huashan Hospital (2022–1038).
Patients
Data were retrospectively collected from the case database of patients diagnosed with PLA who were admitted to Huashan Hospital in Shanghai, China, between 1 June 2012, and 31 May 2022. The study population consisted of patients aged 18–85 years, both male and female, who met the following diagnostic criteria: 1) presence of clinical manifestations, such as fever, chills, or abdominal pain; 2) imaging results such as abdominal ultrasound and/or computed tomography consistent with the imaging characteristics of liver abscesses; 3) confirmation of pathogenic bacteria by blood culture, pus culture, or metagenomic next-generation sequencing (mNGS); and 4) confirmation of an abscess by percutaneous liver biopsy or surgery. Patients with amoebic or tuberculous liver abscesses or incomplete clinical data were excluded.
Data were extracted from patient medical records stored in the electronic medical record system. The data included demographic characteristics (sex, age, body mass index), clinical manifestations (temperature, fever, chills, gastrointestinal symptoms, state of consciousness), underlying diseases (hypertension, diabetes, chronic lung disease, cardiovascular and cerebrovascular diseases, cirrhosis, malignant tumours, chronic renal insufficiency, history of hormone or immunosuppressant use), laboratory test results (haematologic, biochemical, and microbiologic test results), liver imaging results (abscess diameter, location, quantity, separation, presence or absence of gas in the liver), dissemination of infection (to the lung, spleen, brain, skin, or eye), treatment during hospitalization (antibiotic use, percutaneous liver puncture drainage, surgery), and outcome at discharge (recovery or death).
The microbiological characteristics considered included polymicrobial infection, single-microbial infection, anaerobic infection, and infection caused by ESBL-producing microorganisms or carbapenem-resistant microorganisms. Enterobacteriaceae strains resistant to ceftriaxone, cefotaxime, cefoperazone, or other third-generation cephalosporins were considered as carrying ESBL. Polymicrobial infections were defined as the presence of ≥ 2 pathogens cultured from blood or pus specimens. When anaerobic bacteria were present in blood or pus cultures, the patient was considered to have an anaerobic infection. In vitro drug susceptibility testing was performed using the VITEK 2 Compact Microbial Identification System (bioMérieux, Marcy-l’Étoile, France) and the disk diffusion (Kirby – Bauer) method. The antimicrobial disks were purchased from the Oxoid Company (Basingstoke, United Kingdom). The antimicrobial susceptibility testing and interpretation of results were conducted in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines [Citation10].
Parameter definitions
Based on the presumed infection route, the causes of PLA are divided into four categories: 1) direct spread: microorganisms invaded the liver through direct spread from the gallbladder or a nearby abscess; 2) biliary source: any biliary tract disease, such as malignant obstruction, biliary cannulation, or biliary surgery; 3) haematogenous dissemination: dissemination from other distant infection sites in patients with bacteraemia or sepsis; and 4) cryptogenic: without positive blood culture and no other identifiable source of infection.
Statistical analysis
All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, USA) or the R Project for Statistical Computing version 4.1.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria), with statistical significance set at p < .05. Continuous data were expressed as the mean ± standard deviation, and categorical data were expressed as frequencies and percentages. Independent sample t-tests were used to compare continuous variables with a normal distribution between groups, and the Mann – Whitney U-test was used to compare continuous variables with a non-normal distribution between groups. The chi-square test or Fisher’s exact test was used to compare categorical variables between groups. To identify the risk factors for PLA caused by MDRO strains, potential risk factors (including baseline conditions, comorbidities, and infection characteristics) were included as independent variables in the statistical analysis, and logistic regression models were constructed. Significant variables were introduced into the multivariable regression model, and backward stepwise elimination was used to develop the final multivariable regression model.
Results
Baseline characteristics of patients with PLA
From 1 June 2012, to 31 May 2022, 627 patients were diagnosed with liver abscesses, including 239 cases with an identified bacterial pathogen. The baseline characteristics of the patients are provided in . The mean age of the patients was 56.6 years (±14.6 years), and most patients were male (n = 168, 70.3%). The routes of PLA infection were cryptogenic (n = 108, 45.2%), direct dissemination (n = 47, 19.7%), biliary tract origin (n = 71, 29.7%), and haematogenous dissemination (n = 13, 5.4%). Of the 239 patients, 83 (34.7%) had type 2 diabetes, 74 (31.0%) had hypertension, 21 (10.5%) had severe cirrhosis or liver failure, and 57 (23.8%) had malignant tumours. Fever (n = 228, 95.4%) was the most common clinical manifestation, and only 79 patients (33.1%) had abdominal pain. On imaging, 164 patients (68.6%) showed solitary abscesses, and 160 abscesses (66.9%) were in the right lobe. The mean diameter was 66.1 (±31.2) mm. Of the 239 patients, 74 (31.0%) were treated with antimicrobial agents alone, 144 (60.3%) were treated with antimicrobial agents combined with percutaneous liver drainage, and 21 (8.8%) were treated with antimicrobial agents combined with surgical treatment.
Table 1. Baseline characteristics of the study patients (N = 239).
Microbiological characteristics of PLA
The blood culture, pus culture, or mNGS of pus detected 279 pathogens in 239 patients. Of the 239 patients, 204 (85.4%) had a single-pathogen infection, 30 (12.6%) had two pathogens detected, and 5 (2.1%) had three pathogens detected. Of the 279 pathogens, K. pneumoniae was the most common, accounting for 170 cases (60.9%), followed by E. coli (23 cases, 8.2%), Aeromonas spp. (4 cases), Pseudomonas spp. (3 cases), Acinetobacter baumannii (1 case), and other gram-negative bacteria (10 cases, including Klebsiella variabilis, Klebsiella oxytoca, Serratia marcescens, Citrobacter freundii, Citrobacter ljungstromii, Stenotrophomonas maltophilia, Enterobacter aerogenes, and Enterobacter cloacae). Streptococcus was the most common gram-positive genus, accounting for 21 cases (7.5%), followed by Enterococcus faecalis (14 cases, 5.0%), Enterococcus faecium (9 cases, 3.2%), and Staphylococcus aureus (4 cases, 1.4%). Two cases of Nocardia infection were identified using mNGS. Mixed infections occurred mostly in patients with anaerobic bacterial infections or Candida, accounting for 9 cases (3.2%) each ().
Figure 1. Causes of pyogenic liver abscess in the study patients (a). Distribution of multidrug-resistant organisms in patients with pyogenic liver abscess (b). CR, carbapenem-resistant; CRKP, carbapenem-resistant Klebsiella pneumoniae; ESBL, extended-spectrum beta-lactamase (producing); GNB, gram-negative bacteria; KP, Klebsiella pneumoniae; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
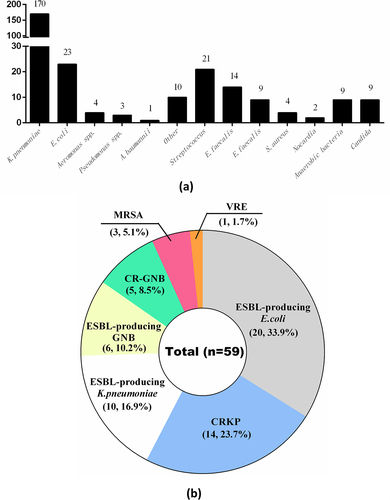
Of the 239 patients, antimicrobial susceptibility results were available for 211, of whom 55 (26.1%) had PLA caused by MDRO. Almost half of the 55 patients with MDRO infection (25 cases, 45.5%) had polymicrobial infections, with a total of 59 pathogens cultured. Four patients had two MDRO isolated each, specifically ESBL-producing E. coli with vancomycin-resistant E. faecium, ESBL-producing K. pneumoniae with carbapenem-resistant A. baumannii, ESBL-producing E. coli with carbapenem-resistant P. aeruginosa, and carbapenem-resistant K. pneumoniae with ESBL-producing E. cloacae. Of the 59 MDRO, ESBL-producing bacteria were the most common, including E. coli (20 cases, 33.9%), K. pneumoniae (10 cases, 16.9%), and other gram-negative bacilli (6 cases, 10.2%), followed by carbapenem-resistant bacteria, including K. pneumoniae (14 cases, 23.7%), Aeromonas spp. (2 cases, 3.4%), Pseudomonas spp. (2 cases, 3.4%), and A. baumannii (1 case, 1.7%). Three cases of methicillin-resistant S. aureus (3/59, 5.1%) and one case of vancomycin-resistant E. faecium (1/59, 1.7%) infection were identified among the gram-positive bacteria ().
Comparison of patients with PLA caused by MDRO and non-MDRO
The patients with culture results showing K. pneumoniae or E. coli were divided into MDRO and non-MDRO groups. Of the 175 patients, 44 were included in the MDRO group and 131 were included in the non-MDRO group. The clinical characteristics of the patients are shown in . Compared with patients in the non-MDRO group, patients in the MDRO group tended to have poorer baseline health and were more likely to have severe cirrhosis, liver failure, or a malignancy. They also had significantly higher Charlson Comorbidity Index scores. Patients in the MDRO group were more likely than those in the non-MDRO group to have a history of hospitalization, invasive procedures, and antibiotic use within the 6 months before hospital admission. In addition, the presumed source of PLA differed between the two groups. MDRO infections were most likely to originate from the biliary tract, whereas non-MDRO infections were most likely to be of cryptogenic origin. The frequency of vomiting and headache were higher in the MDRO group. The MDRO group had lower white blood cell counts, absolute lymphocyte counts, haemoglobin levels, platelet counts, and albumin levels, whereas the alkaline phosphatase levels were higher than those in the non-MDRO group. There were no significant differences between the two groups in the imaging characteristics of the abscesses, except for the presence of intrahepatic gas, which was significantly more frequent in the MDRO group.
Table 2. Comparison of the clinical characteristics, treatment, and clinical outcomes of patients with pyogenic liver abscess caused by multidrug-resistant organisms and non-multidrug-resistant organisms.
Comparison of treatment and clinical outcomes
There were no significant differences in the incidence of sepsis or pleural effusion between the MDRO and non-MDRO groups (). However, the incidence of bacteraemia was higher in the MDRO group. The incidence of metastatic infections, such as lung abscesses, endophthalmitis, or other metastatic foci, such as spleen, lumbar vertebrae, or prostate abscesses, was significantly higher in the non-MDRO group (). The proportion of patients admitted to the intensive care unit did not differ significantly between the two groups. All 175 patients with PLA received antibiotic treatment, with the most receiving combined treatment with two or more antibiotics during the initial stage. Antibiotic treatment did not differ significantly between the MDRO and non-MDRO groups. The proportion of patients who received antibiotics combined with percutaneous drainage was higher in the MDRO group (77.3% vs. 53.4%), whereas the proportion of patients who received antibiotics combined with surgical treatment was higher in the non-MDRO group (9.9% vs. 6.8%). The proportion of mixed infections was higher in the MDRO group than in the non-MDRO group (47.7% vs. 1.5%, p =.001), and the mortality rate was significantly higher in the MDRO group (15.9% vs. 2.3%, p =.03).
Analysis of risk factors for PLA caused by MDRO
Multivariable logistic regression showed that the Charlson Comorbidity Index score, hospitalization within 6 months, invasive procedures within 6 months, and intrahepatic gas were independent risk factors for PLA caused by MDRO ().
Table 3. Logistic regression analysis of risk factors associated with pyogenic liver abscess caused by multidrug-resistant organisms.
To increase the clinical utility of the regression model, it was used to create an interactive nomogram. A score was assigned to each item in the model (), and the sum was the total score. The point on the possibility axis corresponded to the risk of MDRO in patients with PLA (). shows an example of a patient with a liver abscess without intrahepatic gas, no history of hospitalization within the past 6 months, and no surgical procedure history. The patient had a Charlson Comorbidity Index score of 0 and an alkaline phosphatase level of 115 U/L. According to the multivariable logistic regression model, the probability of developing PLA caused by MDRO was estimated to be approximately 1.67%.
Figure 2. Individualized predictive nomogram model for predicting the risk of pyogenic liver abscess caused by multidrug-resistant organisms (a). Calibration plot (b) and receiver operating characteristic (ROC) curve (c) of the nomogram model for predicting the risk of multidrug-resistant organisms in patients with pyogenic liver abscess. The figure was created using R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
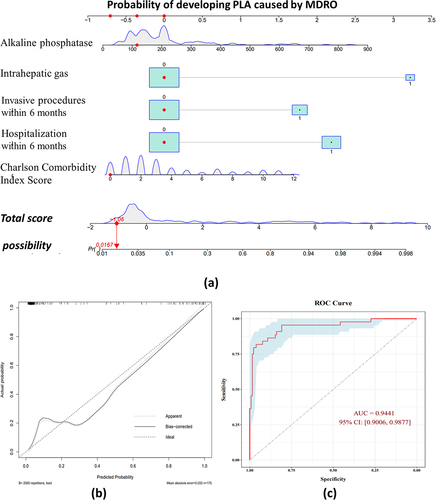
Table 4. Correspondence between risk factors and points.
Table 5. Association between total points and the probability of pyogenic liver abscess being caused by an MDRO.
The Hosmer – Lemeshow goodness-of-fit test for the regression model showed a P-value of .4303, indicating a good fit. Using bootstrap sampling with 2000 repeats, the calibration curve () showed a high degree of fit with the ideal curve, with an average absolute error of 0.033, and the predicted values were generally consistent with the observed values. The receiver operating characteristic curve (ROC) suggested that the area under the curve (AUC) was 0.9441 and the optimal cut-point based on the Youden index was 0.484 (). The diagnostic accuracy of the model for predicting PLA caused by MDRO based on this value is shown in . In addition, variance inflation factors (VIFs) are computed for independent variables to check for multicollinearity. The VIF values ranged from 1.12 to 2.04 and showed no sign of multicollinearity ().
Table 6. Model evaluation at the optimal threshold point according to the Youden index.
Table 7. Variance inflation factors for independent variables.
Discussion
In this study, we conducted an analysis of the pathogens associated with PLA at a tertiary hospital in Shanghai, China. Gram-negative enterobacteria were the most common pathogens, with K. pneumoniae accounting for 60.9% and E. coli accounting for 8.2%. Previous studies have shown that K. pneumoniae is more common than E. coli as the cause of liver abscesses, accounting for 60–80% of all cases of PLA in China and other Asian countries [Citation11–13]. Antibiotic resistance has become a major public health issue worldwide, and the incidence of PLA caused by antimicrobial-resistant bacteria has increased in recent years [Citation8]. In this study, 55 (23%) patients had MDRO infections, and ESBL-producing bacteria were most common, accounting for 61.0% of the MDRO, followed by carbapenem-resistant bacteria (32%). The prevalence of ESBL-producing E. coli was much higher than that of ESBL-producing K. pneumoniae. Carbapenem-resistant K. pneumoniae (CRKP) was the most common of the carbapenem-resistant bacteria. A study conducted in South Korea [Citation14] evaluated 833 patients with PLA from 2008 to 2017 and found that 6.6% of cases of PLA were caused by MDRO, which lower than the prevalence observed in this study. The presence of anaerobic bacteria and other rare pathogens may have been underestimated because conventional bacterial culture may not detect atypical pathogens. In this study, the pus of some patients was subjected to mNGS. This revealed the presence of two cases of Nocardia liver abscess. Nocardia-induced liver abscesses are rarely reported and occur mostly in patients undergoing solid organ transplantation and in those using immunosuppressants [Citation15,Citation16].
The clinical manifestations of PLA are non-specific, with fever as the main manifestation. Other common symptoms include diarrhoea, fatigue, and vomiting. If impaired consciousness or headache are present, the possibility of a brain abscess should be considered because community-acquired K. pneumoniae can lead to liver abscess invasion syndrome [Citation6]. In this study, the incidence of cryptogenic and metastatic abscesses was significantly higher in the non-MDRO group, which may be related to the high colonization rate of highly virulent K. pneumoniae in Asian populations, as well as its ability to cross the intestinal mucosal barrier and travel via the portal vein if the intestinal mucosal barrier is disrupted [Citation17,Citation18]. Some studies have shown that colorectal cancer can cause intestinal mucosal permeability, and PLA may be an early manifestation of colorectal cancer [Citation19,Citation20]. However, in this study, no cases of colorectal cancer were observed in patients with PLA.
The laboratory test results of patients with PLA, such as routine blood tests, liver function, and coagulation function, may be abnormal. The haemoglobin and albumin levels in the MDRO group were lower than those in the MDRO group, which may be related to chronic malnutrition caused by a history of abdominal surgery and malignant tumours in many patients in the MDRO group. The alkaline phosphatase levels were also higher in the MDRO group, which may be related to obstruction of intra- and extrahepatic bile ducts by conditions such as cirrhosis and digestive system tumours.
In this study, the mortality rate was significantly higher in the MDRO group than in the non-MDRO group, possibly due to the combination of the severity of the MDRO PLA and poor baseline health. Two cases of lung abscess due to highly virulent CRKP occurred in the MDRO group. Highly virulent CRKP is characterized by high virulence, multidrug resistance, and high transmissibility, leading to poor clinical outcomes [Citation21]. Previous studies have shown that advanced age, malignancy, biliary origin, and a high APACHE II score are associated with poor prognosis in patients with PLA [Citation22–24]. In this study, the Charlson Comorbidity Index was used to predict the clinical outcomes of PLA. In this study, a high Charlson Comorbidity Index was significantly associated with mortality and PLA caused by MDRO, consistent with the results of a previous study [Citation25].
In this study, the Charlson Comorbidity Index, hospitalization within 6 months, invasive procedures within 6 months, and intrahepatic gas were independent risk factors for PLA caused by MDRO. A previous meta-analysis reported no significant difference in the presence of intrahepatic gas between patients with K. pneumoniae and non-K. pneumoniae PLA, which differs from the results of this study [Citation22]. Nomograms serve as a predictive tool for prognosis by estimating clinical events and integrating key prognostic factors into a statistical prediction model. As part of this study, we constructed a nomogram including the independent risk factors, which were identified using multivariable logistic regression analysis. Individuals with a higher total score on the nomogram had a greater risk of PLA caused by MDRO. This model was stable and reliable in predicting infection risk, as demonstrated by its calibration and receiver operating characteristic curves.
This study has some limitations. It was conducted at a single centre and had a retrospective design, which may have introduced information bias. Prospective multicenter studies are required to confirm these results.
Conclusions
MDRO causing PLA have specific characteristics. E. coli and K. pneumoniae, are the main bacterial MDRO causing PLA. Patients with PLA caused by these strains are more likely to have polymicrobial infections and higher mortality rates. A high Charlson Comorbidity Index score, hospitalization within the past 6 months, invasive procedures within the past 6 months, and the presence of gas in the liver are independent risk factors for predicting PLA caused by MDRO. These findings are important for guiding clinical treatment and preventing PLA caused by MDRO, providing a basis for further research and enhancing strategies for the prevention and treatment of this disease.
Author contributions statement
XQ designed the study. QL, CC, and XZ collected and analysed the data. QL and MH wrote the paper. XQ and MH revised the intellectual content. All authors agree to be accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data supporting the findings of this study are provided in the article and online at https://doi.org/10.7910/DVN/V1EYGB.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (https://doi.org/10.1080/21505594.2024.2363658)
Additional information
Funding
References
- Lee SS, Chen YS, Tsai HC, et al. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis. 2008;47(5):642–10. doi: 10.1086/590932
- Lo JZ, Leow JJ, Ng PL, et al. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J Hepatobiliary Pancreat Sci. 2015;22(2):156–165. doi: 10.1002/jhbp.174
- Shi SH, Feng XN, Lai MC, et al. Biliary diseases as main causes of pyogenic liver abscess caused by extended-spectrum beta-lactamase-producing enterobacteriaceae. Liver Int. 2017;37(5):727–734. doi: 10.1111/liv.13267
- Chang FY, Chou MY. Comparison of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-K. pneumoniae pathogens. J Formos Med Assoc. 1995;94(5):232–237.
- Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913–1916. doi: 10.1001/archinte.1986.00360220057011
- Siu LK, Yeh KM, Lin JC, et al. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881–887. doi: 10.1016/S1473-3099(12)70205-0
- Saccente M. Klebsiella pneumoniae liver abscess, endophthalmitis, and meningitis in a man with newly recognized diabetes mellitus. Clin Infect Dis. 1999;29(6):1570–1571. doi: 10.1086/313539
- Zhang Y, Zhao C, Wang Q, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: Geographic Distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. 2016;60(10):6115–6120. doi: 10.1128/AAC.01127-16
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 32nd ed. Wayne (PA): Clinical and Laboratory Standards Institute; 2022 ed. CLSI supplement M100.
- Tsai FC, Huang YT, Chang LY, et al. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14(10):1592–1600. doi: 10.3201/eid1410.071254
- Yang CC, Yen CH, Ho MW, et al. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect. 2004;37(3):176–184.
- Qian Y, Wong CC, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep. 2016;6(1):38587. doi: 10.1038/srep38587
- Park JW, Kim JH, Jung JH, et al. A multicenter retrospective study on clinical characteristics and outcome of pyogenic liver abscess focusing multidrug-resistant organisms. J Clin Med. 2022;11(4):11. doi: 10.3390/jcm11041114
- Wiesmayr S, Stelzmueller I, Tabarelli W, et al. Nocardiosis following solid organ transplantation: a single-centre experience. Transplant Int. 2005;18(9):1048–1053. doi: 10.1111/j.1432-2277.2005.00177.x
- Hanchanale P, Jain M, Varghese J, et al. Nocardia liver abscess post liver transplantation—A rare presentation. Transpl Infect Dis. 2017;19(2). doi: 10.1111/tid.12670
- Chung DR, Lee H, Park MH, et al. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis. 2012;31(4):481–486. doi: 10.1007/s10096-011-1334-7
- Fung CP, Lin YT, Lin JC, et al. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis. 2012;18(8):1322–1325. doi: 10.3201/eid1808.111053
- Lee JK, Kum J, Ghosh P. Nonmetastatic cancer of the colon associated with pyogenic liver abscess. Am J Gastroenterol. 2008;103:798–799.
- Mohan BP, Meyyur Aravamudan V, Khan SR, et al. Prevalence of colorectal cancer in cryptogenic pyogenic liver abscess patients. Do they need screening colonoscopy? A systematic review and meta-analysis. Dig Liver Dis. 2019;51(12):1641–1645. doi: 10.1016/j.dld.2019.08.016
- Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37–46. doi: 10.1016/S1473-3099(17)30489-9
- Chan KS, Chia CTW, Shelat VG. Demographics, Radiological Findings, and Clinical outcomes of Klebsiella pneumonia vs. Non-Klebsiella pneumoniae Pyogenic Liver Abscess: A systematic review and meta-analysis with trial sequential analysis. Pathogens. 2022;11(9):11. doi: 10.3390/pathogens11090976
- Lee YT, Wang CC, Li CF, et al. Utility of acute physiology and chronic health evaluation (APACHE II) in predicting mortality in patients with pyogenic liver abscess: a retrospective study. J Clin Med. 2021;10(12):10. doi: 10.3390/jcm10122644
- Chen SC, Lee YT, Yen CH, et al. Pyogenic liver abscess in the elderly: clinical features, outcomes and prognostic factors. Age Ageing. 2009;38(3):271–276; discussion. 10.1093/ageing/afp002
- Rossi G, Nguyen Y, Lafont E, et al. Large retrospective study analysing predictive factors of primary treatment failure, recurrence and death in pyogenic liver abscesses. Infection. 2022;50(5):1205–1215. doi: 10.1007/s15010-022-01793-z
