ABSTRACT
The increasing antibiotic resistance poses a significant global health challenge, threatening our ability to combat infectious diseases. The phenomenon of collateral sensitivity, whereby resistance to one antibiotic is accompanied by increased sensitivity to another, offers potential avenues for novel therapeutic interventions against infections unresponsive to classical treatments. In this study, we elucidate the emergence of tobramycin (TOB)-resistant small colony variants (SCVs) due to mutations in the hemL gene, which render S. Typhimurium more susceptible to nitrofurantoin (NIT). Mechanistic studies demonstrate that the collateral sensitivity in TOB-resistant S. Typhimurium SCVs primarily stems from disruptions in haem biosynthesis. This leads to dysfunction in the electron transport chain (ETC) and redox imbalance, ultimately inducing lethal accumulation of reactive oxygen species (ROS). Additionally, the upregulation of nfsA/B expressions facilitates the conversion of NIT prodrug into its active form, promoting ROS-mediated bacterial killing and contributing to this collateral sensitivity pattern. Importantly, alternative NIT therapy demonstrates a significant reduction of bacterial load by more than 2.24-log10 cfu/g in the murine thigh infection and colitis models. Our findings corroborate the collateral sensitivity of S. Typhimurium to nitrofurans as a consequence of evolving resistance to aminoglycosides. This provides a promising approach for treating infections due to aminoglycoside-resistant strains.
Introduction
Non-typhoid Salmonella (NTS) continues to pose a significant global public health threat, accounting for an estimated 93.8 million illnesses and 155,000 deaths annually [Citation1]. As a zoonotic disease, salmonellosis ranks as the second most frequently reported bacterial gastrointestinal disease in the European Union, following campylobacteriosis [Citation2]. Among these cases, Salmonella enterica serovar Typhimurium (S. Typhimurium) stands out as one of the most prevalent NTS serotypes responsible for humans infections [Citation3,Citation4]. The symptoms of S. Typhimurium infection range from diarrhoea and vomiting to potentially fatal dehydration, particularly among children and the elderly [Citation5]. Globally, S. Typhimurium outbreaks have become increasingly prevalent, with a significant proportion associated with multidrug-resistant strains [Citation6–9]. Despite ongoing endeavours to develop novel treatment strategies, antibiotics such as fluoroquinolones, aminoglycosides, and β-lactams remain pivotal in Salmonella Typhimurium therapy [Citation10].
Bacterial small colony variants (SCV) are naturally occurring slow-growing subpopulations of bacteria characterized by smaller colony morphology, typically about 10% the size of wild-type colonies [Citation11]. In addition to the formation of smaller colonies, altered virulence factors and increased antibiotic tolerance or resistance are also the distinctive phenotypic features [Citation12]. At present, the SCV formation has been observed in various bacteria, including Staphylococcus spp., Pseudomonas spp., and Escherichia coli [Citation13]. Moreover, comprehensive phenotypic and mechanistic investigations into SCV formation in S. Typhimurium have also been reported [Citation14–16]. Cano et al. showed that prolonged bacterial residence in the intracellular environment of fibroblasts resulted in the emergence of genetically stable S. Typhimurium SCVs [Citation14]. Mutations in lpd and aroD were associated with SCV intracellular persistence in fibroblasts and reduced susceptibility to aminoglycosides [Citation14]. Previous studies also demonstrated the spontaneous emergence of S. Typhimurium SCVs after exposures to lactoferricin, colistin, and human defensin hNP-1 [Citation17]. In this instances, mutations related to haem biosynthesis and respiration were implicated in SCV formation [Citation17]. In addition, extensive amino acid auxotrophy, as observed in water cultures of S. Typhimurium, can impede bacterial growth and trigger SCV formation characterized by a loss-of-function mutation in the glutamine synthetase gene (glnA) [Citation18]. Such auxotrophic SCVs may lead to increased resistance to host immune responses and antibiotics, contributing to the challenge of treating chronic S. Typhimurium infections.
Antibiotic resistance poses a growing problem that can emerge during antibiotic therapy as a result of gene transfer or the selection of spontaneous mutations [Citation19–21]. The global dissemination of multidrug-resistant S. Typhimurium strains has significantly restricted clinical treatment options, emphasizing the urgent need for alternative therapeutic strategies to improve antibiotic efficacy [Citation9]. The trade-offs associated with antibiotic resistance are increasingly attracting attention for designing antibiotic strategies that suppress the within-host emergence of antibiotic resistance [Citation22]. In this context, collateral sensitivity, where resistance to one antibiotic results in heightened susceptibility to a second antibiotic, has been proposed as a potential strategy for suppressing antibiotic [Citation23].
In the current study, we showed phenotypic trade-offs associated with the use of aminoglycosides that can perturb antibiotic susceptibility profiles in S. Typhimurium SCV mutants. This adaptation leads to collateral sensitivity to nitrofurantoin (NIT) by perturbing nfsA/B expression and haem biosynthesis, resulting in ETC dysfunction, redox imbalance, and ultimately ROS-induced bacterial eradication. Our findings provide proof-of-principle evidence for collateral sensitivity as a potential treatment strategy to address aminoglycoside resistance in S. Typhimurium infections.
Materials and methods
Bacterial culture and growth condition
All S. Typhimurium strains used in this study are detailed in supplemental Table S1. The SCV mutants derived from S. Typhimurium ATCC 14028 were cultured and maintained at 37 °C in Luria-Bertani broth (LB) or agar (LA, Oxoid, Basingstoke, UK). When required, tobramycin (TOB), nitrofurantoin (NIT) or 5-aminolevulinic acid (ALA) was supplemented to the growth media at the specified concentrations.
Plasmids and primers
The primers and plasmids used in this study are listed in Table S2 and S3. All plasmids were extracted using the TIANprep Mini Plasmid Kit (Tiangen, China).
Isolation of SCV mutants and adaptation evolution
TOB was used to select the SCV mutants of S. Typhimurium ATCC 14028 by gradient plate method on LB agar as described [Citation24] with minor modifications. In brief, overnight cultures of ATCC 14028 were streaked onto gradient plate containing 0–512 mg/L of TOB and incubated at 37 °C overnight. Small and extremely small colonies were picked and restreaked on fresh selective media with 64 mg/L TOB to purify. This process was repeated until the SCV phenotype was consistently observed. Notably, the colony sizes of these SCVs were approximately one-tenth of those observed in wild-type bacterial colonies [Citation13]. The SCV mutants of S. Typhimurium ATCC 14028 (designated as TOB-R) were then serially passaged by streaking onto TOB-supplemented plates to assess the phenotypic stability. Stable TOB-R SCV mutants, exhibiting single colonies approximately one-tenth the size of the WT, were picked, purified, and stored in 30% glycerol at −80 °C.
Whole genome sequencing and single nucleotide polymorphism analysis
DNA was extracted from S. Typhimurium ATCC 14028 and SCVs using a commercial genomic DNA purification kit according to the manufacturer’s instructions (Tiangen, Beijing, China). Whole genome sequencing (WGS) was performed with the Illumina HiSeq 2500 system (Novogene, Guangzhou, China) using the paired-end 2 × 150-bp sequencing protocol. The draft genome was de novo assembled using SPAdes version 3.9.0. The complete genome of ATCC 14028 (GenBank accession no. CP034479.1) served as the reference sequence for single nucleotide polymorphism (SNP) analysis of WT and SCVs using Snippy v4.6.0.
Construction of ΔhemL and complemented S. Typhimurium strains
Two plasmid systems consisting of pSGKP_hemL_HR and pCasKP-apr were used to constructed the hemL deletion mutant (ΔhemL) in the ATCC 14028 (WT) background [Citation25]. For pSGKP_hemL, a sgRNA targeting the hemL was constructed in the pSGKp-rif using reverse PCR, and the resulting amplicon was transferred into E. coli DH5α. Subsequently, the plasmid was extracted, confirmed by Sanger sequencing, and designated as pSGKP_hemL. For pSGKP_hemL_HR, the pSGKP_hemL was digested by Sma I, and the ~ 500 bp homologous arm flanking hemL were cloned into the digested pSGKP_hemL. The resulting plasmid was named pSGKP_hemL_HR.
The plasmid pCasKP-apr was electroporated into the WT strain to enable the expression of Cas9 and lambda proteins. To introduce the pSGKP_hemL_HR plasmid into the WT strain harbouring pCasKP-apr, an overnight culture (1 mL) of the pCasKP-apr-harbouring WT strain was diluted into 100 mL of LB broth supplemented with 30 mg/L apramycin and incubated at 30 °C. Upon reaching an OD600 of approximately 0.2, 1 mL of 20% L-arabinose was added to induce the lambda Red recombineering operon of pCasKP-apr. After 2 h of induction at 30 °C and reaching an OD600 of approximately 0.6, the culture was prepared as electrocompetent cells. For electroporation, 100 μL of electrocompetent cells was thawed on ice for 5 min and mixed with 300–500 ng of pSGKP_hemL_HR. The mixture was then transferred into a 1 mm electroporation cuvette and electroporated. Subsequently, the pulsed cells were recovered in 1 mL of antibiotic-free LB broth and incubated at 30 °C for 4 h before being plated onto LB agar plates supplemented with the 30 mg/L apramycin and 50 mg/L rifampicin for overnight culture at 30 °C. The resulting colonies were verified by PCR and Sanger sequencing. To eliminate the pCasKP-apr and pSGKP_hemL_HR plasmids used in the knockout process, the knockout strains were obtained by three successive passages on LB agar plates containing 5% sucrose at 42 °C.
The hemL genes from the WT and TOB-R strains were recombined into a low-copy plasmid pGEN by seamless cloning. Subsequently, they were electroporated into the ΔhemL mutant to generate the complemented strains, designated as ΔhemL (hemLWT) and ΔhemL (hemLTOB-R), respectively.
Antimicrobial susceptibility testing and IC90 determination
The minimal inhibitory concentrations (MICs) of tobramycin and nitrofurantoin with or without ALA (200 µM) against the parental ATCC 14028, TOB-R, ΔhemL mutant, and its complemented strains was determined using the broth microdilution method in accordance with the CLSI M100-S25 guideline [Citation26]. Escherichia coli ATCC 25,922 served as the quality control strain. To map the collateral sensitivity profiles of antibiotic-resistant S. Typhimurium, overnight cultures of the tested strains were grown at 37°C with shaking in 96-well microtiter plates containing various gradient concentrations of antibiotics (amikacin, kanamycin, gentamycin, tobramycin, cefotaxime, cefquinome, ciprofloxacin, tetracycline, tigecycline, polymyxin E, polymyxin B, chloramphenicol, florfenicol, azithromycin, nitrofurantoin and rifampicin). Endpoint absorbance measurements (OD600 nm) were taken after 18 h of incubation using a plate reader (Perkin Elmer, USA) and background-subtracted. The inhibition percentage (%) was calculated as follows: [(OD positive control – OD negative control) – (OD antibiotic - OD negative control)]/(OD positive control – OD negative control) × 100%. The inhibitory concentration was defined as the lowest antibiotic concentration that inhibited 90% of the growth of the tested strain (IC90).
Determination of bacterial growth in the presence of ALA, TOB and NIT
The WT, TOB-R, ΔhemL mutant, and its complemented strains were cultured to exponential phase and adjusted to an OD600 nm to 0.5 (equivalent to ~ 108 cfu/mL). The cultures were then diluted to ~ 106 cfu/mL in fresh broth, with or without the addition of 200 µM ALA. Bacterial growth curves were monitored by measuring the OD600 nm every 20 minutes at 37 °C to evaluate the impact of ALA on S. Typhimurium growth. Bacterial suspensions were then serially diluted to ~ 103 cfu/mL, and 100 μL of the suspensions were spread evenly on agar plates with or without 200 µM ALA to observe colony size and identify the SCV phenotype. In addition, serial dilutions of bacterial cultures were spotted onto agar plates containing TOB (4 mg/L) or NIT (8 mg/L), respectively, to validate the collateral sensitivity of these strains. All plates were cultured at 37 °C overnight and subsequently photographed.
Heme concentration determination
The haem concentration was measured using the porphyrin fluorescence assay [Citation27]. This assay involves generating the fluorescent product protoporphyrin from haem by heating samples in oxalic acid. Briefly, overnight cultures of bacterial strains were diluted 1:100 and then incubated at 37 °C and 180 rpm for 4 h. Following incubation, bacterial cells were washed, collected and mixed with 500 µL of 20 mM oxalic acid at 4 °C for 16 h. The samples were then divided into two 500 μL aliquots. One set of samples were heated at 95 °C for 30 min to detect the total fluorescence value of porphyrin and haem, while the second set served as the control group stored at room temperature to measure the fluorescence value of porphyrin concentration. After cooling, the supernatant was centrifuged, and fluorescence was measured with excitation at 400 nm and emission at 620 nm using a Perkin Elmer fluorescence spectrometer. The absolute difference between the fluorescence values of the two groups represented the haem concentration.
Membrane depolarization assay
The membrane depolarization assay was performed following a previously established protocol [Citation28]. Briefly, an overnight bacterial culture was diluted 1:100 in fresh LB broth and incubated for 4 h at 37 °C with agitation at 180 rpm. Bacterial cells were then harvested, washed twice, and resuspended to obtain an OD600 nm of 0.5 with 5 mM HEPES buffer (containing 5 mM glucose, pH 7.0). After incubation for 30 min, a final concentration of 5 μM 3,3-dipropylthiadicarbocyanine iodide [DiSC3(5)] was added at 37 °C in the dark. Subsequently, the dissipated membrane potential of each tested S. Typhimurium strain was determined by measuring the fluorescence intensity using an EnSight Multimode plate reader (Perkin Elmer, USA), with excitation and emission wavelengths set at 622 nm and 670 nm, respectively.
Hydrogen peroxide killing assay
The resistance to hydrogen peroxide (H2O2) killing was determined as described previously [Citation29]. Bacterial cells in exponential phase were diluted to ~ 10 6 cfu/mL in fresh LB broth, and H2O2 was added at a final concentration of 2 mM for 1 h. After H2O2 treatment, the bacterial cultures were serially diluted 10-fold in PBS, and 100 μL aliquots from each dilution were plated on LB plates and then incubated at 37 °C for 16 h to quantify colony counts.
Total ROS determination
Total ROS levels in S. Typhimurium strains were determined by the fluorescence probe 2,’7’-dichlorofluorescein diacetate (DCFH-DA) [Citation30]. Exponential phase bacteria were washed twice with PBS and adjusted to an OD600 nm of 0.5. The DCFH-DA was then added at a final concentration of 10 µM, and the mixture was further incubated at 37 °C for 30 min. After washing with PBS for three times, fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 525 nm using an EnSight Multimode plate reader (Perkin Elmer, USA).
RNA isolation and RT-qPCR
The mRNA levels of soxS, marA, rob, nfsA and nfsB in TOB-R, ΔhemL mutant and its complemented strains relative to the WT were quantified by RT-qPCR analysis using the SYBR Green Master Mix (Vazyme, China). Bacterial cultures were grown in antibiotic-free LB broth until reaching the exponential phase (i.e. OD600 = 0.5). Total RNAs were extracted using the OMEGA Total RNA Kit I (Omega, China), followed by reverse transcription of 500 ng total RNA using the HiScript III All-in-one RT SuperMix Perfect (Vazyme, China). The primers used for RT-qRCR were listed in Table S3. Relative gene expression levels were determined using the 2−ΔΔCt method [Citation31].
Nitroreductase overexpression
The open reading frames of nfsA and nfsB were separately amplified from the WT chromosome using primers containing EcoR I restriction sites. The plasmid pBAD24 was then digested by EcoR I, and the amplified nfsA and nfsB sequences were individually cloned into the digested pBAD24. The resulting plasmids were designated as pBAD:nfsA and pBAD:nfsB, respectively. These plasmids were then electroporated into the WT to generate nfsA and nfsB overexpression strains.
Murine infection models
Six-week-old specific-pathogen-free ICR mice (Guangdong Medical Lab Animal Center, Guangzhou, China) were used for the in vivo studies. All animal experiments were approved by the Animal Research Committees of the South China Agricultural University (SCAU-IACUC). Murine thigh infection and colitis models were established to assess the differential efficacy of NIT against the WT, TOB-R, ΔhemL mutant and its complemented strains ΔhemL (hemLWT) and ΔhemL (hemLTOB-R).
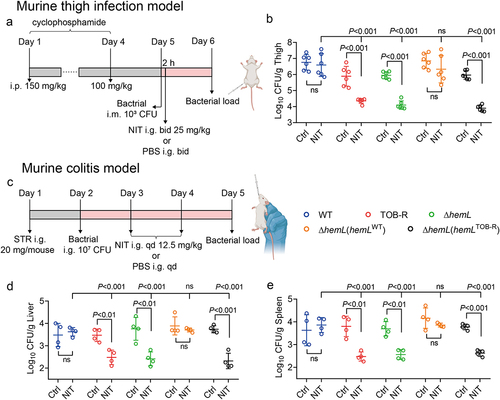
For the murine thigh infection model, mice were rendered neutropenic by the injection of cyclophosphamide intraperitoneally 4 days (150 mg/kg) and 1 day (100 mg/kg) prior to infection [Citation32]. Thigh infections were induced by the intramuscular injection of 0.10 mL of bacterial suspension (104 cfu/mL) into the thighs of neutropenic mice. Control groups received only the same volume of PBS inoculation. At 2 h post-infection, mice were randomized to receive (i) control treatment with PBS vehicle, intragastrically (i.g.) twice a day (bid), or (ii) NIT at 25 mg/kg i.g. bid. Control and NIT-treated mice were euthanized by CO2 inhalation at 24 h after start of therapy. Thighs were aseptically removed, homogenized, and quantitatively cultured to determine the mean log10 cfu/g tissue (± standard deviation; n = 6).
For the murine colitis model, mice were administered 20 mg streptomycin in 100 μL of sterile water by gavage 24 h prior to S. Typhimurium infection [Citation33]. Each mouse was then orally gavaged with 0.10 mL of bacterial suspension (~10 8 cfu/mL). Control groups received only the same volume of PBS inoculation. At 24 h post-infection, mice were randomized to receive (i) control treatment with PBS i.g. once a day (qd), or (ii) NIT at 12.5 mg/kg i.g. qd. The treatments last for 2 days. Mice were euthanized by CO2 inhalation and their liver and spleen were harvested, homogenized, serially diluted, and quantitatively cultured to determine the mean log10 cfu/g tissue (± standard deviation; n = 4).
Ethics statement
Animal experiments were conducted in strict compliance with the ARRIVE guidelines 2.0. Animals were maintained in accordance with the National Standards for Laboratory Animals of China. The Animal Research Committee (IACUC) of the South China Agricultural University (SCAU) approved these studies (#2022B121 and #2022C014).
Statistical analysis
All experiments were performed with three biological replicates. Statistical analysis was performed using SPSS software (IBM, USA). Unless stated otherwise, the statistical significance of comparison was assessed using the Student’s t-test, one-way or two-way analysis of variance. The MIC variables were analysed using the chi-square test, as appropriate (p < 0.05, p < 0.01, p < 0.001, ns: not significant).
Results
TOB-resistant S. Typhimurium SCV with a point mutation in hemL showed collateral sensitivity to NIT
Three evolutionary-derived S. Typhimurium ATCC 14028 mutants (TOB-R) exhibiting stable resistance to TOB were selected under the gradient selective pressure of TOB. Notably, these TOB-R mutant strains presented a tip-like morphology, with smaller and more diffuse colonies on both LB and blood agar plates compared to the WT ( and S1), indicative of the SCV phenotype [Citation13]. WGS and Snippy-SNP spectrum analysis of the WT and three TOB-R mutants consistently showed a SNP mutation (T:A>C:G) in the hemL gene (Table S4). This mutation led to a change from cysteine (Cys) to arginine (Arg) at amino acid position 212 (HemL-C212R).
Figure 1. TOB-resistant S. Typhimurium SCV shows collateral sensitivity to NIT.
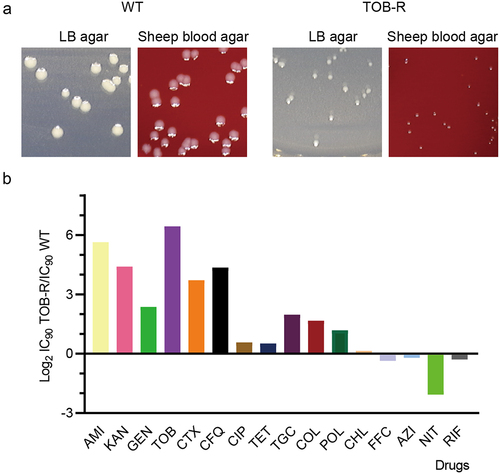
For the TOB-R SCV, we further performed dose-response curves to determine the IC90 changes for 16 antibiotics ( and S2). The S. Typhimurium SCV mutant that evolved resistance to TOB, a commonly used treatment for multidrug-resistant Gram-negative bacteria, exhibited increased sensitivity to four unrelated antibiotics from diverse chemical classes, including NIT, RIF, FFC and AZI (). While most observed collateral sensitivity resulted in a less than twofold reduction in IC90 relative to WT, a fourfold reduction of IC90 was observed for the TOB-R SCV against NIT. Expectedly, all three evolutionarily derived ATCC 14028 mutants (TOB-R) of S. typhimurium showed resistance to TOB and susceptibility to NIT (Table S5). Importantly, the enduring collateral sensitivity between TOB and NIT persisted even after 28 days of consecutive passaging (data not shown). This result indicated that NIT could be a promising sequential-treatment approach against such resistant pathogens when S. Typhimurium SCV mutants develop resistance to aminoglycosides. Of concern, apart from antibiotics belonging to the aminoglycosides (AMI, KAN and GEN), we also observed collateral resistance with a > 16-fold increase in the IC90 for the TOB-R SCV mutant compared to the WT (). In particular, a significant level of collateral resistance to β-lactams (CTX and CFQ; ) was noted for the TOB-R SCV mutant. Combinations of β-lactam and aminoglycoside classes are commonly used in clinical practice to broaden the antimicrobial spectrum [Citation34]. Hence, this collateral resistance may lead to treatment failure due to the selection of resistant S. Typhimurium SCV mutants during TOB treatment.
The ΔhemL induces SCV formation and NIT collateral sensitivity
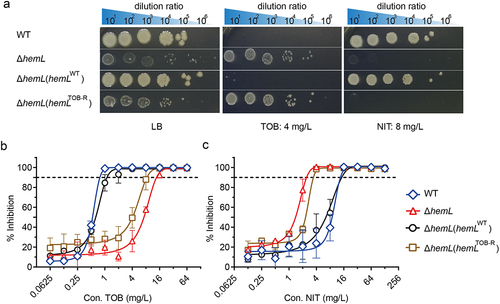
To explore the impacts of the hemL gene on SCV formation and collateral sensitivity to NIT, we used CRISPR/Cas-9 to construct a hemL knockout strain of S. Typhimurium ATCC 14028 (ΔhemL). We then complemented this strain with plasmids containing either the WT or mutated hemL, generating the ΔhemL (hemLWT) and ΔhemL (hemLTOB-R) strains, respectively. Similar to the TOB-R SCV strain (), both the ΔhemL and ΔhemL (hemLTOB-R) colonies exhibited irregular and smaller morphologies on LB agar plates compared to the WT (). However, complementation of the ΔhemL strain with hemLWT restored colony morphology similar to that of the WT (). Consistent with ΔhemL, the ΔhemL (hemLTOB-R) strain exhibited resistance to TOB (). In contrast, an increased sensitivity to NIT was observed for both ΔhemL and ΔhemL (hemLTOB-R) strains, with a 3.78 to 4.35-fold reduction in the IC90 value of NIT (; p < 0.05). This suggests that the intact hemL gene and its involvement in synthesizing ALA and haem are crucial for the collateral sensitivity between TOB and NIT.
Figure 2. S. Typhimurium ΔhemL mutant confers resistance to TOB and sensitivity to NIT.
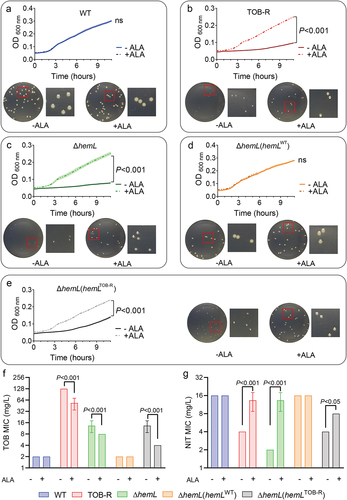
In the haem synthesis pathway of S. Typhimurium, HemL catalyzes the conversion of glutamate-1-semialdehyde into ALA. We therefore speculated that dysfunction in the hemL function leads to intracellular deficiencies of ALA and haem, potentially prompting the formation of TOB-resistant SCV [Citation14]. After exogenous addition of 200 µM ALA, all hemL-deficient strains including TOB-R, ΔhemL and ΔhemL (hemLTOB-R), exhibited growth restoration comparable to the WT level (). However, ALA supplementation had no discernible effect on the growth of the functionally intact WT or ΔhemL (hemLWT) strain (). Similarly, the provision of ALA in agar plates inhibited SCV formation and substantially restored colony morphology for these hemL-deficient strains (; p < 0.001). In addition, the resistance to TOB and collateral sensitivity to NIT, as evidenced by changes in MIC, could be reversed by ALA supplementation (; p < 0.001, p < 0.05). In particular, the MICs of TOB-R and ΔhemL strains reverted nearly to the WT level in the presence of 200 µM ALA ().
Figure 3. ALA deficiency induces S. Typhimurium SCV phenotype and collateral sensitivity to NIT.
Reduced PMF generated by the ETC leads to TOB resistance
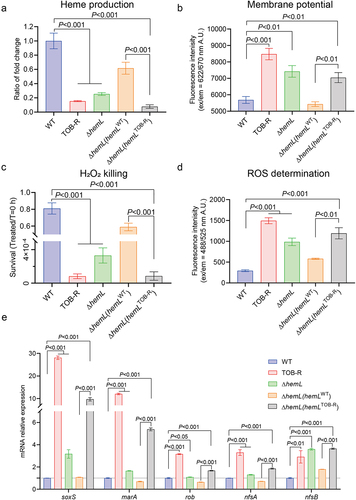
The ETC protein complexes rely on haem for their function and thus depend on porphyrin-haem biosynthesis [Citation35]. The hemL encodes glutamate-1-semialdehyde aminomutase, which catalyzes the final step in ALA synthesis, a crucial precursor in the haem biosynthetic pathway [Citation36]. As expected, intracellular haem levels were decreased significantly in TOB-R, ΔhemL and ΔhemL (hemLTOB-R) strains compared to WT (; p < 0.001). Since TOB internalization usually requires a PMF generated by the ETC [Citation37,Citation38], we therefore measured the cytoplasmic membrane potential (ΔΨ) of the tested S. Typhimurium strains using the fluorescent probe DiSC3(5). Compared to the WT, the TOB-R and ΔhemL strains exhibited a 1.31 to 1.50-fold increase in fluorescence (; p < 0.01), indicating a loss of electric potential in the hemL-deficient S. Typhimurium strains. The membrane potential depolarization is related to PMF production, which facilitates aminoglycoside uptake through its transporter [Citation39], potentially contributing to increased TOB resistance in hemL-deficient strains.
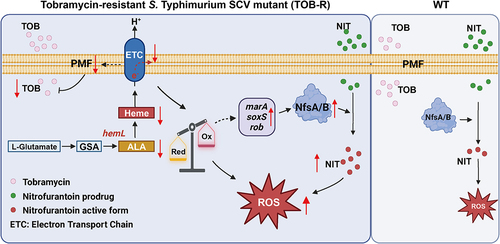
Figure 4. Mechanism of collateral sensitivity to NIT in TOB-resistant S. Typhimurium SCV mutant strain.
Intracellular redox imbalance and increased nfsA/B expression contribute to NIT collateral sensitivity
The dissipation of membrane potential generally leads to an imbalance in redox homoeostasis, culminating in bacterial death [Citation40]. Thus, we compared the sensitivity to H2O2 for the WT, TOB-R, ΔhemL and their complemented S. Typhimurium strains. Under the exogenous oxidative stress, the survival rates of TOB-R and ΔhemL strains were significantly lower than that of the WT (; p < 0.001). Similar results were observed between the ΔhemL (hemLTOB-R) and ΔhemL (hemLWT) strains (p < 0.001). Further analysis with the DCFH-DA probe demonstrated that the hemL-deficient strains, including TOB-R, ΔhemL and ΔhemL (hemLTOB-R), accumulated higher intracellular ROS levels compared to the WT and ΔhemL (hemLWT) strains (; p < 0.05).
The conversion of nitrofurantoin from its prodrug to active form necessitates intracellular processing by bacterial nitroreductases NfsA and NfsB [Citation41]. As expected, the overexpression of nfsA and nfsB resulted in increased sensitivity to nitrofurantoin (Figure S3 and Table S6). We also observed higher expression levels of nfsA and nfsB genes in the TOB-R and ΔhemL strains compared to the WT (). Consistently, complementation of the ΔhemL strain with hemLTOB-R significantly elevated the expressions of nfsA and nfsB compared to the hemLWT-complemented ΔhemL strain (; p < 0.001). In addition, increased expression levels of key genes (soxS, marA and rob) that positively regulate nitroreductases were observed in all hemL-deficient S. Typhimurium strains (; p < 0.05). Based on these results, a schematic model illustrating the possible mechanisms underlying NIT collateral sensitivity is shown in . In TOB-resistant S. Typhimurium SCV, the hemL mutation decreases haem biosynthesis, leading to the ETC dysfunction, redox imbalance, and intracellular ROS accumulation. Upregulated expression of nfsA/B facilitates the conversion of NIT into its active form, thereby promoting ROS-induced bacterial killing. These factors collectively contributed to the collateral sensitivity of S. Typhimurium to NIT.
Figure 5. Graphic illustration of the possible mechanism of collateral sensitivity to NIT in TOB-resistant S. Typhimurium SCV induced by HemL deficiency.
Importantly, in vivo experiments further demonstrated the enhanced efficacy of NIT against TOB-R SCV infections (). In the murine thigh infection model, NIT treatment had no significant effects on WT and ΔhemL (hemLWT) infections, but significantly decreased bacterial loads in the thighs of TOB-R, ΔhemL, and ΔhemL (hemLTOB-R) infected mice by 1.54 to 2.06-log10 cfu/g (, p < 0.001). Similar results were observed in the murine colitis model, where NIT treatment markedly reduced bacterial loads in the liver and spleen of TOB-R, ΔhemL and ΔhemL (hemLTOB-R) infected mice by 0.99 to 1.42-log10 cfu/g (, p < 0.01). Therefore, NIT may represent a promising strategy for combating aminoglycoside-resistant S. Typhimurium SCV infections.
Figure 6. Comparison of NIT efficacy in murine models of thigh infection and colitis due to the WT, TOB-R, ΔhemL and its complemented strains.
Discussion
Collectively, this study delineates an experimentally isolated haem-auxotrophic S. Typhimurium SCV under the selective pressure of TOB, revealing its pronounced collateral sensitivity to NIT. Since the initial discovery of bacterial SCV from Eberthella typhosa (now known as S. Typhimurium) in 1910, SCV formation has been observed across various bacteria from clinical specimens [Citation13,Citation42]. Proctor et al. highlighted a persistent and relapsing infection associated with S. aureus SCVs, immediately recognizing the clinical importance of SCV infection in considering therapeutic strategies [Citation43]. With respect to the underlying mechanisms, previous studies have showed that ALA deficiency due to the hemL mutation induces S. Typhimurium SCV formation in fibroblasts [Citation14]. This is in line with our findings in the TOB-resistant S. Typhimurium SCVs, as we also identified a point mutation in the hemL gene in this study. Importantly, our study verified that supplementation with exogenous ALA can restore the small colony morphology of S. Typhimurium SCV mutants. Furthermore, we elucidated the molecular mechanisms by which ALA deficiency-induced S. Typhimurium SCVs lead to tobramycin resistance and nitrofurantoin sensitivity.
Intracellular bacterial adaptation and alteration in energy metabolism are associated with phenotype switching to SCV [Citation44]. In particular, the ETC dysfunction is frequently correlated with SCV formation and persistent infections [Citation45]. Previous studies have demonstrated that SCV isolates of E. coli, P. aeruginosa, and S. aureus with ETC deficiencies exhibit lower growth rates under aerobic conditions [Citation46–48]. These deficiencies lead to a rapid reduction in membrane potential, diminish the transmembrane H+ gradient, and impair energy-dependent drug transport processes. Since aminoglycoside uptake relies on both ATP and pH [Citation49], even minor ETC dysfunction can impact ATP production and aminoglycoside susceptibility during SCV formation. This inference was supported by results presented herein. The haem-deficient S. Typhimurium strains showed reduced PMF and increased resistance to TOB. Complementation of the ΔhemL strain with the intact hemLWT gene restored growth and all other key SCV phenotypic features, including colony size, haem levels, and PMF. These findings parallel previous studies showing reduced aminoglycoside susceptibility in clinically isolated S. aureus and P. aeruginosa SCV strains from cystic fibrosis patients [Citation50].
Deficiencies in the bacterial ETC can also affect antibiotic susceptibility through disrupting redox balance and energy homoeostasis [Citation51]. A recent study demonstrated that E. coli mutants with impaired ubiquinone biosynthesis grow poorly under aerobic conditions due to oxidative stress [Citation52]. Similarly, in this study, S. Typhimurium strains with hemL mutations were notably sensitive to H2O2 treatment. In addition, the ETC defects exacerbate the leakage of electrons, impair cellular redox-buffering capacity, and ultimately result in uncontrolled ROS-induced bacterial killing [Citation53]. This is consistent with our observation of elevated ROS levels in the hemL-deficient S. Typhimurium strains. Subsequent ROS accumulation may promote bacterial killing through oxidative damage, thus heightening the bactericidal activity of NIT.
In principle, drug switches can restore control of evolving pathogen populations and may be pre-planned according to the collateral sensitivity profiles. This approach could prolong the efficacy of existing antibiotics and help to predict the evolutionary directions of emerging resistance genes [Citation54]. To our knowledge, this study provides the first demonstration of an intriguing collateral sensitivity strategy involving nitrofurans exploited by a stable haem-auxotrophic S. Typhimurium SCV isolate under constant exposure to aminoglycosides. In fact, this collateral sensitivity to nitrofurans herein could potentially represent a functional evolutionary trade-off to aminoglycoside resistance. While the precise mechanism of nitrofurans remains to be elusive, their bactericidal activity is believed to primarily involve ROS-mediated oxidative DNA damage [Citation41]. However, the efficacy of NIT depends on the intracellular conversion of the prodrug to its active form by bacterial nitroreductases NfsA/B [Citation55,Citation56]. In S. Typhimurium, the expressions of nfsA and nfsB genes are regulated by three activators, marA, rob, and soxS [Citation57]. Interestingly, our study showed significant upregulation of multiple regulons (marA/rob/soxS) in hemL-deficient S. Typhimurium strains, which further promoted the expressions of nfsA and nfsB. Consequently, more NIT prodrug could be converted to its active component, thereby boosting NIT activity against S. Typhimurium mutants. Furthermore, the nitroreduction process generates the highly reactive byproducts of aerobic metabolism, including ROS from the oxidation of amines [Citation58]. This process could synergistically interact with haem deficiency-induced redox imbalance. Since excessive ROS production and redox imbalance can result in DNA damage, it is plausible that heterogeneity in the expression of DNA repair or ROS detoxification enzymes contribute to collateral sensitivity [Citation51].
Conclusions
In summary, the present study demonstrated the formation of TOB-resistant S. Typhimurium SCV and their collateral sensitivity to NIT caused by mutations in the hemL gene. Mechanistic studies revealed that hemL mutation decreases haem biosynthesis, leading to the ETC dysfunction, redox imbalance and the accumulation of lethal ROS. The upregulated nfsA/B expression further facilitates the production of activated NIT, promoting ROS-induced bacterial killing, and ultimately contributing to the collateral sensitivity of S. Typhimurium SCVs to NIT. This approach may provide a promising avenue for combination therapy to combat resistance evolution. Nevertheless, future in vivo and clinical studies are warranted to determine whether this antibiotic collateral sensitivity strategy could help address antibiotic resistance in clinical settings.
Author contributions
Yu-Feng Zhou and Xiao-Ping Liao completed the conception and design of this study. Chang-Zhen Wang, Liang-Xing Fang and Jian Sun analysed the data. Chang-Zhen Wang and Yu-Feng Zhou drafted the manuscript. Chang-Zhen Wang, Yue-Jun Zhang, Yue-Fei Chu, Long-Gen Zhong, Jin-Peng Xu, Liu-Yan Liang and Teng-Fei Long carried out the experiments. Xiao-Ping Liao revised it critically for intellectual content. Yu-Feng Zhou final approval of the version to be published. All authors agreed to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (1.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Whole genome sequencing data of the WT, TOB-R, TOB-R1 and TOB-R2 strains have been submitted to the NCBI under the BioSample accession number SAMN 40,697,886, SAMN 39,637,111, SAMN40697887, and SAMN 40,697,888. Derived data supporting the findings of this study are available at https://doi.org/10.6084/m9.figshare.25650747.v1.
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2356692
Additional information
Funding
References
- Gong B, Li H, Feng Y, et al. Prevalence, serotype distribution and antimicrobial resistance of non-typhoidal salmonella in hospitalized patients in conghua district of Guangzhou, China. Front Cell Infect Microbiol. 2022;12:805384. doi: 10.3389/fcimb.2022.805384
- Beikzadeh B. Immunoinformatics design of multi-epitope vaccine using OmpA, OmpD and enterotoxin against non-typhoidal salmonellosis. BMC Bioinf. 2023;24(1):63. doi: 10.1186/s12859-023-05183-6
- Schiller LR. A germy world—food-borne infections in 2009. Nat Rev Gastroenterol Hepatol. 2009;6(4):197–15. doi: 10.1038/nrgastro.2009.40
- Ao TT, Feasey NA, Gordon MA, et al. Global burden of invasive nontyphoidal salmonella disease, 20101. Emerg Infect Dis. 2015;21(6):941–949. doi: 10.3201/eid2106.140999
- Kurtz JR, Goggins JA, McLachlan JB. Salmonella infection: interplay between the bacteria and host immune system. Immunol Lett. 2017;190:42–50. doi: 10.1016/j.imlet.2017.07.006
- Martínez MC, Retamal P, Rojas-Aedo JF, et al. Multidrug-resistant outbreak-associated salmonella strains in irrigation water from the metropolitan region, Chile. Zoonoses Public Health. 2017;64(4):299–304. doi: 10.1111/zph.12311
- Folster JP, Grass JE, Bicknese A, et al. Characterization of resistance genes and plasmids from outbreaks and illness clusters caused by salmonella resistant to ceftriaxone in the United States, 2011–2012. Microb Drug Resist. 2017;23(2):188–193. doi: 10.1089/mdr.2016.0080
- Alt K, Simon S, Helmeke C, et al. Outbreak of uncommon O4 non-agglutinating salmonella typhimurium linked to minced pork, saxony-anhalt, Germany, January to April 2013. PLOS ONE. 2015;10(6):e0128349. doi: 10.1371/journal.pone.0128349
- Xiang Y, Li F, Dong N, et al. Investigation of a salmonellosis outbreak caused by multidrug resistant salmonella typhimurium in China. Front Microbiol. 2020;11:801. doi: 10.3389/fmicb.2020.00801
- Zafar S, Ajab H, Mughal ZU, et al. Prediction and evaluation of multi epitope based sub-unit vaccine against salmonella typhimurium. Saudi J Biol Sci. 2022;29(2):1092–1099. doi: 10.1016/j.sjbs.2021.09.061
- Besse A, Groleau MC, Déziel E, et al. Emergence of small colony variants is an adaptive strategy used by pseudomonas aeruginosa to mitigate the effects of redox imbalance. mSphere. 2023;8(2):e0005723. doi: 10.1128/msphere.00057-23
- Richter K, Thomas N, Zhang G, et al. Deferiprone and Gallium-Protoporphyrin have the capacity to potentiate the activity of antibiotics in staphylococcus aureus small colony variants. Front Cell Infect Microbiol. 2017;7:280. doi: 10.3389/fcimb.2017.00280
- Proctor RA, von Eiff C, Kahl BC, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4(4):295–305. doi: 10.1038/nrmicro1384
- Cano DA, Pucciarelli MG, Martínez-Moya M, et al. Selection of small-colony variants of salmonella enterica serovar typhimurium in nonphagocytic eucaryotic cells. Infect Immun. 2003;71(7):3690–3698. doi: 10.1128/IAI.71.7.3690-3698.2003
- Li W, Li Y, Wu Y, et al. Phenotypic and genetic changes in the life cycle of small colony variants of salmonella enterica serotype typhimurium induced by streptomycin. Ann Clin Microbiol Antimicrob. 2016;15(1):37. doi: 10.1186/s12941-016-0151-3
- Pränting M, Andersson DI. Escape from growth restriction in small colony variants of salmonella typhimurium by gene amplification and mutation. Mol Microbiol. 2011;79(2):305–315. doi: 10.1111/j.1365-2958.2010.07458.x
- Pränting M, Andersson DI. Mechanisms and physiological effects of protamine resistance in salmonella enterica serovar typhimurium LT2. J Antimicrob Chemother. 2010;65(5):876–887. doi: 10.1093/jac/dkq059
- Aurass P, Düvel J, Karste S, et al. glnA truncation in salmonella enterica results in a small colony variant phenotype, attenuated host cell entry, and reduced expression of flagellin and SPI-1-Associated effector genes. Appl Environ Microbiol. 2018;84(2):e01838–17. doi: 10.1128/AEM.01838-17
- Tueffers L, Barbosa C, Bobis I, et al. Pseudomonas aeruginosa populations in the cystic fibrosis lung lose susceptibility to newly applied β-lactams within 3 days. J Antimicrob Chemother. 2019;74(10):2916–2925. doi: 10.1093/jac/dkz297
- Liu YY, Wang Y, Walsh TR, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7
- Bloemberg GV, Keller PM, Stucki D, et al. Acquired resistance to bedaquiline and delamanid in therapy for tuberculosis. New Engl J Med. 2015;373(20):1986–1988. doi: 10.1056/NEJMc1505196
- Sanz-García F, Gil-Gil T, Laborda P, et al. Translating eco-evolutionary biology into therapy to tackle antibiotic resistance. Nat Rev Microbiol. 2023;21(10):671–685. doi: 10.1038/s41579-023-00902-5
- Imamovic L, Sommer MO. Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Sci Transl Med. 2013;5(204):204ra132. doi: 10.1126/scitranslmed.3006609
- Szybalski W, Bryson V. Genetic studies on microbial cross resistance to toxic agents I. J Bacteriol. 1952;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952
- Wang Y, Wang S, Chen W, et al. CRISPR-Cas9 and CRISPR-Assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl Environ Microbiol. 2018;84(23):e01834–18. doi: 10.1128/AEM.01834-18
- CLSI. Performance standards for antimicrobial susceptibility testing. 31st ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute; 2021.
- Weissman Z, Pinsky M, Donegan RK, et al. Using genetically encoded heme sensors to probe the mechanisms of heme uptake and homeostasis in Candida albicans. Cell Microbiol. 2021;23(2):e13282. doi: 10.1111/cmi.13282
- Te Winkel JD, Gray DA, Seistrup KH, et al. Analysis of antimicrobial-triggered membrane depolarization using voltage sensitive dyes. Front Cell Dev Biol. 2016;4:29. doi: 10.3389/fcell.2016.00029
- Bitoun JP, Liao S, Xie GG, et al. Deficiency of BrpB causes major defects in cell division, stress responses and biofilm formation by streptococcus mutans. Microbiol (Reading). 2014;160(1):67–78. doi: 10.1099/mic.0.072884-0
- Li Q, Chen S, Zhu K. Collateral sensitivity to pleuromutilins in vancomycin-resistant enterococcus faecium. Nat Commun. 2022;13(1):1888. doi: 10.1038/s41467-022-29493-0
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262
- Zhou YF, Tao MT, He YZ, et al. In vivo bioluminescent monitoring of therapeutic efficacy and pharmacodynamic target assessment of antofloxacin against Escherichia coli in a neutropenic murine thigh infection model. Antimicrob Agents Chemother. 2018;62(1):e01281–17. doi: 10.1128/AAC.01281-17
- Barthel M, Hapfelmeier S, Quintanilla-Martínez L, et al. Pretreatment of mice with streptomycin provides a salmonella enterica serovar typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71(5):2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003
- Barthel A, Ursenbach A, Kaeuffer C, et al. Characteristics and treatment of gordonia spp bacteremia, France. Emerg Infect Dis. 2023;29(5):1025–1028. doi: 10.3201/eid2905.221901
- Elliott T, Avissar YJ, Rhie GE, et al. Cloning and sequence of the salmonella typhimurium hemL gene and identification of the missing enzyme in hemL mutants as glutamate-1-semialdehyde aminotransferase. J Bacteriol. 1990;172(12):7071–7084. doi: 10.1128/jb.172.12.7071-7084.1990
- Morales EH, Pinto CA, Luraschi R, et al. Accumulation of heme biosynthetic intermediates contributes to the antibacterial action of the metalloid tellurite. Nat Commun. 2017;8(1):15320. doi: 10.1038/ncomms15320
- Taber HW, Mueller JP, Miller PF, et al. Bacterial uptake of aminoglycoside antibiotics. Microbiol Rev. 1987;51(4):439–457. doi: 10.1128/mr.51.4.439-457.1987
- Xia Y, Wang D, Pan X, et al. TpiA is a key metabolic enzyme that affects virulence and resistance to aminoglycoside antibiotics through CrcZ in pseudomonas aeruginosa. MBio. 2020;11(1):e02079–19. doi: 10.1128/mBio.02079-19
- Meylan S, Porter CBM, Yang JH, et al. Carbon sources tune antibiotic susceptibility in pseudomonas aeruginosa via tricarboxylic acid cycle control. Cell Chem Biol. 2017;24(2):195–206. doi: 10.1016/j.chembiol.2016.12.015
- Liu Y, Tong Z, Shi J, et al. Reversion of antibiotic resistance in multidrug-resistant pathogens using non-antibiotic pharmaceutical benzydamine. Commun Biol. 2021;4:1328. doi: 10.1038/s42003-021-02854-z
- Ren H, Zhong Z, Zhou S, et al. CpxA/R-Controlled nitroreductase expression as target for combinatorial therapy against uropathogens by promoting reactive oxygen species generation. Adv Sci. 2023;10(25):e2300938. doi: 10.1002/advs.202300938
- Bryan LE, Kwan S. Aminoglycoside-resistant mutants of pseudomonas aeruginosa deficient in cytochrome d, nitrite reductase, and aerobic transport. Antimicrob Agents Chemother. 1981;19(6):958–964. doi: 10.1128/AAC.19.6.958
- Proctor RA, van Langevelde P, Kristjansson M, et al. Persistent and relapsing infections associated with small-colony variants of staphylococcus aureus. Clin Infect Dis. 1995;20:95–102. doi: 10.1093/clinids/20.1.95
- Vulin C, Leimer N, Huemer M, et al. Prolonged bacterial lag time results in small colony variants that represent a sub-population of persisters. Nat Commun. 2018;9:4074. doi: 10.1038/s41467-018-06527-0
- Zheng X, Fang R, Wang C, et al. Resistance profiles and biological characteristics of rifampicin-resistant staphylococcus aureus small-colony variants. Infect Drug Resist. 2021;14:1527–1536. doi: 10.2147/IDR.S301863
- Noaman KA, Alharbi NS, Khaled JM, et al. The transmutation of Escherichia coli ATCC 25922 to small colony variants (SCVs) E. coli strain as a result of exposure to gentamicin. J Infect Public Health. 2023;16(11):1821–1829. doi: 10.1016/j.jiph.2023.08.024
- Tuchscherr L, Löffler B, Proctor RA. Persistence of staphylococcus aureus: multiple metabolic pathways impact the expression of virulence factors in small-colony variants (SCVs). Front Microbiol. 2020;11:1028. doi: 10.3389/fmicb.2020.01028
- Lozano C, Azcona-Gutiérrez JM, Van Bambeke F, et al. Great phenotypic and genetic variation among successive chronic pseudomonas aeruginosa from a cystic fibrosis patient. PLOS ONE. 2018;13(9):e0204167. doi: 10.1371/journal.pone.0204167
- Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069
- Schneider M, Mühlemann K, Droz S, et al. Clinical characteristics associated with isolation of small-colony variants of staphylococcus aureus and pseudomonas aeruginosa from respiratory secretions of patients with cystic fibrosis. J Clin Microbiol. 2008;46(5):1832–1834. doi: 10.1128/JCM.00361-08
- Fino C, Vestergaard M, Ingmer H, et al. PasT of Escherichia coli sustains antibiotic tolerance and aerobic respiration as a bacterial homolog of mitochondrial Coq10. Microbiologyopen. 2020;9(8):e1064. doi: 10.1002/mbo3.1064
- Nitzschke A, Bettenbrock K, Weiner JH. All three quinone species play distinct roles in ensuring optimal growth under aerobic and fermentative conditions in E. coli K12. PLOS ONE. 2018;13(4):e0194699. doi: 10.1371/journal.pone.0194699
- Jastroch M, Divakaruni AS, Mookerjee S, et al. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. doi: 10.1042/bse0470053
- Hernando-Amado S, Laborda P, Martínez JL. Tackling antibiotic resistance by inducing transient and robust collateral sensitivity. Nat Commun. 2023;14(1):1723. doi: 10.1038/s41467-023-37357-4
- Thulin E, Sundqvist M, Andersson DI. Amdinocillin (mecillinam) resistance mutations in clinical isolates and laboratory-selected mutants of Escherichia coli. Antimicrob Agents Chemother. 2015;59(3):1718–1727. doi: 10.1128/AAC.04819-14
- McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother. 1994;33(Suppl A):23–30. doi: 10.1093/jac/33.suppl_A.23
- Roemhild R, Linkevicius M, Andersson DI, et al. Molecular mechanisms of collateral sensitivity to the antibiotic nitrofurantoin. PLOS Biol. 2020;18(1):e3000612. doi: 10.1371/journal.pbio.3000612
- Zuma NH, Aucamp J, N’Da DD. An update on derivatisation and repurposing of clinical nitrofuran drugs. Eur J Pharm Sci. 2019;140:105092. doi: 10.1016/j.ejps.2019.105092
