ABSTRACT
The global surge in multidrug-resistant bacteria owing to antibiotic misuse and overuse poses considerable risks to human and animal health. With existing antibiotics losing their effectiveness and the protracted process of developing new antibiotics, urgent alternatives are imperative to curb disease spread. Notably, improving the bactericidal effect of antibiotics by using non-antibiotic substances has emerged as a viable strategy. Although reduced nicotinamide adenine dinucleotide (NADH) may play a crucial role in regulating bacterial resistance, studies examining how the change of metabolic profile and bacterial resistance following by exogenous administration are scarce. Therefore, this study aimed to elucidate the metabolic changes that occur in Edwardsiella tarda (E. tarda), which exhibits resistance to various antibiotics, following the exogenous addition of NADH using metabolomics. The effects of these alterations on the bactericidal activity of neomycin were investigated. NADH enhanced the effectiveness of aminoglycoside antibiotics against E. tarda ATCC15947, achieving bacterial eradication at low doses. Metabolomic analysis revealed that NADH reprogrammed the ATCC15947 metabolic profile by promoting purine metabolism and energy metabolism, yielding increased adenosine triphosphate (ATP) levels. Increased ATP levels played a crucial role in enhancing the bactericidal effects of neomycin. Moreover, exogenous NADH promoted the bactericidal efficacy of tetracyclines and chloramphenicols. NADH in combination with neomycin was effective against other clinically resistant bacteria, including Aeromonas hydrophila, Vibrio parahaemolyticus, methicillin-resistant Staphylococcus aureus, and Listeria monocytogenes. These results may facilitate the development of effective approaches for preventing and managing E. tarda–induced infections and multidrug resistance in aquaculture and clinical settings.
Introduction
The Edwardsiella genus, first reported in Japan in the 1960s, received detailed elaboration in 1965 by Ewing, McWhorter, Escobar, and Lubin [Citation1]. Comprising only three taxa, namely Edwardsiella tarda, E. ictaluri, and E. hoshinae, The Edwardsiella genus has a broad host range, infecting various fish species, reptiles, birds, and mammals [Citation2,Citation3]. E. tarda, generally considered the causative agent of edwardsiellosis in fish, has been detected in over 25 fish species across seven continents [Citation4,Citation5]. In addition, it is considered a moderately zoonotic bacterium capable of infecting various age groups and individuals with compromised immune systems [Citation6]. Although vaccine development is still in its juvenile stage, antibiotics remain the primary treatment for E. tarda–induced fish and human diseases [Citation7]. However, extensive use of antibiotics has led to an increasing emergence of antibiotic-resistant strains. Moreover, E. tarda exhibits innate resistance to various antibiotics [Citation8,Citation9], resulting in suboptimal antibiotic treatment outcomes.
Tetracyclines, macrolides, sulphonamides, quinolones, and aminoglycoside antibiotics are widely used in aquaculture [Citation10]. Among aminoglycoside antibiotics, neomycin has been widely used to control bacterial and viral infections in the aquaculture industry. For example, neomycin is effective against Aeromonas species and cyprinid herpesvirus 2 infections in fish [Citation11–13] and has demonstrated efficacy in treating E. tarda–induced infections [Citation14,Citation15]. Despite the current susceptibility of most E. tarda strains to neomycin [Citation16,Citation17], the substantial use of this antibiotic in aquaculture increases the development of resistance, potentially compromising its effectiveness. Moreover, a fraction of antibiotics are utilized, with the majority being discharged into the water environment, causing serious environmental problems. Therefore, addressing the issue of enhancing the efficacy of low-dose antibiotics is imperative and warrants further research.
In recent years, changes in the metabolic pathways of drug-resistant bacteria have been proposed to be related to the regulation of drug resistance [Citation18–20]. Several studies have confirmed that the sensitivity of bacteria to antibiotics can be altered by the regulation of their metabolic pathways. For example, experiments have demonstrated that enhancement of fatty acid synthesis in bacteria can facilitate the acquisition of drug resistance [Citation21,Citation22]. Furthermore, adenosine monophosphate deaminase-driven shifts in nucleotide metabolism have been demonstrated to enhance the eradication of tolerant and persistent bacteria [Citation23]. We found that exogenous addition of carbon sources such as alanine and/or glucose increased the sensitivity of E. tarda EIB202 to kanamycin by promoting the tricarboxylic acid (TCA) cycle [Citation19]. Notably, this alanine-induced enhancement was attenuated after inhibition of the TCA cycle through the knockout of sucA and sucB [Citation24]. In addition, metabolites such as indole, nitric oxide, and gaseous ammonia can regulate the sensitivity of Escherichia coli to antibiotics by changing their metabolic state [Citation25,Citation26].
Reduced nicotinamide adenine dinucleotide (NADH) is an important product of cellular energy metabolism and is typically synthesized from the TCA cycle and glycolysis. Its use was approved by the US Food and Drug Administration in 1998 and has been used clinically to treat diseases [Citation27–29]. However, limited studies have been conducted regarding the mechanism underlying the effect of NADH on bacterial resistance. Exogenous carbon sources have been demonstrated to change the sensitivity of Gram-positive and Gram-negative bacteria to antibiotics, a phenomenon closely related to changes in intracellular NADH concentrations [Citation19,Citation30–32]. Conversely, several studies have revealed that bacterial resistance often involves a diminished energy metabolic state accompanied by decreased intracellular NADH concentrations [Citation33,Citation34]. Therefore, we inferred that NADH plays a crucial role in the regulation of bacterial resistance. However, studies examining how the metabolic profile and resistance of drug-resistant bacteria are altered following the exogenous administration of NADH remain scarce. It is therefore necessary to elucidate these aspects.
In recent years, metabolomics has gradually attracted attention and has been applied to the detection of cellular metabolic changes [Citation32,Citation35–37]. Metabolomic analysis enables a comprehensive understanding of the changes in intracellular metabolic pathways. Therefore, in this study, we aimed to elucidate the metabolic changes that occur in E. tarda ATCC15947 following the exogenous addition of NADH, and how these alterations enhance the lethality of neomycin against ATCC15947 using metabolomics. Furthermore, we investigated the potential of NADH to restore the sensitivity of ATCC15947 to aminoglycosides, tetracyclines, and chloramphenicols. The findings of this study provide new insights for the development of therapeutic strategies to combat infections caused by this bacterium.
Results
NADH reverted ATCC15947 resistance to neomycin
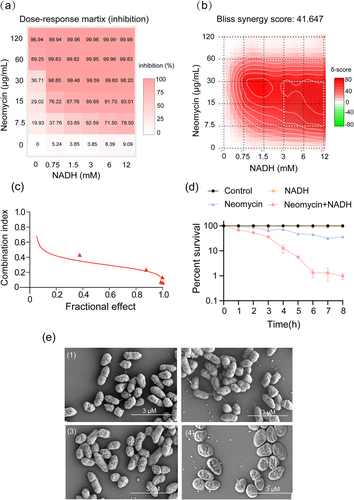
ATCC15947 cells were cultured with neomycin and NADH to observe the effect of NADH on neomycin activity against ATCC15947. SynergyFinder 3.0 and Calcusyn software were used to analyse synergistic effects [Citation38]. The mean dose-response survival rates of the bacteria treated with NADH, neomycin, or their combination are presented in Table S1. The optimal conditions for promoting neomycin sensitivity and the highest level of synergy are shown in . The comprehensive synergy score for NADH combined with neomycin was calculated at 41.65 across a broad concentration range, indicating strong synergy between the two agents. Furthermore, the combination of 3 mM NADH and 30 μg/mL neomycin yielded the best synergistic effects (). A combination index (CI) illustrating the effects of the combinations on ATCC15947 is shown in . The CI value for NADH plus neomycin ranged from 0.5 to 0.1, indicating a strong synergy between neomycin and NADH [Citation39]. In addition to neomycin, NADH reversed the resistance of ATCC15947 to doxycycline and florfenicol (Figure S1a and b). Moreover, a time-gradient experiment was conducted to explore the temporal impact of the synergistic effect. The results revealed optimal efficacy at 6 h (). In addition, scanning electron microscopy revealed intact cell structures in the control and NADH groups. However, the structure of the minority cells treated with neomycin alone was disrupted, and the degree of disruption was further intensified by neomycin and NADH combination treatment (). These results demonstrate that the combination of NADH and neomycin induces structural damage to bacterial cells, potentially leading to bacterial death.
Figure 1. NADH reverts the resistance of ATCC15947 to neomycin. (a) Dose-response matrix (inhibition) for NADH & neomycin. The inhibition ratio is positively relative to the degree of red. (b) The drug interaction landscapes based on the Bliss model on ATCC15947. The synergy score is showed by red (>0) and green (<0). (c) Calcusyn analysis of NADH combined with neomycin synergy on ATCC15947 (Combined R = 0.95, NADH R = 0.72, neomycin R = 0.94). (d) Time gradient sterilization experiment of 3 mM NADH and 30 μg/mL neomycin. (e) SEM of ATCC15947: (1) Control group, (2) NADH group of 3 mM, (3) Neomycin group of 30 μg/mL, (4) NADH combined with neomycin group. Data are presented as mean ± SEM (n = 3 biological replicates).
NADH reverted antibiotic resistance in ATCC15947 by reprogramming its metabolic profile
Metabolomics was employed to analyse metabolic changes following treatment of ATCC15947 with NADH. Unsupervised hierarchical clustering identified 113 metabolites in ATCC15947 (Figure S2a). These metabolites were divided into five categories: amino acids, carbohydrates, nucleotides, fatty acids and lipids, and others with proportions of 20.2, 25.4, 21.0, 24.6 and 8.8%, respectively (Figure S2b). A total of 69 metabolites exhibited differential abundance (), with 36 present in high abundance and 33 in low abundance following NADH treatment (). Among these, the top five upregulated metabolites were L-methylguanine, lipoamide, adenosine diphosphate (ADP), N-4 acetylcytidine, and glucosamine, whereas the top five downregulated metabolites were PE (9:0/9:0), glutamylcysteine, octanedioic acid, 10-heptadecenoic acid, and decanoic acid. These differential metabolites were also divided into five categories: amino acids, carbohydrates, nucleotides, fatty acids and lipids, and others with proportions of 18.8, 24.6, 22.0, 24.6 and 10.0%, respectively ().
Figure 2. Metabolome analysis of ATCC15947 between control and NADH group. (a) Heat map showing differential abundance of metabolites. Blue and yellow colors indicate lower and higher abundances of the metabolites relative to the mean level of the control group, respectively. (b) Z-score plots of changes in differential metabolites based on control. The data were respectively scaled to the mean and standard deviation of control. Each point represents one biological repeat. Different treatments are distinguished by the color. (c) Categories of differential abundance of different metabolites. (d) Significantly enriched metabolic pathways in response to NADH treatment (p < 0.05). (e) Changes in differential metabolites involved in the significantly enriched pathways. Yellow colour and blue colour indicate increased and decreased metabolites, respectively.
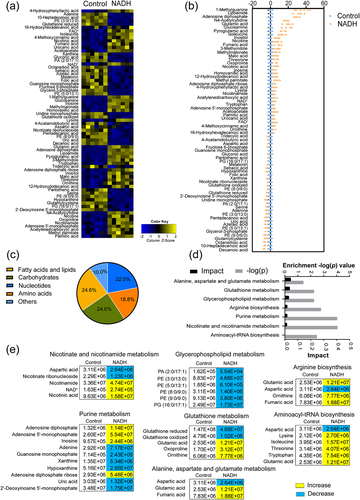
Seven enriched metabolic pathways were identified based on the obtained data: alanine, aspartate, and glutamate metabolism; glutathione metabolism; glycerophospholipid metabolism; arginine biosynthesis; purine metabolism; nicotinate and nicotinamide metabolism; and aminoacyl-tRNA biosynthesis. Changes in the abundance of differential metabolites in these pathways were analysed. Among the seven pathways, purine metabolism exhibited the highest number of differential metabolites (). Notably, the abundance of adenosine 5”-monophosphate (AMP), ADP, inosine, and adenosine diphosphate ribose was upregulated, whereas that of adenine, guanosine monophosphate, xanthine, hypoxanthine, uric acid, and 2”−deoxyinosine 5’-monophosphate was downregulated. Furthermore, the abundance of AMP and ADP, which are associated with adenosine 5′-triphosphate (ATP) synthesis, was upregulated during purine metabolism (). Orthogonal partial least squares discriminant analysis (OPLS-DA) strategy was adopted to provide insights into separations between NADH and control group. NADH and control group were clearly separated from each other, which mean these two groups had different metabolism characteristics (Figure S2c). The discriminating variables were presented in the S-plots (Figure S2d). Cutoff values for the absolute value of the covariance p and correlation p (corr) were ≥ 0.05. The axes that are plotted in the S-plot from the predictive component are p vs. p (corr), representing the magnitude (modelled covariation) and reliability (modelled correlation), respectively. A total of 10 potential biomarkers were identified. The abundance of flavin adenine dinucleotide (FAD), 4-hydroxyphenyllactic acid, palmitic acid, methyl palmitate, and ethinyl dicarboxylic acid were increased (Figure S2e) and the abundance of PE (3:0/13:0) adenine, 10-heptadecenoic acid, glutathione reduced and glutamylcysteine were decreased (Figure S2f). These results suggest that NADH reprogrammed the metabolome of ATCC15947, particularly by altering purine metabolism, which could potentially contribute to the reversal of drug resistance in ATCC15947.
Exogenous NADH activated purine metabolism
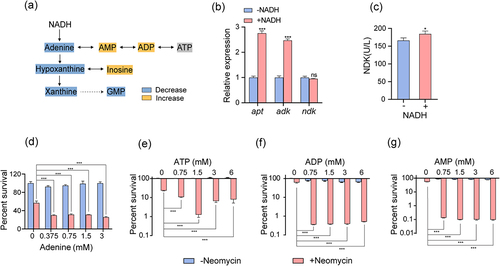
The metabolic network associated with purine metabolism was mapped using enrichment pathway analysis (). The expression levels of the purine metabolism-associated genes were measured to verify the association between exogenous NADH and purine metabolism. NADH treatment led to elevated ade phosphoribosyltransferase (apt) and ado kinase gene (adk) (). Nucleoside diphosphate kinase (NDK/NDPK), encoded by nucleoside diphosphate kinase gene (ndk), is important in purine metabolism as it facilitates ATP production; specifically, it can catalyse the production of ADP and ATP from inosine monophosphate [Citation40]. Although the expression level of ndk remained relatively unchanged, NDK enzyme activity increased significantly following treatment with NADH (). These results indicate that purine metabolism activation is related to NADH and that exogenous NADH likely stimulates purine metabolism to generate ATP. To test this hypothesis, intermediates involved in purine metabolism, such as adenine, AMP, ADP, and ATP, were introduced separately into the medium to assess their roles. The results revealed that these metabolites independently promoted the bactericidal effects of neomycin on ATCC15947 (). These results support the notion that purine metabolism plays an important role in reversing ATCC15947 resistance, which is likely attributable to the ATP generated from purine metabolism.
Figure 3. NADH promotes purine metabolism. (a) Changes in purine metabolism after addition of NADH in ATCC15947. (b) qRT-PCR for expression of genes in purine metabolism in the absence and presence of NADH. (c) Activity of NDK were detected with and without 3 mM NADH. (d)–(g) the concentration effect of adenine/ATP/ADP/AMP plus 30 μg/mL neomycin on the bactericidal efficacy. Data are presented as mean ± SEM (n = 3 biological replicates). *p < 0.05.**p < 0.01. ***p < 0.001.
NADH enhanced bacterial respiration
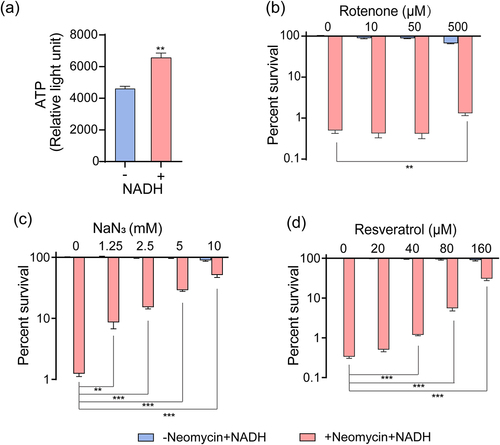
In addition to purine metabolism, NADH undergoes oxidative phosphorylation through the respiratory chain to generate ATP [Citation41]. Our results revealed that exogenous NADH increased the intracellular ATP levels in ATCC15947 cells (). Therefore, inhibitors were used to further investigate the effects of NADH on bacterial respiration. Rotenone and NaN3 act as inhibitors of the respiratory chain, whereas resveratrol inhibits ATP synthase [Citation19,Citation42]. The potent bactericidal effects of NADH and neomycin were eliminated when these substances were introduced into the reaction system (). These results demonstrate that NADH activates the respiratory chain and generates additional ATP through oxidative phosphorylation, thereby enhancing the bactericidal effect of neomycin.
Figure 4. NADH promotes respiratory chain and ATP generation. (a) Effects of NADH on ATP content in ATCC15947. (b)–(d) the concentration effect of rotenone/NaN3/resveratrol on the bactericidal efficacy of 3 mM NADH combined with 30 μg/mL neomycin. Data are presented as mean ± SEM (n = 3 biological replicates). *p < 0.05.**p < 0.01. ***p < 0.001.
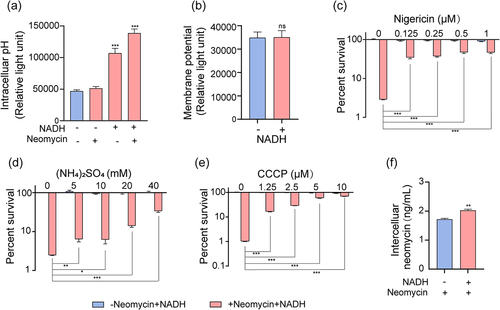
NADH increased neomycin uptake through the increase of proton motive force
Previous studies have reported that bacterial uptake of aminoglycosides is associated with proton motive force (PMF), established PMF comprises both electrical (ΔΨout–in) and pH (ΔpHout–in) gradients [Citation31,Citation43]. In our study, compared to the control group, the addition of NADH increased the intracellular pH, leading to an elevated ΔpH (). However, no change in the membrane potential (ΔΨ) was observed (). Considering that nigericin/excess K+ and (NH4)2SO4 can disrupt the ΔpH of bacteria [Citation30,Citation44], we speculated that the bactericidal effects of NADH and neomycin would be attenuated by their addition. As expected, nigericin/excess K+ and (NH4)2SO4 abolished the synergistic effect of NADH and neomycin on ATCC15947 (). Given that ΔΨ remained unchanged, the elevated ΔpH induced an increase in PMF. Carbonyl cyanide-chlorophenyl hydrazone (CCCP), a well-known inhibitor of PMF dissipation [Citation45], was subsequently introduced into the medium to examine the role of PMF. The results revealed that NADH-induced elimination of ATCC15947 by neomycin decreased following CCCP treatment (). Consistently, exogenous NADH increased intracellular neomycin content in cells (). These results suggest that NADH increases PMF by elevating ΔpH, thereby enhancing neomycin uptake and potentiating its bactericidal effects.
Figure 5. NADH increased PMF and intracellular neomycin content. (a) Changes in intercelluar pH with effect of neomycin or NADH in ATCC15947. (b) Membrane potential changes after treating with NADH. (c)–(d) the concentration effect of nigericin/(NH4)2SO4/CCCP on the bactericidal efficacy of 3 mM NADH combined with 30 μg/mL neomycin. (f) Intracellular neomycin content was detected in presence of NADH and neomycin. Data are presented as mean ± SEM (n = 3 biological replicates). *p < 0.05.**p < 0.01. ***p < 0.001.
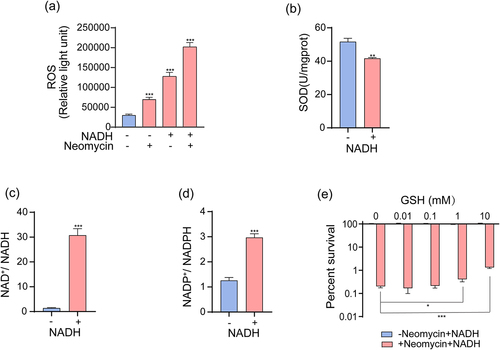
Exogenous NADH induced oxidative – antioxidative system imbalance
Superoxides are produced during cellular respiration [Citation46]. Activation of the respiratory chain by NADH may lead to significant production of superoxides, thereby disrupting the oxidative – antioxidative system in bacteria. To measure cellular oxidative stress levels, we evaluated intercellular reactive oxygen species (ROS) levels and quantified NAD+/NADH and NADP+/NADPH ratios. The intercellular ROS levels increased following NADH treatment (). Furthermore, the activity of the ROS-scavenging enzyme superoxide dismutase was determined, revealing a consistently lower activity than that in the control group after treatment with NADH (). Moreover, the NAD+/NADH and NADP+/NADPH ratios were significantly increased by NADH compared to those in the control (). Elevated ROS levels and NAD+/NADH and NADP+/NADPH ratios suggest an imbalance in the oxidative – antioxidative system in vivo, which potentially enhances the bactericidal effects of aminoglycosides. Considering that GSH is a ROS scavenger [Citation47], we used it for further analysis. Upon addition of GSH, the enhanced bactericidal effects induced by NADH and neomycin exposure were impaired ().
Figure 6. NADH sensitizes ATCC15947 to neomycin killing through inducing imbalance of oxidative – antioxidative system. (a) Changes in ROS levels with effect of neomycin or NADH in ATCC15947. (b) Activity of SOD were detected with and without 3 mM NADH. (c) and (d) NADH affected the ratio of NAD+/NADH and NADP+/NADPH. (e) The concentration effect of GSH on the bactericidal efficacy of 3 mM NADH combined with 30 μg/mL neomycin. Data are presented as mean ± SEM (n = 3 biological replicates). *p < 0.05.**p < 0.01. ***p < 0.001.
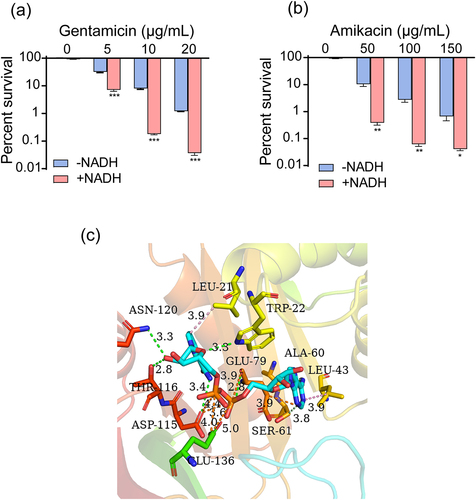
Exogenous NADH promoted the bactericidal effect of aminoglycosides against ATCC15947 and altered aminoglycoside acetyltransferases activity
In the presence of exogenous NADH, highly synergistic effects were observed with other aminoglycosides such as gentamicin and amikacin (). Given that NADH exhibits high synergy with various aminoglycosides, we hypothesized that NADH may inhibit the activity of aminoglycoside-modifying enzymes. Among the three types of aminoglycoside-modifying enzymes: aminoglycoside nucleotidyltransferases (ANTs), aminoglycoside phosphotransferases (APHs), and aminoglycoside acetyltransferases (AACs), capable of rendering aminoglycosides inactive [Citation48,Citation49], only AACs are expressed in ATCC15947. AutoDock analysis was performed using AACs as receptors and NADH as ligands to explore potential attachment interactions between NADH and AACs. NADH exhibited robust binding to AACs (binding energy, − 10 kcal/mol), interacting with several key amino residues (ASN120, LEU2, TRP22, THR116, GLU79, ALA60, LEU43, ASP115, GLU136, SER61) ().
Figure 7. NADH can inhibit inactivation of aminoglycosides caused by AACs. (a) and (b) Synergistic bactericidal efficacy of different concentrations of gentamicin/amikacin with 3 mM NADH. (c) 3D schematic diagram of NADH binding to the active site of AACs. Data are presented as mean ± SEM (n = 3 biological replicates). *p < 0.05.**p < 0.01. ***p < 0.001.
Discussion
The development of new antibiotics has been scarce for decades [Citation50]. Therefore, alternative methods to address bacterial resistance have been developed. Studies have revealed that exogenous addition of non-antibiotic substances can reprogram the metabolome of drug-resistant bacteria, rendering them sensitive to antibiotics [Citation51,Citation52]. Instead of relying solely on inhibiting antibiotic hydrolysis or efflux pumps to maintain intracellular antibiotic levels, metabolome reprogramming can facilitate a direct increase in intracellular antibiotic levels [Citation51,Citation53]. Many exogenous metabolites have been shown to affect NADH, PMF, ATP formation, and other cellular processes [Citation53]. For instance, exogenous metabolites can reverse or change antibiotic resistance in Edwardsiella and other gram-negative bacteria by affecting metabolic processes such as increasing citric acid cycle flux, NADH production, PMF, and the expression of outer membrane proteins [Citation19,Citation35]. Studies have also demonstrated that in combination with specific metabolites, such as glucose and pyruvate, the killing efficiency of antibiotics against E. coli and Staphylococcus aureus can be improved [Citation54,Citation55]. These studies highlight how exogenous metabolites can render drug-resistant bacteria sensitive to antibiotics, with concomitant changes in intracellular NADH content.
In this study, upon direct application of exogenous NADH, which acts as a key metabolic regulator, we successfully reversed the resistance of ATCC15947 to multiple antibiotics. NADH also could enhance the bactericidal activity of doxycycline and florfenicol against ATCC15947 (Figure S3). Our findings demonstrate that NADH reprogrammed the bacterial metabolome, inducing changes in several metabolic pathways. Notably, our study suggests that the activation of purine metabolism plays a vital role in reversing resistance to neomycin in ATCC15947 cells. The expression of genes and enzyme activities involved in purine metabolism, including apt, adk expression and NDK activity, were upregulated following NADH addition. These genes and enzymes are involved in regulating purine metabolism to produce ATP. Consistent with these results, an increase in ATP content was observed. Therefore, the activation of purine metabolism can increase the intracellular ATP content, representing a significant mechanism for enhancing intracellular energy metabolism. Similarly, activation of the respiratory chain by NADH also contributes to an increase in intracellular ATP content. This heightened ATP production results in an enhanced metabolic rate, ultimately contributing to cell death [Citation20,Citation52]. The improvement of energy metabolism and ATP generation through purine metabolism and respiratory chain reactions is significant in reversing ATCC15947 resistance.
In addition to purine metabolism, the introduction of NADH overactivates the respiratory chain, leading to changes in PMF and causing an imbalance in the oxidative – antioxidative system in bacteria [Citation33,Citation54]. PMF comprises ΔpH and ΔΨ, and contributes to aminoglycoside uptake [Citation30,Citation56]. NADH-induced ΔpH change leads to an increase in PMF, thereby elevating the intracellular level of neomycin, ultimately leading to bacterial death. Our recent study found that L-glutamine in enhancing gentamicin uptake through disrupting ΔpH and increasing cell membrane permeability, increasing antibacterial effect of aminoglycoside antibiotics on MRSA [Citation57]. Disruption of the oxidative – antioxidative system not only elevates ROS levels but also alters the ratios of NAD+/NADH and NADP+/NADPH [Citation58,Citation59]. Furthermore, ROS accumulation induced by elevated intracellular NADH levels enhances the bactericidal effects of aminoglycosides [Citation60,Citation61]. Similar to the β-lactam resistance mechanism of gram-negative bacteria, in which hydrolases destroy antibiotics, a protein capable of hydrolysing aminoglycoside antibiotics in ATCC15947 exists. Aminoglycoside antibiotics are chemically modified by aminoglycoside-modifying enzymes, including APHs, ANTs, and AACs [Citation62–64]. Among these, only AACs were expressed in ATCC15947 cells in this study. Notably, a potential interaction may exist between NADH and AACs through molecular docking, which may lead to inhibition of AACs activity. One limitation of this study is the inability to identify whether purine metabolism or the respiratory chain plays a pivotal role in enhancing the bactericidal effect of neomycin under NADH treatment. This uncertainty arises from the association between purine metabolism and the respiratory chain and ROS and their ability to generate a substantial amount of ATP [Citation65–69]. Further investigation is necessary to elucidate this aspect.
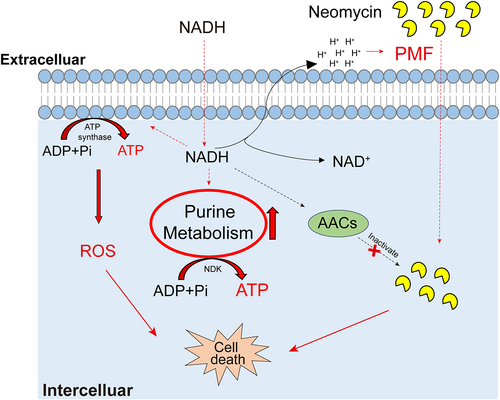
In summary, our study revealed that exogenous NADH reprogrammed the metabolome of ATCC15947 and activated purine metabolism and respiratory chain. This, in turn, results in an increase in intracellular ATP, ROS levels, and antibiotic content, while also potentially inhibiting the activity of AACs (). Therefore, NADH has emerged as a potent metabolite for use in combination with antibiotics, offering potential insights into effective approaches to combating E. tarda–induced infections in aquaculture and addressing infections caused by other pathogenic bacteria in both aquaculture and clinical settings.
Figure 8. Proposed mechanism of NADH-promoted neomycin killing ATCC15947. Exogenous NADH promotes purine metabolism and cellular respiration, causing an increase in intracellular ATP as well as neomycin content and intracellular ROS level. Increased ATP level elevated neomycin bactericidal efficiency. NADH also inhibits the activity of AACs, which prevents neomycin from inactivation caused by AACs, allowing keep high concentration of neomycin in cells.
Materials and methods
Bacterial strains, culture conditions, and chemicals
E. tarda ATCC15947 used in this study was obtained from Associate Prof. Chao Wang, Shandong Freshwater Fisheries Research Institute, Jinan; Methicillin-resistant Staphylococcus aureus (MRSA) USA300 was kindly provided by Dr. Hua Zhou, Zhejiang University; Listeria monocytogenes was kindly provided by Dr. Xinhai Chen, from Shenzhen Bay Lab; Aeromonas hydrophila Ah-HN1 was kindly provided by Prof. Xianliang Zhao, from Shantou University; Vibrio parahaemolyticus ATCC17802 was purchased from HuanKai Microbiology Technology Co., Lid (Guangzhou, China). These strains were cultured at 37°C for 16 h in Trypticase Soy Broth (TSB) broth [Citation69] (HuanKai Microbiology Technology Co., Ltd., Guangdong, China) or Luria-Bertani (LB) broth with shaking at 220 rpm. NADH, neomycin, adenosine triphosphate (ATP), adenosine 5′-monophosphate (AMP), adenosine diphosphate (ADP), (NH4)2SO4, KCl, resveratrol, glutathione (GSH) and other antibiotics were purchased from Macklin (Shanghai, China). 2′,7′-dichlorofluorescein diacetate (DCFH-DA), NaN3 (Sigma, USA), nigericin (APExBIO), bis-(1,3-dibutylbarbituric acid) trimethine oxonol (DiBAC4(3), Glpbio), carboxyfluorescein succinimidyl ester (CFSE, Yuanye, China), and carbonyl cyanide m-chlorophenyl hydrazone (CCCP, Selleck, USA) were used in this study.
Bactericidal study
Antibacterial assays were performed as previously described [Citation31]. Briefly, a single colony was picked and grown in 30 mL broth in 100 mL flasks at 37°C with shaking at 220 rpm overnight. After centrifugation for 5 min at 8,000 rpm to remove the supernatant, the cells was washed twice with sterile saline and resuspended in M9 medium (containing 10 mM acetate, 1 mM MgSO4, and 100 µM CaCl2) to an OD600 of 0.2. The cells were treated with NADH, antibiotics, and/or other chemical agents (such as CCCP, NaN3, rotenone, etc) in M9 medium at 37°C, 220 rpm for 6 h. Finally, 100 μL of the bacterial cultures were collected and serially diluted. An aliquot (10 μL) of each culture was plated on TSB/LB agar to determine the bacterial count.
Analysis of combined drug effects
In order to learn the effect of drug combination, Synergy Finder 3.0 (https://synergyfinder.fimm.fi/) and Calcusyn were used. The process of obtaining the synergy score and maps has been described previously [Citation70]. Briefly, the mean percentage inhibition of a pair of drugs (neomycin and NADH) and their combinations at different doses was input. Following the software recommendation, the bliss reference model was selected for this study. Then, the comprehensive synergy score and synergy maps of the drug combinations were obtained automatically by the software using the Bliss model. According to the user guide on the website, a synergy score > 10 means that the interaction between the two drugs was likely to be synergistic [Citation71]. Synergy scoring can be visualized as either a two-dimensional or a three-dimensional interaction surface over the dose matrix. The depth of the colour reveals the degree of percentage inhibition in the two-dimensional image, and the height of the 3D drug interaction landscape is standardized as the percentage of inhibition to compare the degrees of interaction among drug combinations. The combination index (CI) method was performed as previously described [Citation72]. Data obtained from the bactericidal experiments were used to perform these analyses and were expressed in the form of inhibition rate.
Scanning electron microscopy analysis
Bacterial morphology was observed using a scanning electron microscope (Hitachi SU8100; Hitachi, Japan). E. tarda ATCC15947 was grown in 30 mL TSB broth at 37°C, 220 rpm overnight. Bacteria that had been cultivated overnight were collected by centrifugation and resuspended in M9 medium to OD600 = 0.2. NADH and neomycin were added, and the incubated at 37°C for 6 h. After 6 h of culture, bacteria were collected by centrifugation, washed with PBS three times, and then fixed using electron microscopy for 2 h at room temperature. Fixed samples were drifted through 0.1 M phosphate buffer (pH = 7.4) three times for 15 min each time. Then transfer samples into 1% OsO4 in 0.1 M phosphate buffer for 1–2 h at room temperature to complete post-fixation, and dehydrated with ascending concentrations of ethanol (30%, 50%, 70%, 80%, 90%, 95%, 100%) and isoamyl acetate for 15 min [Citation73]. Finally, the samples were freeze-dried and gold-coated. The ultrastructure was observed using scanning electron microscopy (SEM).
Metabolomics and data analysis
The metabolome sample preparation is described below. Six single colonies were inoculated into 30 mL TSB and cultured at 37°C, 220 rpm for 16 h until saturation. Cells were collected by centrifugation, washed three times with 0.85% NaCl, TSB was completely removed, and then resuspended in M9 medium to OD600 = 1.0. Adding NADH was added to M9 medium and then incubated together at 37°C for 6 h. After 6 h of culture, bacteria were collected by centrifugation at 4°C, 12,000 rpm, washed with PBS three times. The collected cells were quenched with liquid nitrogen and then stored at −80°C after cold methanol added. The purpose of the steps below was to allow the sample to undergo derivatization. Sonication (200 W, 2-s pulse, and 3-s pause over ice, 5 min) was used to lyse the cells to obtain metabolites. Cold methanol (1 mL) containing 10 μg of ribitol as an internal reference standard was used to extract metabolites from the cell lysate [Citation35]. The samples obtained in the above steps were centrifuged at 4°C and 12,000 rpm to remove the precipitate. A vacuum centrifuge was used to prepare supernatant samples. Mix The dried sample with 50 μL of methoxyamine hydrochloride solution (20 mg/mL, pyridine solution) and incubated together at 37°C for 1.5 h. Finally, 50 μL N-methyl-N-(trimethylsilyl) trifluoroacetamide was added to the sample and reacted at 37°C for 1 h to complete sample derivatization [Citation19]. The Derivatized samples were collected by centrifugation at 12000 g for 15 min at 4°C to remove the supernatant. The pellets were used for subsequent experiments. In the subsequent G Agilent 7890A GC and Agilent 5975C inert XL mass selector (Agilent Technologies) analysis, 1 μL of the derivatized sample was used. inlet temperature was set to 300°C, split ratio of the carrier gas (high-purity helium) was 5: 1 and constant linear velocity was 40.0 cm/s respectively [Citation74]. A DB-5 MS capillary column (30 m × 250 μm × 0.25 μm, J&W Scientific Inc., USA) was used to separate metabolites. Metabolite mass signals were read using a mass spectrometer in the full scan mode. The retention times of the bacterial metabolites were related to the retention times of n-alkanes. Thus, the relevant metabolite content signal can be obtained from n-alkane retention events. Finally, by analysing the light diesel sample with the same instrumental parameters as above, the retention index (equivalent to the retention time of n-alkanes) of the bacterial metabolites can be obtained [Citation75].
Statistical analysis was performed as described previously [Citation76]. Initial peak detection and mass spectral deconvolution were performed using Agilent software (Agilent 6.0). Agilent software (Agilent 6.0) was used to perform initial peak detection and mass spectral deconvolution. Metabolites were identified by spectral matching and retention time (RT) indexing of the National Institute of Standards and Technology (NIST) library using NIST MS Search 2.0. After removing known artefact peaks and relating them to identical compounds, the metabolites were determined. Data were analysed using IBM SPSS Statistics (version 22.0; SPSS Inc., Chicago, IL, USA) for statistical analysis, and data with a p value less than 0.05 were regarded as significant. Hierarchical clustering analysis was performed using the R Studio software (version 4.0.3). SIMCA – P 14+ was used to analyse the principal components and orthogonal partial least squares. Analysis of the Z-score can be performed in Microsoft Excel by labelling the differences of means. Metabolic pathway enrichment was performed using MetaboAnalyst (version 6.0; http://www.metaboanalyst.ca/). Graphs were drawn using Microsoft Excel and GraphPad Prism 8.0 (San Diego, CA,UAS).
Measurement of ATP
ATP levels were determined using BacTiter-GloTM Microbial Cell Viability Assay (Promega, USA) [Citation75]. Overnight bacterial cultures were collected by centrifugation at 8,000 rpm for 5 min, resuspended in M9 medium, and diluted to an OD600 of 0.2. NADH was added to medium and incubated at 37°C, 220 rpm for 6 h. After 6 h of culture, 50 µL of the samples and 50 µL of the kit solution were mixed in a 96-well plate. Finally, absorbance was measured immediately using a microplate reader (Biotek, Synergy HT, Vermont, USA) according to the manufacturer’s instructions.
Measurement of intracellular pH
Carboxyfluorescein succinimidyl ester (CFSE) was used to detect intracellular pH, which decreases with decreasing pH [Citation77]. As described above, bacteria that were resuspended in M9 medium with an OD600 of 0.2 were obtained. Then NADH and 30 μg/mL neomycin were added to medium and cultured at 37°C, 220 rpm for 6 h. After 6 h, 198 μL of culture was removed, and 2 μL of 10 mM CFSE was added and mixed in a 96-well plate, followed by incubation at 37°C for 1 h in the dark. Fluorescence was measured immediately using a fluorescence plate reader (Biotek, Synergy HT, Vermont, USA) at an excitation wavelength of 500 nm and an emission wavelength of 520 nm.
Measurement of membrane potential
Membrane potential was measured using DiBAC4(3), as described previously [Citation78]. In brief, overnight bacteria were collected at an OD600 value of 0.2, and incubated in 5 mL of M9 medium with/without NADH for 6 h at 37°C, 220 rpm. Cells (198 μL) containing 1 × 107 CFU were stained with 2 μL of 10 mM DiBAC4(3) for 1 h in the dark. Finally, a fluorescence plate reader (Biotek, Synergy HT, Vermont, USA) was used to measure fluorescence at an excitation wavelength of 490 nm and an emission wavelength of 516 nm.
Measurement of intracellular neomycin concentration
A rapid ELISA test kit (Mlbio, Shanghai, China) was used to detect the intracellular neomycin concentration. Bacteria from overnight cultures were collected as previously described [Citation79], bacteria were resuspended in M9 medium to OD600 = 1.0. Next, neomycin was added and incubated for 6 h at 37°C and 220 rpm with or without NADH. Cultures were collected by centrifugation at 8,000 rpm, 37°C for 5 min and then washed three times with PBS (pH = 7.2) to ensure that extracellular antibiotics had been completely removed. Thirty millilitres of bacteria were harvested and lysed by sonication (200 W total power with 50% output, 2 s pulse, and 3 s pause) on ice for 10 min. After sonic fragmentation, the supernatant was collected by centrifugation at 12,000 g, 4°C. We then followed the instructions of the kit. Finally, neomycin concentration was determined based on the rapid diagnostic kit instructions. The absorbance was measured at 450 nm using a plate reader (BioTek, Synergy HT, Vermont, USA).
Measurement of ROS
ROS detection was performed as previously described [Citation80]. Overnight bacterial cultures were collected by centrifugation at 8,000 rpm for 5 min. After washing three times with 0.85% sterile saline, the bacteria were resuspended in M9 minimal medium to an OD600 of 0.2. NADH or/and neomycin were added to medium and incubated at 37°C, 220 rpm for 6 h. Then, 196 µL of cultures and 4 µL of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were added to a 96-well plate and incubated at 37°C for 30 min in the dark. Fluorescence units were immediately measured at an excitation wavelength of 485 nm and an emission wavelength of 515 nm using a plate reader (Biotek, Synergy HT, Vermont, USA).
Measurement of NAD+ /NADH and NADP+ /NADPH
NAD+/NADH assay kit with WST-8 (Beyotime, Shanghai, China) and NADP+/NADPH assay kit with WST-8 (Beyotime, Shanghai, China) were used to measure NAD+/NADH and NADP+/NADPH. For NAD+/NADH and NADP+/NADPH, the steps were identical, except for the two different assay kits used. Ten millilitres of bacteria with OD600 = 1.0, which were incubated in M9 medium at 37°C for 6 h with/without NADH, were collected as described above [Citation80]. Cells were resuspended in 500 µL of PBS and then were lysed by sonication for 10 min on ice, and then centrifuged at 12,000 g for 5 min at 4°C for supernatants. The absorbance was measured at 450 nm using a plate reader (BioTek, Synergy HT, Vermont, USA).
Enzyme assay
Cultured bacterial cells were harvested and resuspended in M9 medium to an OD600 of 1.0. Samples (30 mL) were collected by centrifugation at 8,000 rpm for 5 min. The cells were resuspended in PBS and lysed by sonication (200 W total power with 50% output, 2 s pulse, and 3 s pause) for 8 min on ice, followed by centrifugation at 12,000 rpm for 10 min. The resulting supernatants were used for enzyme assays. Superoxide dismutase (SOD) and NDK activities were measured using the SOD Assay Kit (Solarbio, Beijing, China) and NDK Assay Kit (Mlbio, Shanghai, China), respectively. According to the manufacturer’s instructions, the carefully prepared supernatants were used for SOD and NDK measurements. A reading value of 450 nm was available for the enzyme activity of SOD and NDK.
Quantitative RT-PCR analysis
Quantitative real-time PCR (qRT-PCR) was performed as previously described with modifications [Citation81]. Bacterial cells (1.5 mL) were harvested at OD600 = 1.0 by centrifugation (12,000 g, 4°C, 3 min) and immediately quenched in liquid nitrogen. The cells were then lysed to extract total RNA using TRIZOL reagent (Invitrogen Life Technologies) according to the manufacturer’s protocol. Reverse transcription-PCR was conducted with 1 µg of total RNA using the Evo M-mLV RT Mix Kit with gDNA Clean for qPCR (Accurate Biotechnology Co., Ltd., Guangdong, China), according to the manufacturer’s instructions. RT-PCR was carried out in 96-well plates, with each well containing a total volume of 10 µL liquid composed of 5 µL 2× SYBR Green Pro Taq HS Premix, 4 µL cDNA template, and 0.5 µL each of primer (10 μM). The primer sequences are listed in Table S2. As per the manufacturer’s instructions, three biological replicates were used, and all assays were performed using a CFX Connect Real-Time System (Bio-Rad, USA). Cycling parameters were as follows: an initial denaturation at 95°C for 30 s, 45 cycles at 95°C for 5 s, and 58°C for 30 s mRNA levels of target genes were normalized to that of 16s rRNA gene, which is constitutively and steadily expressed under the conditions analysed.
Molecular docking
A molecular docking assay was performed to analyse the interactions between AACs and NADH. The Auto Dock 4.2.6 software from The Scripps Research Institute, was used as previously described [Citation82,Citation83]. Briefly, the two-dimensional structure of NADH downloaded from the PubChem online database was converted to the PDB format using Chem3D software (Chem3D 18.0.0.231). The X-ray crystal structure of aminoglycoside N(6‘)-acetyltransferase type 1 (1S3Z) was obtained from the PDB database. Once the receptor molecule and ligand files were prepared, the Glide software (PyMOL 2.5, DeLano Scientific LLC, CA, USA) was used to set a grid box to restrict the rotation and movement of the ligand. Molecular docking was performed using AutoDock4. Finally, PyMOL 2.5 software was used for visual simulation analysis, and the Protein-Ligand Interaction Profiler tool was used to analyse receptor-ligand binding via hydrophobic interactions and hydrogen bonds.
Authors’ contributions
Yu-bin Su and Man-jun Yang conceptualized and designed the study. Yu-bin Su and Yi-lin Zhong wrote this manuscript. Yu-bin Su and Yi-lin Zhong interpreted the data and performed data analyses. Yi-lin Zhong, Juan Guo, Zi-yi Zhang, and Yu Zheng performed experiments.
Supplemental Material
Download MS Word (959.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data generated during the study is available at repository name “Metabolomic data of ATCC15947” at https://doi.org/10.6084/m9.figshare.25484686
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2367647
Additional information
Funding
References
- Xiao JF, Wang Q, Liu Q, et al. Isolation and identification of fish pathogen edwardsiella tarda from mariculture in China. Aquacult Res. 2008;40(1):13–17. doi: 10.1111/j.1365-2109.2008.02101.x
- Reichley SR, Ware C, Steadman J, et al. Comparative phenotypic and genotypic analysis of Edwardsiella isolates from different hosts and geographic origins, with emphasis on isolates formerly classified as E. tarda, and evaluation of diagnostic methods. J Clin Microbiol. 2017;55(12):3466–3491. doi: 10.1128/JCM.00970-17
- Jung WJ, Kwon J, Giri SS, et al. Isolation and characterization of a highly virulent Edwardsiella piscicida strain responsible for mass mortality in marbled eel (Anguilla marmorata) cultured in Korea. Aquaculture. 2022;555:738199. doi: 10.1016/j.aquaculture.2022.738199
- Tucker CS, Hargreaves JA. Biology and culture of channel catfish. USDA National Wildlife Research Center - Staff Publications; 2004.
- Griffin MJ, Greenway TE, Wise DJ. Edwardsiella spp. Fish viruses and bacteria: pathobiology and protection. Wallingford (UK): CABI (Centre for Agriculture and Biosciences International) Digital Library; 2017. p. 190–210.
- Nucci C, Da Silveira W, da Silva Correa S, et al. Microbiological comparative study of isolates of Edwardsiella tarda isolated in different countries from fish and humans. Vet Microbiol. 2002;89(1):29–39. doi: 10.1016/S0378-1135(02)00151-7
- Hoseinifar SH, Ashouri G, Marisaldi L, et al. Reducing the use of antibiotics in European aquaculture with vaccines, functional feed additives and optimization of the gut microbiota. J Marine Sci Eng. 2024;12(2):204. doi: 10.3390/jmse12020204
- Li Q, Li SS, Li SW, et al. Antimicrobial and anti-inflammatory cyclic tetrapeptides from the co-cultures of two marine-derived fungi. J Natural Prod. 2024;87(2):365–370. doi: 10.1021/acs.jnatprod.3c01115
- Leung KY, Wang Q, Zheng X, et al. Versatile lifestyles of Edwardsiella: Free-living, pathogen, and core bacterium of the aquatic resistome. Virulence. 2022;13(1):5–18. doi: 10.1080/21505594.2021.2006890
- Adenaya A, Berger M, Brinkhoff T, et al. Usage of antibiotics in aquaculture and the impact on coastal waters. Mar Pollut Bull. 2023;188:114645. doi: 10.1016/j.marpolbul.2023.114645
- Sun PH, Yu F, Lu JF, et al. In vivo effects of neomycin sulfate on non-specific immunity, oxidative damage and replication of cyprinid herpesvirus 2 in crucian carp (Carassius auratus gibelio). Aquacul and Fish. 2019;4(2):67–73. doi: 10.1016/j.aaf.2018.09.003
- Zhai JL, Xiao ZD, Xue M, et al. Morganella morganii, a bacterial pathogen in diseased Chinese soft-shelled turtles (Pelodiscus sinensis). Aquaculture. 2024;579:740190. doi: 10.1016/j.aquaculture.2023.740190
- Zhao XL, Chen H, Jin ZH, et al. GC‐MS‐based metabolomics analysis reveals L‐aspartate enhances the antibiotic sensitivity of neomycin sulfate‐resistant Aeromonas hydrophila. J Fish Dis. 2018;41(12):1831–1841. doi: 10.1111/jfd.12894
- Krause KM, Serio AW, Kane TR, et al. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):6. doi: 10.1101/cshperspect.a027029
- Bondad‐Reantaso MG, MacKinnon B, Karunasagar I, et al. Review of alternatives to antibiotic use in aquaculture. Rev In Aquaculture. 2023;15(4):1421–1451. doi: 10.1111/raq.12786
- Manzoor K, Rasool F, Khan N, et al. Resistance patterns of frequently applied antimicrobials and occurrence of antibiotic resistance genes in Edwardsiella tarda detected in Edwardsiellosis-infected tilapia species of fish farms of Punjab in Pakistan. J Microbiol Biotechnol. 2023;33(5):668. doi: 10.4014/jmb.2301.01008
- Ezzat M, Rm E-T, ELmasry NM. Antibiotic resistance and antibiotic resistance genes among Edwardsiella tarda isolated from fish. Suez Canal Vet Med J SCVMJ. 2021;26(1):171–188. doi: 10.21608/scvmj.2021.184978
- Bhargava P, Collins JJ. Boosting bacterial metabolism to combat antibiotic resistance. Cell Metab. 2015;21(2):154–155. doi: 10.1016/j.cmet.2015.01.012
- Peng B, Su YB, Li H, et al. Exogenous alanine and/or glucose plus kanamycin kills antibiotic-resistant bacteria. Cell Metab. 2015;21(2):249–262. doi: 10.1016/j.cmet.2015.01.008
- Stokes JM, Lopatkin AJ, Lobritz MA, et al. Bacterial metabolism and antibiotic efficacy. Cell Metab. 2019;30(2):251–259. doi: 10.1016/j.cmet.2019.06.009
- Wang QX, Lin MY, Shen PH, et al. Elevation of fatty acid biosynthesis metabolism contributes to zhongshengmycin resistance in Xanthomonas oryzae. Antibiotics. 2021;10(10):1166. doi: 10.3390/antibiotics10101166
- Su YB, Kuang SF, Ye JZ, et al. Enhanced biosynthesis of fatty acids is associated with the acquisition of ciprofloxacin resistance in Edwardsiella tarda. mSystems. 2021;6(4):e00694–00621. doi: 10.1128/mSystems.00694-21
- Kitzenberg DA, Lee JS, Mills KB, et al. Adenosine awakens metabolism to enhance growth-independent killing of tolerant and persister bacteria across multiple classes of antibiotics. MBio. 2022;13(3):e00480–00422. doi: 10.1128/mbio.00480-22
- Ye JZ, Su YB, Lin XM, et al. Alanine enhances aminoglycosides-induced ROS production as revealed by proteomic analysis. Front Microbiol. 2018;9:29. doi: 10.3389/fmicb.2018.00029
- Lee HH, Collins JJ. Microbial environments confound antibiotic efficacy. Nat Chem Biol. 2012;8(1):6–9. doi: 10.1038/nchembio.740
- Shatalin K, Shatalina E, Mironov A, et al. H2S: a universal defense against antibiotics in bacteria. Science. 2011;334(6058):986–990. doi: 10.1126/science.1209855
- Woźniacka A, Sysa‐Jędrzejowska A, Adamus J, et al. Topical application of NADH for the treatment of rosacea and contact dermatitis. Clin Exp Dermatol. 2003;28(1):61–63. doi: 10.1046/j.1365-2230.2003.01118.x
- Forsyth LM, Preuss HG, MacDowell AL, et al. Therapeutic effects of oral NADH on the symptoms of patients with chronic fatigue syndrome. Ann Allergy Asthma Immunol. 1999;82(2):185–191. doi: 10.1016/S1081-1206(10)62595-1
- Qiu Y, Wang HY, Pan HY, et al. NADH improves AIF dimerization and inhibits apoptosis in iPscs-derived neurons from patients with auditory neuropathy spectrum disorder. Hear Res. 2024;441:108919. doi: 10.1016/j.heares.2023.108919
- Deng WY, Fu TW, Zhang Z, et al. L-lysine potentiates aminoglycosides against Acinetobacter baumannii via regulation of proton motive force and antibiotics uptake. Emerging Microbes & Infect. 2020;9(1):639–650. doi: 10.1080/22221751.2020.1740611
- Fan LY, Pan ZY, Liao X, et al. Uracil restores susceptibility of methicillin-resistant Staphylococcus aureus to aminoglycosides through metabolic reprogramming. Front Pharmacol. 2023;14:1133685. doi: 10.3389/fphar.2023.1133685
- Kuang SF, Xiang J, Chen YT, et al. Exogenous pyruvate promotes gentamicin uptake to kill antibiotic-resistant Vibrio alginolyticus. Int J Antimicrob Agents. 2024;63(1):107036. doi: 10.1016/j.ijantimicag.2023.107036
- Arce-Rodríguez A, Pankratz D, Preusse M, et al. Dual effect: high NADH levels contribute to efflux-mediated antibiotic resistance but drive lethality mediated by reactive oxygen species. MBio. 2022;13(1):e02434–02421. doi: 10.1128/mbio.02434-21
- Chen XW, Wu JH, Liu YL, et al. Fructose promotes ampicillin killing of antibiotic-resistant Streptococcus agalactiae. Virulence. 2023;14(1):2180938. doi: 10.1080/21505594.2023.2180938
- Su YB, Peng B, Han Y, et al. Fructose restores susceptibility of multidrug-resistant Edwardsiella tarda to kanamycin. J Proteome Res. 2015;14(3):1612–1620. doi: 10.1021/pr501285f
- Li L, Su YB, Peng B, et al. Metabolic mechanism of colistin resistance and its reverting in Vibrio alginolyticus. Environ Microbiol. 2020;22(10):4295–4313. doi: 10.1111/1462-2920.15021
- Kok M, Maton L, Peet van der M, et al. Unraveling antimicrobial resistance using metabolomics. Drug Discov Today. 2022;27(6):1774–1783. doi: 10.1016/j.drudis.2022.03.015
- Xia T, Xu LL, Guo PY, et al. Synergism of amlodipine and telmisartan or candesartan on blood pressure reduction by using SynergyFinder 3.0 and probability sum test in vivo. Pharmacol Res & Perspectives. 2023;11(2):e01064. doi: 10.1002/prp2.1064
- Genovese S, Epifano F, Preziuso F, et al. Gercumin synergizes the action of 5-fluorouracil and oxaliplatin against chemoresistant human cancer colon cells. Biochem Biophys Res Commun. 2020;522(1):95–99. doi: 10.1016/j.bbrc.2019.11.068
- Zhang L, Song MF, Yang N, et al. Nucleoside Diphosphate Kinases (ndk) reveals a key role in adhesion and virulence of Aeromonas veronii. Microbial Pathogenesis. 2020;149:104577. doi: 10.1016/j.micpath.2020.104577
- Kaila VR, Wikström M. Architecture of bacterial respiratory chains. Nature Rev Microbiol. 2021;19(5):319–330. doi: 10.1038/s41579-020-00486-4
- Dadi PK, Ahmad M, Ahmad Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int j biol macromol. 2009;45(1):72–79. doi: 10.1016/j.ijbiomac.2009.04.004
- Krulwich TA, Sachs G, Padan E. Molecular aspects of bacterial pH sensing and homeostasis. Nature Rev Microbiol. 2011;9(5):330–343. doi: 10.1038/nrmicro2549
- Bao XR, Bové M, Coenye T. Organic acids and their salts potentiate the activity of selected antibiotics against Pseudomonas aeruginosa biofilms grown in a synthetic cystic fibrosis sputum medium. Antimicrob Agents Chemother. 2022;66(1):e01875–01821. doi: 10.1128/AAC.01875-21
- Li ZY, Wu L, Huang ZJ, et al. CCCP facilitates aminoglycoside to kill late stationary-phase Escherichia coli by elevating hydroxyl radical. ACS Infect Dis. 2023;9(4):801–814. doi: 10.1021/acsinfecdis.2c00522
- Larosa V, Remacle C. Insights into the respiratory chain and oxidative stress. Biosci Rep. 2018;38: BSR20171492. doi: 10.1042/BSR20171492
- Kwon DH, Lee H, Park C, et al. Glutathione induced immune-stimulatory activity by promoting M1-like macrophages polarization via potential ROS scavenging capacity. Antioxidants. 2019;8(9):413. doi: 10.3390/antiox8090413
- Cox G, Ejim L, Stogios PJ, et al. Plazomicin retains antibiotic activity against most aminoglycoside modifying enzymes. ACS Infect Dis. 2018;4(6):980–987. doi: 10.1021/acsinfecdis.8b00001
- Aishwarya KVL, Geetha PV, Eswaran S, et al. Spectrum of aminoglycoside modifying enzymes in gram-negative bacteria causing human infections. J Lab Physicians. 2020;12(1):27–31. doi: 10.1055/s-0040-1713687
- Wasan H, Reeta KH, Gupta YK. Strategies to improve antibiotic access and a way forward for lower middle-income countries. J Antimicrob Chemother. 2024;79(1):1–10. doi: 10.1093/jac/dkad291
- Peng B, Li H, Peng XX., et al. Functional metabolomics: from biomarker discovery to metabolome reprogramming. Protein & Cell. 2015;6(9):628–637. doi: 10.1007/s13238-015-0185-x
- Douafer H, Andrieu V, Phanstiel IVO, et al. Antibiotic adjuvants: make antibiotics great again! J Med Chem. 2019;62(19):8665–8681. doi: 10.1021/acs.jmedchem.8b01781
- Peng B, Li H, Peng XX., et al. Call for next-generation drugs that remove the uptake barrier to combat antibiotic resistance. Drug Discov Today. 2023;28(10):103753. doi: 10.1016/j.drudis.2023.103753
- Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216–220. doi: 10.1038/nature10069
- Jiang M, Su YB, Ye JZ, et al. Ampicillin-controlled glucose metabolism manipulates the transition from tolerance to resistance in bacteria. Sci Adv. 2023;9(10):eade8582. doi: 10.1126/sciadv.ade8582
- Mates SM, Eisenberg ES, Mandel LJ, et al. Membrane potential and gentamicin uptake in Staphylococcus aureus. In: Proceedings of the National Academy of Sciences of the United States of America; 1982; Vol. 79. p. 6693–6697. doi: 10.1073/pnas.79.21.6693
- Fan LY, Pan ZY, Zhong YL, et al. L-glutamine sensitizes Gram-positive-resistant bacteria to gentamicin killing. Microbiol Spectr. 2023;11(6):e01619–01623. doi: 10.1128/spectrum.01619-23
- Imlay JA. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nature Rev Microbiol. 2013;11(7):443–454. doi: 10.1038/nrmicro3032
- Long YM, Hu LG, Yan XT, et al. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. Int J Nanomed. 2017;Volume 12:3193–3206. doi: 10.2147/IJN.S132327
- Lv BY, Huang XB, Lijia CC, et al. Heat shock potentiates aminoglycosides against gram-negative bacteria by enhancing antibiotic uptake, protein aggregation, and ROS. In: Proceedings of the National Academy of Sciences of the United States of America; 2023; Vol. 120. p. e2217254120. doi: 10.1073/pnas.2217254120
- Donkor GY, Anderson GM, Stadler M, et al. The novel silver-containing antimicrobial potentiates aminoglycoside activity against Pseudomonas aeruginosa. bioRxiv. 2023;03(15):532855.
- Ojdana D, Sieńko A, Sacha P, et al. Genetic basis of enzymatic resistance of E. coli to aminoglycosides. Adv Med Sci. 2018;63(1):9–13. doi: 10.1016/j.advms.2017.05.004
- Bordeleau E, Stogios PJ, Evdokimova E, et al. ApmA is a unique aminoglycoside antibiotic acetyltransferase that inactivates apramycin. MBio. 2021;12(1):02705–02720. doi: 10.1128/mbio
- Bassenden AV, Dumalo L, Park J, et al. Structural and phylogenetic analyses of resistance to next-generation aminoglycosides conferred by AAC (2′) enzymes. Sci Rep. 2021;11(1):11614. doi: 10.1038/s41598-021-89446-3
- Furuhashi M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am J Physiol Endocrinol Metab. 2020;19(5):E827–E834. doi: 10.1152/ajpendo.00378.2020
- Mantena RK, Wijburg OL, Vindurampulle C, et al. Reactive oxygen species are the major antibacterials against Salmonella Typhimurium purine auxotrophs in the phagosome of RAW 264.7 cells. Cell Microbiol. 2008;10(5):1058–1073. doi: 10.1111/j.1462-5822.2007.01105.x
- Seregina TA, Petrushanko IY, Zaripov PI, et al. Activation of purine biosynthesis suppresses the sensitivity of E. coli gmhA mutant to antibiotics. Int J Mol Sci. 2023;24(22):16070. doi: 10.3390/ijms242216070
- Liu Y, Yang KN, Jia YQ, et al. Thymine sensitizes Gram-negative pathogens to antibiotic killing. Front Microbiol. 2021;12:622798. doi: 10.3389/fmicb.2021.622798
- Wang C, Dong XS, Yang YY, et al. Metabolites in the TCA cycle promote resistance to chloramphenicol of Edwardsiella tarda. J Proteome Res. 2020;20(1):972–981. doi: 10.1021/acs.jproteome.0c00725
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4
- Ke J, Li MT, Huo YJ, et al. The synergistic effect of Ginkgo biloba extract 50 and aspirin against platelet aggregation. Drug Design Develop Therapy. 2021;15:3543–3560. doi: 10.2147/DDDT.S318515
- Pandita A, Kumar B, Manvati S, et al. Synergistic combination of gemcitabine and dietary molecule induces apoptosis in pancreatic cancer cells and down regulates PKM2 expression. PLOS ONE. 2014;9(9):e107154. doi: 10.1371/journal.pone.0107154
- Zheng YD, Zhong TR, Wu HM, et al. Crizotinib shows antibacterial activity against Gram-positive bacteria by reducing ATP production and targeting the CTP synthase PyrG. Microbiol Spectr. 2022;10(3):e00884–00822. doi: 10.1128/spectrum.00884-22
- Ye JZ, Su YB, Peng XX, et al. Reactive oxygen species-related ceftazidime resistance is caused by the pyruvate cycle perturbation and reverted by Fe3+ in Edwardsiella tarda. Front Microbiol. 2021;12:654783. doi: 10.3389/fmicb.2021.654783
- Yang J, Zeng ZH, Yang MJ, et al. NaCl promotes antibiotic resistance by reducing redox states in Vibrio alginolyticus. Environ Microbiol. 2018;20(11):4022–4036. doi: 10.1111/1462-2920.14443
- Zeng ZH, Du CC, Liu SR, et al. Glucose enhances tilapia against Edwardsiella tarda infection through metabolome reprogramming. Fish & Shellfish Immunol. 2017;61:34–43. doi: 10.1016/j.fsi.2016.12.010
- Westcott MM, Henry CJ, Amis JE, et al. Dendritic cells inhibit the progression of listeria monocytogenes intracellular infection by retaining bacteria in major histocompatibility complex class II-rich phagosomes and by limiting cytosolic growth. Infect Immun. 2010;78(7):2956–2965. doi: 10.1128/IAI.01027-09
- Zhang S, Yang MJ, Peng B, et al. Reduced ROS -mediated antibiotic resistance and its reverting by glucose in Vibrio alginolyticus. Environ Microbiol. 2020;22(10):4367–4380. doi: 10.1111/1462-2920.15085
- Zhang S, Wang J, Jiang M, et al. Reduced redox-dependent mechanism and glucose-mediated reversal in gentamicin-resistant Vibrio alginolyticus. Environ Microbiol. 2019;21(12):4724–4739. doi: 10.1111/1462-2920.14811
- Kou TS, Wu JH, Chen XW, et al. Exogenous glycine promotes oxidation of glutathione and restores sensitivity of bacterial pathogens to serum-induced cell death. Redox Biol. 2022;58:102512. doi: 10.1016/j.redox.2022.102512
- Li SH, Xiang J, Zeng YY, et al. Elevated proton motive force is a tetracycline resistance mechanism that leads to the sensitivity to gentamicin in Edwardsiella tarda. Microb Biotechnol. 2024;17(1):e14379. doi: 10.1111/1751-7915.14379
- Liu P, Zhong LS, Xiao J, et al. Ethanol extract from Artemisia argyi leaves inhibits HSV-1 infection by destroying the viral envelope. Virol J. 2023;20(1):1–15. doi: 10.1186/s12985-023-01969-5
- Wang Y, Li F, Wang ZX, et al. Luteolin inhibits herpes simplex virus 1 infection by activating cyclic guanosine monophosphate-adenosine monophosphate synthase-mediated antiviral innate immunity. Phytomedicine. 2023;120:155020. doi: 10.1016/j.phymed.2023.155020
