ABSTRACT
The emergence of multidrug-resistant bacteria poses a significant threat to human health, necessitating a comprehensive understanding of their underlying mechanisms. Uropathogenic Escherichia coli (UPEC), the primary causative agent of urinary tract infections, is frequently associated with multidrug resistance and recurrent infections. To elucidate the mechanism of resistance of UPEC to beta-lactam antibiotics, we generated ampicillin-resistant UPEC strains through continuous exposure to low and high levels of ampicillin in the laboratory, referred to as Low AmpR and High AmpR, respectively. Whole-genome sequencing revealed that both Low and High AmpR strains contained mutations in the marR, acrR, and envZ genes. The High AmpR strain exhibited a single additional mutation in the nlpD gene. Using protein modeling and qRT-PCR analyses, we validated the contributions of each mutation in the identified genes to antibiotic resistance in the AmpR strains, including a decrease in membrane permeability, increased expression of multidrug efflux pump, and inhibition of cell lysis. Furthermore, the AmpR strain does not decrease the bacterial burden in the mouse bladder even after continuous antibiotic treatment in vivo, implicating the increasing difficulty in treating host infections caused by the AmpR strain. Interestingly, ampicillin-induced mutations also result in multidrug resistance in UPEC, suggesting a common mechanism by which bacteria acquire cross-resistance to other classes of antibiotics.
Introduction
Overuse or misuse of antibiotics has led to the development of antibiotic resistance in bacteria, which has become a global crisis. Therefore, understanding the underlying resistance mechanisms is critical for addressing the current challenges in antibiotic resistance. Antibiotic resistance can arise through genetic mutations or the acquisition of resistance genes from other bacteria [Citation1]. Bacteria become resistant to antibiotics through various mechanisms, including limiting access to antibiotics, modifying antibiotic target sites, degrading antibiotics, or promoting antibiotic efflux [Citation2]. When bacteria develop resistance to one type of antibiotic, they often exhibit resistance to other types of antibiotics. Thus, there is a need to understand the underlying mechanisms that drive multidrug antibiotic resistance.
Uropathogenic Escherichia coli (UPEC) is a bacterium that commonly causes urinary tract infections (UTIs). UTIs are among the most common bacterial infections in humans and affect millions of people worldwide [Citation3,Citation4]. In the host, UPEC has developed specific adaptations that enable it to colonize and infect the urinary tract, leading to symptoms such as pain, frequent urination, and fever. During colonization of epithelial cells, UPEC employs type 1 fimbriae to establish infection in the urinary tract. A key player in this process is FimH, an adhesin protein located at the tip of type 1 fimbriae that enables UPEC to recognize and attach to host cells [Citation5]. UTIs are classified according to their symptoms, ranging from urosepsis syndrome (the most severe form) to pyelonephritis (kidney infection) and cystitis (bladder infection) [Citation6]. E. coli CFT073, isolated from a patient with pyelonephritis [Citation7], has been widely used as a model strain to study the virulence mechanisms and pathogenesis of UPEC infections.
A significant challenge in the treatment of UPEC infections is the increasing prevalence of antibiotic resistance. UPEC strains have the ability to acquire resistance to multiple antibiotics, making treatment options more limited and challenging. The treatment of UTIs relies on the use of antibiotics such as β-lactams, fluoroquinolones, and trimethoprim-sulfamethoxazole (TMP-SMZ), which are selected based on the resistance profiles of UPEC [Citation8]. The prevalence of antibiotic resistance in UPEC strains has been increasing globally over the years, and these antibiotic-resistant strains are a significant concern for human health [Citation9]. Recently, UPEC strains isolated from pregnant women with a history of recurrent UTIs were found to be resistant to ampicillin, tetracycline, amikacin, ciprofloxacin, and gentamicin [Citation10]. This highlights the urgent need for effective strategies to address antibiotic resistance in UTIs.
Here, we generated beta-lactam antibiotic-resistant UPEC strains isolated in 25 µg/ml or 50 µg/ml ampicillin (Low AmpR and High AmpR respectively) in the laboratory to investigate the underlying mechanisms of antibiotic resistance of UPEC. Using whole-genome sequencing (WGS), we identified various point mutations, including missense and nonsense mutations, within the AmpR UPEC strains. The ampicillin-resistant UPEC strains harbor mutations in the marR, acrR, envZ, and nlpD genes, all of which lead to ampicillin resistance. By investigating the molecular genetic processes underlying UPEC resistance to ampicillin, we aimed to understand how these mechanisms contribute to multidrug resistance.
Materials and methods
Bacteria strains, plasmids, oligodeoxynucleotides, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table S2. All uropathogenic Escherichia coli (UPEC) CFT073 strains were derived from the wild-type strain CFT073 [Citation7]. Bacteria were grown at 37°C in Luria-Bertani (LB) broth and MacConkey agar. For ampicillin-resistant bacteria, 25 µg/ml ampicillin was used in LB broth and 50 µg/ml ampicillin was used on LB solid medium unless otherwise indicated. For measuring antibiotic induced mRNA expression, ampicillin was used at 4 or 8 µg/ml, and ciprofloxacin was used at 0.06 or 0.12 µg/ml.
Isolation of ampicillin-resistant strains
Escherichia coli (UPEC) CFT073 was cultured in LB broth medium. After 18 h of culture, cells were spread on LB agar medium containing 12.5 µg/ml ampicillin. Plates were incubated for 2–7 days until resistant colonies started to grow on the ampicillin-containing plate. Resistant colonies were purified on LB agar medium containing 12.5 µg/ml ampicillin and inoculated in LB broth medium with the same concentration of ampicillin. This process was repeated with increasing ampicillin concentrations up to 25 µg/ml (Low AmpR) or 50 µg/ml (High AmpR).
Determination of minimal inhibitory concentration (MIC)
In vitro antimicrobial susceptibility testing was performed by measuring the minimal inhibitory concentration (MIC) using the broth microdilution method [Citation11]. Briefly, the strains were cultured in Mueller-Hinton (MH) broth. Overnight-grown bacteria were diluted in broth so that each tube contains approximately 5 × 105 colony-forming units per ml (CFU/ml) (OD600 = 0.005) and dispensed at 100 µl per well in a 96-well plate. Ampicillin was dissolved and then diluted in MH medium to a 2 × top concentration desired in the test. Using a multi-pipette, ampicillin was mixed into the wells of column 1 by sucking up and down 6–8 times. Fifty microliter of sample from column 1 was withdrawn and added to column 2. We repeated the procedure down to column 10. After incubating the plate overnight at 37°C, resazurin (0.015%) [Citation12] was added to all wells (30 μl per well) to monitor bacterial growth and further incubated for 2–4 h for the observation of color change. Pink color indicates bacterial growth and blue color indicates inhibition of bacterial growth.
Real-time quantitative reverse transcription PCR (qRT-PCR)
Total RNA was isolated using the RNeasy kit (Qiagen) according to the manufacturer’s instructions. The purified RNA was quantified using a NanoDrop spectrophotometer (Thermo Fisher). Five hundred nanogram of cDNA was synthesized using a PrimeScript RT reagent kit (TaKaRa). Transcripts were quantified by real-time PCR using the SYBR green PCR master mix (Applied Biosystems) on a StepOnePlus real-time PCR system (Applied Biosystems) with the following PCR protocol: 95°C for 20 sec followed by 40 cycles of 95°C for 2 sec and 60°C for 30 sec. The primers used for the detection of transcripts of each gene are listed in Table S2. The mRNA levels of each target gene were calculated using a standard curve of UPEC CFT073 genomic DNA at known concentrations, and the data were normalized to 16S rRNA levels of the rrsH gene. All real-time qRT-PCR results were derived from three biological replicates.
Whole genome sequencing
Genomic DNA from each strain was extracted using a G-spinTM Genomic DNA extraction Kit (iNtRON). Sixty μg/μl of genomic DNA was processed to construct a sequencing library using a TruSeq Nano DNA Sample Prep Kit (#20015964, Illumina, USA). Sequencing was performed using the NovaSeq 6000 platform (Illumina, USA) on a 151 bp paired-end platform. We obtained an average of 5.62 Gb of data (BioProject accession number PRJNA987582).
The genome of the reference strain, uropathogenic E. coli CFT073 was assembled using SPAdes v3.14.1 [Citation13] and annotated using Prokka [Citation14]. The generated raw reads were adapter- and quality-trimmed using Trimmomatic v.0.36 [Citation15]. Cleaned reads were mapped to the reference genome using CLC genomics workbench 21 (Qiagen) and showed an average 99.45% mapping rate, which can cover approximately over 1,000 × depth per sample. Variant calling and comparison were performed using the Basic Variant Detection tool in the CLC genomics workbench 21 (Qiagen).
Transmission electron microscopy (TEM)
Bacterial samples for TEM were prepared as described previously with some modifications [Citation16,Citation17]. Briefly, bacteria were cultured in LB broth supplemented with 0.1% glucose to promote type 1 fimbriae expression overnight. The bacterial samples were loaded onto a carbon-coated grid for 2 min and washed with distilled water. The remaining water was removed using filter paper. Then, the type 1 fimbriae were negatively stained with 3% of uranyl acetate (UA) or 1% of phosphotungstic acid (PTA) for 10 and 1–2 sec, respectively. After staining, samples were rinsed twice with distilled water and dried at room temperature. Finally, the samples were visualized by TEM (80 kV, JEOL, JEM1010).
Determination of UPEC colony-forming units (CFUs) in the mouse bladder
To assess bacterial loads in the mouse bladder, this study employed the C57BL/6J mouse strain, which is used to investigate bacterial cystitis [Citation18,Citation19]. C57BL/6J female mice aged eight weeks were used for in vivo experiments. Mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (Hanlim Pharmaceuticals). To investigate the persistence of UPEC during antibiotic treatment, the anesthetized mice were transurethrally inoculated with 1 × 108 UPEC using the designated UPEC strains. Subsequently, the mice were administered ampicillin (200 mg/kg) through oral gavage and ciprofloxacin (10 mg/kg) through subcutaneous injection twice per day at 8-hr intervals over a period of three days. One day after the last treatment, the bladders were harvested and homogenized in 0.1% Triton X-100 PBS solution using a bead-beating homogenizer. The UPEC CFUs in the homogenized bladders were determined by plating the appropriately diluted homogenate on MacConkey agar plates that were incubated overnight at 37°C.
Ethic statement
The experimental procedures involving animals were conducted in accordance with the guidelines set forth by the Korea University Institutional Animal Care & Use Committee (KUIACUC-2022–0100), receiving approval on 26 December 2022. In this study, we complied with the ARRIVE 2.0 guidelines for animal experiments.
Determination of UPEC CFUs in the 5637 human bladder cell line
5 × 104 cells per well of human 5637 bladder epithelial cells (ATCC, HTB-9) were seeded in a 24 well plate. After overnight incubation, the cells were treated with media containing 1 × 107 CFU of wild-type or High AmpR CFT073 bacteria (corresponding to a multiplicity of infection (MOI) of 200). The whole plate was centrifuged for 5 min at 1200 rpm. After 1 h of incubation, the medium was changed to RPMI-1640 containing 200 µg/ml gentamicin to kill extracellular bacteria (control), 100 µg/ml ampicillin, or 10 µg/ml ciprofloxacin. After 30 min, the medium was changed to RPMI-1640 containing 50 µg/ml gentamicin or the same amount of ampicillin or ciprofloxacin. The cells were then incubated until 24 hr of initial infection. Then, media were removed, and 500 µl of 0.1% triton X-100 in PBS was added into each well. Using a pipette tip, wells were scraped. The extracted solution was plated on MacConkey agar, and the CFU was calculated accordingly.
Quantification and statistical analysis
The data underwent analysis, and several graphs were generated utilizing Prism software (GraphPad Software). Statistical significance was determined by considering p values less than 0.05. In the graphs representing in vitro experiments, each dot represents samples obtained from two to three independent experiments. In the graphs depicting mouse experiments, the number of mice utilized to obtain bladder samples is indicated by the dots. Statistical significance was assessed using either the unpaired Student’s t-test () or the one-way ANOVA with Tukey’s multiple comparisons test (). Each dot on the graphs representing mouse experiments indicates the number of mice used to obtain bladder samples.
Results
Generating mutations in UPEC strain to confer resistance to ampicillin
To study antibiotic resistance generated by continuous exposure to ampicillin, we incubated E. coli CFT073 with increasing concentrations of ampicillin and repeatedly isolated colonies that had developed resistance to ampicillin (). Using this process, we isolated a strain named Low AmpR, which displayed resistance to 25 μg/ml ampicillin. Taking this a step further, we treated Low AmpR to 50 μg/ml ampicillin and isolated a strain with even higher resistance, which we named High AmpR.
Figure 1. The ampicillin-resistant UPEC strains generated in the laboratory.
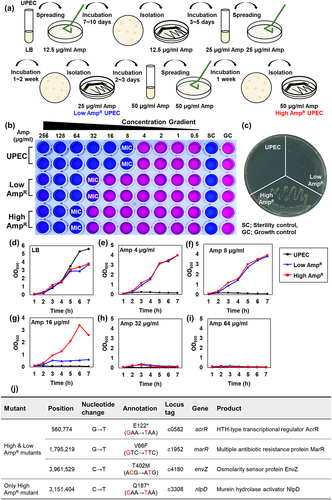
To evaluate the levels of resistance in the generated strains, we performed a minimal inhibitory concentration (MIC) assay for ampicillin on the wild-type UPEC, Low AmpR, and High AmpR strains, which were visualized by adding 0.015% resazurin. The MIC of the wild-type strains was 8 μg/ml, whereas the Low AmpR and High AmpR strains exhibited 4-fold and 8-fold increases in resistance, respectively (32 and 64 μg/ml) (). We further examined their resistance by culturing the wild-type and AmpR strains on LB ampicillin agar plates (50 μg/ml). The wild-type strain did not exhibit any growth at this concentration, whereas the Low AmpR strain formed pale colonies. In contrast, the High AmpR strain formed colonies of similar size and shape as the wild-type strain grown on LB plates without ampicillin ().
We compared the growth curves of AmpR strains with those of the wild-type strain in liquid media. In LB medium without ampicillin, the mutants displayed slightly lower growth than the wild-type (). However, in the presence of 4 and 8 μg/ml ampicillin, the AmpR strains exhibited robust growth, similar to that observed in the absence of ampicillin. Under these conditions, the wild-type strain did not grow, similar to what was observed on LB solid medium (). Additionally, the High AmpR strain showed greater resistance than the Low AmpR strain at 16 μg/ml ampicillin in LB media (). Above 32 μg/ml, none of the strains exhibited significant growth ().
Identification of mutations leading to ampicillin resistance in the low and high AmpR strains
To identify the genetic mutations responsible for ampicillin resistance, we performed whole-genome sequencing (WGS) of AmpR strains. The analysis revealed specific mutations in the acrR, marR, and envZ genes in the Low AmpR strain (). In acrR, a G-to-T transversion mutation led to a nonsense mutation, causing a change from glutamate to a stop codon (GAA-TAA) at position 122. This results in the production of truncated AcrR proteins. Similarly, the marR gene exhibited a point mutation, with a change from valine to phenylalanine (GTC-TTC) at position 66. The envZ mutation involved a change from threonine to methionine (ACG-ATG) at position 402.
Given that the High AmpR strain was derived from the Low AmpR strain, it retained all the mutations found in the Low AmpR strain. However, it also harbors an additional C to T transition nonsense mutation in the nlpD gene. This mutation resulted in a change from glutamine to a stop codon (CAA-TAA) at position 187 of NlpD. These results suggest that the identified mutations in the AmpR strains could be responsible for conferring resistance to ampicillin.
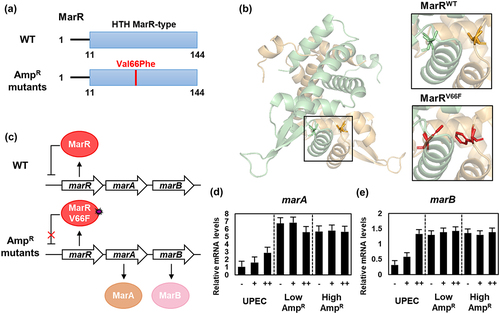
Mutation in MarR of ampicillin-resistant UPEC leads to constitutive expression of MarA and MarB
MarR is a helix-turn-helix (HTH) type repressor of the marRAB operon that is involved in resistance to multiple antibiotics [Citation20]. Among the marRAB operon, MarA acts as a global regulatory protein that controls various genes associated with antibiotic resistance, including the acrAB operon [Citation21]. Additionally, MarA serves as an autoregulator that activates the marRAB operon [Citation22]. MarB is a periplasmic protein with unknown function.
The mutation site observed in MarR (V66F) in the AmpR strains is located within the DNA binding domain. This substitution introduced an aromatic ring structure of phenylalanine, which was expected to reduce the affinity of MarR for DNA, rendering it unable to repress the marRAB operon (). To test this hypothesis, we performed qRT-PCR to measure the expression levels of the MarR-regulated genes marA and marB. In the wild-type strain, the mRNA levels of marA and marB increased in a dose-dependent manner with increasing ampicillin concentrations. However, in the AmpR strains, the expression of marA and marB genes was already significantly elevated even without ampicillin treatment, and the addition of ampicillin did not further increase their expression levels (). This suggests that the MarR V66F mutation abolished repression of the marRAB operon, leading to constitutive expression of MarA and MarB ().
Figure 2. The transcription of the marA and marB genes is up-regulated by the mutation of MarR in the ampicillin-resistant UPEC strains.
Mutation in AcrR of ampicillin-resistant UPEC leads to constitutive expression of AcrA and AcrB
AcrR is an additional global transcriptional regulator that acts as a repressor of the acrAB operon [Citation23] and is controlled by MarA [Citation21]. Together with TolC, AcrA and AcrB constitute the AcrAB-TolC multidrug efflux pump, which plays a crucial role in multidrug resistance [Citation24–26]. The AmpR strains harbored a nonsense mutation in the acrR gene, leading to the translation of a truncated AcrR protein (AcrR1–121) lacking dimerization and ligand-binding domain (). As a result, truncated AcrR1–121 was unable to repress the acrAB operon. This led to the expression of AcrA and AcrB, which form the AcrAB-TolC multidrug efflux pump ().
Figure 3. The transcription of the acrA and acrB genes is up-regulated due to the mutation of AcrR in the ampicillin-resistant UPEC strains.
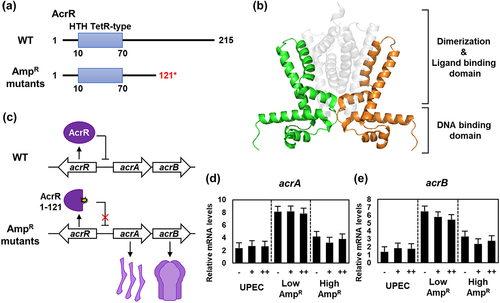
To test this hypothesis, we performed qRT-PCR to measure the mRNA levels of acrA and acrB, which are regulated by AcrR. Unlike marA and marB, there was no significant change in the mRNA levels of acrA and acrB observed in the wild-type, even when treated with ampicillin. However, the Low AmpR strain exhibited upregulated acrA and acrB genes regardless of the presence of ampicillin. Although the High AmpR strain showed slightly lower mRNA levels of acrA and acrB genes compared to the Low AmpR strain, they still maintained higher expression levels irrespective of the presence of ampicillin compared to the wild-type strain (). Therefore, the truncated AcrR protein was unable to repress the acrAB operon, leading to the constitutive expression of AcrA and AcrB. Elevated levels of these two proteins form the AcrAB-TolC multidrug efflux pump, which is likely to confer resistance to ampicillin.
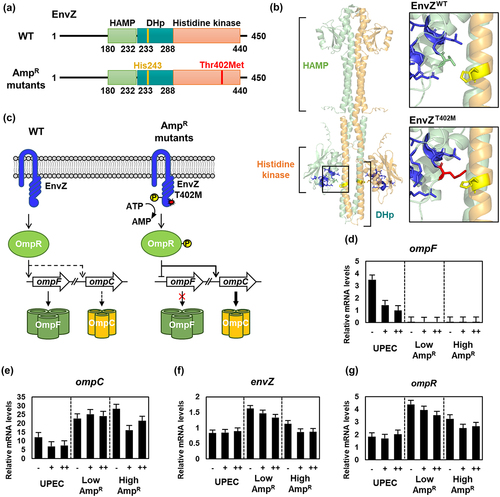
Mutation in EnvZ of ampicillin-resistant UPEC decreases OmpF expression
EnvZ-OmpR is a two-component system responsible for regulating osmotic stress by controlling the expression of the porin proteins OmpF and OmpC [Citation27]. EnvZ is a histidine kinase that comprises the HAMP, dimerization and histidine phosphotransfer (DHp), and histidine kinase domains. Under osmotic stress conditions, EnvZ autophosphorylates at a histidine residue located at position 243 within the DHp domain, and subsequently phosphorylates the transcriptional regulator OmpR [Citation28]. Phosphorylated OmpR (OmpR-P) inhibits the expression of the large-pore protein OmpF while promoting the expression of the small-pore protein OmpC [Citation29]. OmpF, known for its ability to transport various substrates including sugars, nutrients, and antibiotics, exhibits higher permeability than OmpC [Citation30]. On the other hand, OmpC has lower permeability, thus preventing the entry of antibiotics [Citation31–33].
The mutation site of EnvZ T402M in the AmpR strains resides in the histidine kinase domain. This mutation was suspected to affect the phosphorylation of the histidine residue due to the increased length of the side chain, which includes a sulfur atom (). To investigate the impact of this mutation on EnvZ function, we performed qRT-PCR to measure the mRNA levels of ompF, ompC, envZ and ompR. In the wild-type strain, the mRNA levels of ompF and ompC decreased upon ampicillin treatment (), while no significant difference was observed in the transcription of envZ and ompR (). In contrast, the AmpR strains showed complete abolition of OmpF expression, irrespective of the presence or absence of ampicillin (), while OmpC expression remained consistently upregulated (). The expression of envZ was slightly increased or nearly unchanged, whereas the expression of ompR was slightly elevated and reduced upon ampicillin treatment (). These qRT-PCR results suggest that the mutated EnvZ in the AmpR strains is active as a kinase regardless of the presence of ampicillin. Consequently, only the small pore protein OmpC was produced, resulting in decreased membrane permeability and prevention of external antibiotics from entering the cell ().
Figure 4. The transcription of the ompF gene was down-regulated, while ompC, envZ, and ompR were up-regulated due to the mutation of EnvZ in the ampicillin-resistant UPEC strains.
NlpD mutation impacts membrane permeability and ampicillin resistance in the high AmpR strain
NlpD is a murein hydrolase activator, which is required for septal peptidoglycan cleavage and cell division in the cytokinesis process and participates in cell lysis induced by β-lactam antibiotics [Citation34]. NlpD consists of two functional domains, LysM and degenerate LytM (dLytM) (). The LysM domain is responsible for localization, whereas the dLytM domain activates murein hydrolase AmiC () [Citation35]. The High AmpR strain exclusively contains NlpD1–186, lacking the dLytM domain required for interaction with AmiC, likely resulting in inefficient murein hydrolysis. This rigid peptidoglycan structure is expected to decrease membrane permeability and inhibit ampicillin-induced cell lysis. In the wild-type strain, the mRNA level of nlpD increased slightly in response to varying ampicillin concentrations, whereas no significant changes were observed in the AmpR strains. The Low AmpR strain exhibited higher expression levels of nlpD compared to the wild-type, while the High AmpR strain showed similar levels to the wild-type ().
Figure 5. The mutation of NlpD was found only in the High AmpR strain, leading to the loss of murein hydrolysis function.
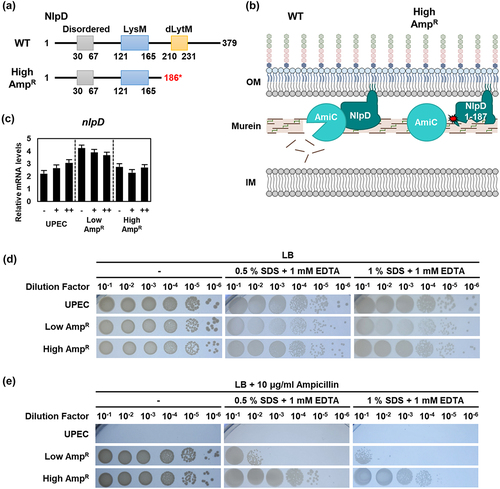
To investigate the impact of NlpD1–186 on membrane permeability, we treated the strains with the detergent sodium dodecyl sulfate (SDS) and the metal chelating agent ethylenediaminetetraacetic acid (EDTA). In the absence of ampicillin, no significant differences were observed between strains (). However, at an ampicillin concentration of 10 μg/ml, the wild-type strain exhibited poor growth, while the AmpR strains showed robust growth. Notably, the High AmpR strain demonstrated significantly greater survival in the presence of ampicillin than the Low AmpR strain (). Therefore, the NlpD mutation likely contributes to reduced membrane permeability and increased resistance to ampicillin by inhibiting the lysis of the murein layer ().
Maintenance of the AmpR UPEC strain during antibiotic treatment in urinary tract infection
To assess the pathogenicity of the AmpR strains, we transurethrally inoculated C57BL/6J mice with the wild-type and High AmpR strains. Following an intravesical infection with 1 × 108 CFU/bladder in C57BL/6J mice, oral administration of ampicillin was given twice daily for three days. On the fourth day post-inoculation, the bladders were harvested, homogenized, serially diluted, and then cultured on MacConkey agar medium. The CFU of the wild-type strain within the bladder showed a significant decrease after ampicillin treatment compared to no antibiotic treatment (). However, in the case of the High AmpR strain, despite exhibiting a lower level of infection within the bladder compared to the wild-type, there was no significant reduction even after ampicillin treatment (). Moreover, when we determined the CFUs of UPEC strains within the 5637 bladder carcinoma cell line, CFU of the High AmpR strain was slightly lower than that of the wild-type in the absence of ampicillin but more than 3-log higher than that of the wild-type in the presence of ampicillin ().
Figure 6. The High AmpR strain maintains bacterial burden in the bladder during antibiotic treatment despite decreasing type 1 fimbriae expression.
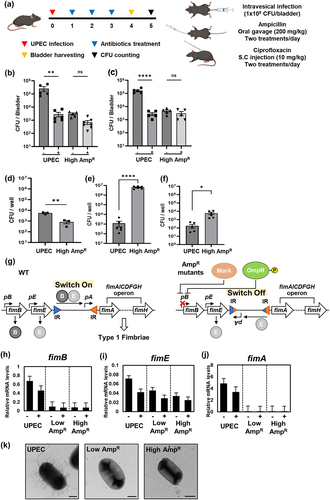
In the High AmpR strain, the decrease in adhesion to the mouse bladder could be due to the lower expression of type 1 fimbriae, which is responsible for the attachment of UPEC to the host cell [Citation36]. To test this hypothesis, we measured mRNA levels of the type 1 fimbriae operon (fimAICDFGH) and its regulators (fimB and fimE) (). The mRNA levels of fimB, encoding a regulatory protein for type 1 fimbriae, slightly decreased in the wild-type strain after ampicillin treatment but were still maintained at a significant level. However, in the AmpR strains, fimB mRNA levels were much lower than those in the wild-type regardless of the presence of ampicillin (). In contrast, the mRNA levels of fimE, which encodes another regulatory protein for type 1 fimbriae, showed a slight decrease after ampicillin treatment, but there was no apparent difference between the wild-type and AmpR strains (). Given that the expression of the type 1 fimbrial structural protein, FimA, is determined by the FimB/FimE ratio () [Citation37], fimA mRNA was highly expressed in the wild-type but nearly undetectable in the AmpR strains (). Similarly, other genes in the type 1 fimbriae operon (fimC, fimD, fimF, and fimG genes) were downregulated in the AmpR strains (Fig. S1). When we detected fimbriae by negative staining, the AmpR strains produced fewer fimbriae than the wild-type ( and S2). This is in agreement with previous reports that both MarA and OmpR-P decrease type 1 fimbriae expression [Citation38,Citation39], given that the levels of both MarA and OmpR-P are likely elevated in the AmpR strains ().
A shared mechanism of multidrug resistance in the AmpR UPEC
In the previous section, we demonstrated that ampicillin resistance in the AmpR strains originated from the higher expression of components in the multidrug efflux pump (via marR and acrR mutations), limiting the access of ampicillin by decreasing membrane permeability (via envZ mutation), or inhibiting cell lysis (via nlpD mutation). This underlying mechanism of ampicillin resistance led us to examine whether the identified AmpR strains would exhibit resistance to other classes of antibiotics.
Previously, it was established that mutations in the acrR gene are associated with resistance to fluoroquinolone antibiotics [Citation40]. Many multidrug-resistant clinical isolates exhibit mutations in the mar operon and genes involved in outer membrane permeability [Citation41]. Additionally, several genes such as marR, acrR, and envZ have been implicated in antibiotic and organic solvent resistance in the previous studies [Citation40,Citation42,Citation43]. Therefore, we performed MIC assays to investigate whether the AmpR strains displayed resistance to other antibiotics with different mechanisms of action, including ciprofloxacin, kanamycin, chloramphenicol, tetracycline, trimethoprim, and nitrofurantoin. As expected, the AmpR strains exhibited higher MIC values for the tested antibiotics than the wild-type strain. However, the AmpR strains showed a decreased MIC for kanamycin compared to the wild-type strain, although the High AmpR strain showed higher resistance than the Low AmpR strain. The MIC of nitrofurantoin was unaffected by the AmpR mutations (Table S1).
Using ciprofloxacin as a representative example, we performed further experiments. The MIC of ciprofloxacin for the wild-type strain was 0.015 μg/ml. In contrast, both the Low and High AmpR strains exhibited an MIC of 0.12 μg/ml, with no significant difference between the two AmpR strains (). Using qRT-PCR analysis, we measured the changes in mRNA levels of the nine aforementioned genes (excluding fimbrial genes) in response to ciprofloxacin. The marA, marB, acrA, and acrB genes exhibited lower expression levels in the wild-type strain regardless of the ciprofloxacin concentration, whereas the AmpR strains maintained high expression levels of these genes (). Conversely, the mRNA levels of the ompF and ompC genes decreased in the wild-type strain upon exposure to ciprofloxacin. However, the AmpR strains exhibited undetectable levels of ompF and constitutively high expression levels of ompC regardless of the presence of ciprofloxacin (). The envZ and ompR genes in the wild-type strain were transcribed at similar levels regardless of ciprofloxacin treatment, whereas in the AmpR strains, their transcripts were slightly higher than those in the wild-type strain (). Lastly, ciprofloxacin reduced the mRNA levels of nlpD, but the AmpR strains maintained higher expression levels than the wild-type strain (). Overall, these data indicate that the expression behaviors of the AmpR strains in response to ciprofloxacin are similar to those in response to ampicillin.
Figure 7. AmpR UPEC strains gain resistance to other classes of antibiotics including ciprofloxacin.
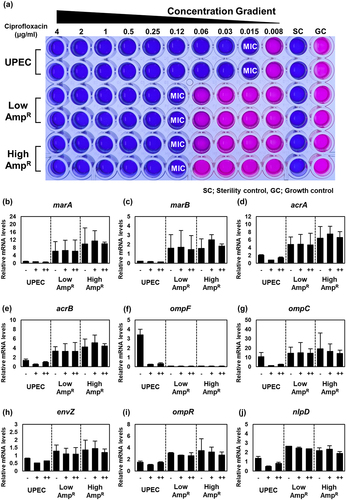
Similar to what we observed with ampicillin treatment, the High AmpR strain maintained bacterial CFU even after ciprofloxacin treatment in the mouse bladder () and increased bacterial burden in the 5637 urothelial cell line (). These data suggest that acquired mutations during ampicillin exposure render the AmpR strain resistant to other antibiotics as well and pose a challenge to antibiotic treatment.
Discussion
In this study, we generated strains that exhibited resistance to ampicillin by continuous exposure to ampicillin in the laboratory. Instead of acquiring a plasmid-associated expected-spectrum beta-lactamase (ESBL) gene [Citation44], we identified four chromosomal mutations in the marR, acrR, envZ, and nlpD genes. These mutations indirectly confer resistance to β-lactam antibiotics by altering bacterial physiology, such as reducing membrane permeability or increasing the expression of efflux pumps. Previous studies have shown that E. coli strains evolved by ampicillin or biocide treatment often harbor mutations in the marR, acrR, envZ, and ompR genes [Citation45,Citation46]. In both cases, the isolated strains exhibited cross-resistance to a variety of other antibiotics [Citation45,Citation46], supporting the notion that reducing membrane permeability or increasing the expression of efflux pumps are common regulatory mechanisms of multidrug resistance. Our findings provide additional evidence that nlpD mutation significantly enhances ampicillin resistance. Using the MIC assay, we determined that the four mutations induced by ampicillin also conferred resistance to several other antibiotics with different mechanisms of action. This suggests that these genes are also involved in the acquisition of multidrug resistance as well.
Among the various mutations in marR, mutations occurring at amino acid positions 69 and 73 within the helix-turn-helix (HTH) motif lead to the separation of DNA-binding domains, resulting in the loss of DNA-binding activity [Citation47]. Similarly, in the case of MarRV66F mutation, an aromatic ring structure was introduced near the DNA-binding domain. It is expected that this modification will decrease the DNA binding affinity, rendering MarRV66F unable to act as a repressor, thus allowing the expression of the marRAB operon (). The expression of MarA leads to the up-regulation of various multidrug resistance-related genes, including acrA, acrB, and tolC, which are components of the AcrAB-TolC efflux pump [Citation48]. Additionally, AcrR1–121, which lacks the dimerization domain, was unable to repress the acrAB operon and instead promoted the expression of acrA and acrB (). Furthermore, MarA is involved in DNA repair, enabling the repair of DNA damage caused by antibiotics and the regulation of membrane permeability [Citation49]. Although the expression level of OmpF can also be decreased by MarA [Citation50], this does not fully explain the complete repression of OmpF and the increased expression of OmpC. Therefore, it is speculated that EnvZT402M promotes the phosphorylation of OmpR; however, further studies are required to elucidate this mechanism.
Figure 8. A proposed model of multidrug resistance mechanisms in the laboratory-evolved AmpR UPEC strains.
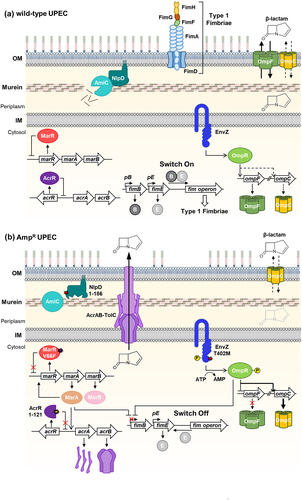
In terms of the connection between NlpD and antibiotics, previous studies have shown that the dLytM domain of NlpD plays a crucial role in ampicillin-induced cell lysis [Citation34]. In our study, we confirmed that the High AmpR strain, lacking the dLytM domain in NlpD (NlpD1–186), exhibited increased resistance to ampicillin. Moreover, the nlpD mutation not only conferred increased resistance to antibiotics but also resistance to the detergent SDS.
In an in vivo experiment using C57Bl/6J mice, the AmpR strain exhibited reduced infection efficiency compared to the wild-type strain. This is likely due to the decrease in type 1 fimbriae expression under the influence of MarA and OmpR-P [Citation38,Citation39]. However, once the infection was established, the increase in antibiotic resistance of the mutants posed a greater challenge for the treatment of the mouse bladder infection ().
The prevalence of multidrug-resistant uropathogenic Escherichia coli (UPEC) is increasing in developing countries, particularly with respect to resistance to beta-lactam, fluoroquinolone, and trimethoprim-sulfamethoxazole (TMP-SMX) antibiotics [Citation9]. In our study, we identified ampicillin-resistant mutants that displayed resistance not only to ampicillin but also to other antibiotics, including ciprofloxacin, trimethoprim, chloramphenicol, and tetracycline. This suggests that multidrug-resistant uropathogenic Escherichia coli (UPEC) may arise due to shared regulatory mechanisms, such as decreased membrane permeability, increased expression of drug efflux pumps, or inhibition of cell lysis. Mutations in envZ and acrB have been reported to result in decreased resistance to kanamycin [Citation43], similar to what we observed in the AmpR strains (Table S1). Interestingly, susceptibility to nitrofurantoin remained unchanged. This is likely due to the conversion of nitrofurantoin into electrophilic intermediates by nitroreductase, leading to the generation of radical molecules that can target various cellular components [Citation51]. Finally, understanding the mechanisms underlying multidrug resistance and pathogenesis in laboratory-evolved UPEC strains will provide insights into the development of potential therapeutic strategies.
Author contributions
E.-J.L. designed the research, analyzed the data, and wrote the manuscript; N.C. and E.C. performed the experiments and wrote the manuscript; Y.-J.C. analyzed the whole-genome sequencing; H.W.C wrote the manuscript; M.J.K. performed the in vivo experiment.
Supplemental Material
Download Zip (2.1 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available in the NCBI SRA database at https://www.ncbi.nlm.nih.gov/sra (reference number PRJNA987582).
Supplemental data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2367648
Additional information
Funding
References
- Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78(1):119–17. doi: 10.1146/annurev.biochem.78.082907.145923
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42–51. doi: 10.1038/nrmicro3380
- Micali S, Isgro G, Bianchi G, et al. Cranberry and recurrent cystitis: more than marketing? Crit Rev Food Sci Nutr. 2014;54(8):1063–1075. doi: 10.1080/10408398.2011.625574
- Flores-Mireles AL, Walker JN, Caparon M, et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol. 2015;13(5):269–284. doi: 10.1038/nrmicro3432
- Connell I, Agace W, Klemm P, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci, USA. 1996;93(18):9827–9832. doi: 10.1073/pnas.93.18.9827
- Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566
- Mobley HL, Green DM, Trifillis AL, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58(5):1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990
- Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366(11):1028–1037. doi: 10.1056/NEJMcp1104429
- Kot B. Antibiotic resistance among uropathogenic Escherichia coli. Pol J Microbiol. 2019;68(4):403–415. doi: 10.33073/pjm-2019-048
- Habibi A, Khameneie MK. Antibiotic resistance properties of uropathogenic Escherichia coli isolated from pregnant women with history of recurrent urinary tract infections. Trop J Pharm Res. 2016;15(8):1745–1750. doi: 10.4314/tjpr.v15i8.21
- Peck KR, Kim MJ, Choi JY, et al. In vitro time-kill studies of antimicrobial agents against blood isolates of imipenem-resistant Acinetobacter baumannii, including colistin- or tigecycline-resistant isolates. J Med Microbiol. 2012;61(Pt 3):353–360. doi: 10.1099/jmm.0.036939-0
- Elshikh M, Ahmed S, Funston S, et al. Resazurin-based 96-well plate microdilution method for the determination of minimum inhibitory concentration of biosurfactants. Biotechnol Lett. 2016;38(6):1015–1019. doi: 10.1007/s10529-016-2079-2
- Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi: 10.1089/cmb.2012.0021
- Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformat. 2014;30(14):2068–2069. doi: 10.1093/bioinformatics/btu153
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformat. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170
- Han Y, Lee EJ. Detecting salmonella type II flagella production by transmission electron microscopy and immunocytochemistry. J Microbiol. 2020;58(4):245–251. doi: 10.1007/s12275-020-9297-y
- Vega-Hernández R, Ochoa SA, Valle-Rios R, et al. Flagella, type I fimbriae and curli of uropathogenic Escherichia coli promote the release of proinflammatory cytokines in a coculture system. Microorgan. 2021;9(11):2233. doi: 10.3390/microorganisms9112233
- Garofalo CK, Hooton TM, Martin SM, et al. Escherichia coli from urine of female patients with urinary tract infections is competent for intracellular bacterial community formation. Infect Immun. 2007;75(1):52–60. doi: 10.1128/IAI.01123-06
- Walters MS, Lane MC, Vigil PD, et al. Kinetics of uropathogenic Escherichia coli metapopulation movement during urinary tract infection. MBio. 2012;3(1). doi: 10.1128/mBio.00303-11
- Beggs GA, Brennan RG, Arshad M. MarR family proteins are important regulators of clinically relevant antibiotic resistance. Protein Sci. 2020;29(3):647–653. doi: 10.1002/pro.3769
- Reyes-Fernández EZ, Schuldiner S. Acidification of cytoplasm in Escherichia coli provides a strategy to cope with stress and facilitates development of antibiotic resistance. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-66890-1
- Martin RG, Jair KW, Wolf RE, et al. Autoactivation of the marRAB multiple antibiotic resistance operon by the MarA transcriptional activator in Escherichia coli. J Bacteriol. 1996;178(8):2216–2223. doi: 10.1128/jb.178.8.2216-2223.1996
- Ma D, Alberti M, Lynch C, et al. The local repressor AcrR plays a modulating role in the regulation of acrAB genes of Escherichia coli by global stress signals. Mol Microbiol. 1996;19(1):101–112. doi: 10.1046/j.1365-2958.1996.357881.x
- Fralick JA. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178(19):5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996
- Sulavik MC, Houseweart C, Cramer C, et al. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrob Agents Chemother. 2001;45(4):1126–1136. doi: 10.1128/AAC.45.4.1126-1136.2001
- Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin Infect Dis. 1998;27(Supplement_1):S32–S41. doi: 10.1086/514920
- Aiba H, Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, stimulates the transcription of the ompF and ompC genes in Escherichia coli. FEBS Lett. 1990;261(1):19–22. doi: 10.1016/0014-5793(90)80626-T
- Cai SJ, Inouye M. Inouye M EnvZ-OmpR interaction and osmoregulation in Escherichia coli. J Biol Chem. 2002;277(27):24155–24161. doi: 10.1074/jbc.M110715200
- Lan C-Y, Igo MM. Differential expression of the OmpF and OmpC porin proteins in Escherichia coli K-12 depends upon the level of active OmpR. J Bacteriol. 1998;180(1):171–174. doi: 10.1128/JB.180.1.171-174.1998
- Brigitte Roux B. The binding of antibiotics in OmpF porin. Structure. 2013;21(1):76–87.
- Ferenci T, Phan K. How porin heterogeneity and trade-offs affect the antibiotic susceptibility of gram-negative bacteria. Genes (Basel). 2015;6(4):1113–1124. doi: 10.3390/genes6041113
- Chetri S, Singha M, Bhowmik D, et al. Transcriptional response of OmpC and OmpF in Escherichia coli against differential gradient of carbapenem stress. BMC Res Notes. 2019;12(1). doi: 10.1186/s13104-019-4177-4
- Choi U, Lee C-R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli. Front Microbiol. 2019;10:953. doi: 10.3389/fmicb.2019.00953
- Uehara T, Dinh T, Bernhardt TG. LytM-domain factors are required for daughter cell separation and rapid ampicillin-induced lysis in Escherichia coli. J Bacteriol. 2009;191(16):5094–5107. doi: 10.1128/JB.00505-09
- Tsang M-J, Yakhnina AA, Bernhardt TG, et al. NlpD links cell wall remodeling and outer membrane invagination during cytokinesis in Escherichia coli. PloS Genet. 2017;13(7):e1006888. doi: 10.1371/journal.pgen.1006888
- Connell I, Agace W, Klemm P, et al. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci USA. 1996;93(18):9827–9832. doi: 10.1073/pnas.93.18.9827
- Schwan WR, Shibata S, Aizawa S-I, et al. The two-component response regulator RcsB regulates type 1 piliation in Escherichia coli. J Bacteriol. 2007;189(19):7159–7163. doi: 10.1128/JB.00705-07
- Vila J, Soto SM. Salicylate increases the expression of marA and reduces in vitro biofilm formation in uropathogenic Escherichia coli by decreasing type 1 fimbriae expression. Virulence. 2012;3(3):280–285. doi: 10.4161/viru.19205
- Rentschler AE, Lovrich SD, Fitton R, et al. OmpR regulation of the uropathogenic Escherichia coli fimB gene in an acidic/high osmolality environment. Microbiology. 2013;159(Pt_2):316–327. doi: 10.1099/mic.0.059386-0
- Olliver A, Vallé M, Chaslus-Dancla E, et al. Role of an acrR mutation in multidrug resistance of in vitro-selected fluoroquinolone-resistant mutants of salmonella enterica serovar typhimurium. FEMS Microbiol Lett. 2004;238(1):267–272. doi: 10.1111/j.1574-6968.2004.tb09766.x
- Chetri S, Das BJ, Bhowmik D, et al. Transcriptional response of mar, sox and rob regulon against concentration gradient carbapenem stress within Escherichia coli isolated from hospital acquired infection. BMC Res Notes. 2020;13(1). doi: 10.1186/s13104-020-04999-2
- Wales A, Davies R. Co-selection of resistance to antibiotics, biocides and heavy metals, and its relevance to foodborne pathogens. Antibiotics. 2015;4(4):567–604. doi: 10.3390/antibiotics4040567
- Adler M, Anjum M, Andersson DI, et al. Combinations of mutations in envZ, ftsI, mrdA, acrB and acrR can cause high-level carbapenem resistance in Escherichia coli. J Antimicrob Chemother. 2016;71(5):1188–1198. doi: 10.1093/jac/dkv475
- Meier S, Weber R, Zbinden R, et al. Extended-spectrum beta-lactamase-producing gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39(4):333–340. doi: 10.1007/s15010-011-0132-6
- Li M, Liu Q, Teng Y, et al. The resistance mechanism of Escherichia coli induced by ampicillin in laboratory. Infect Drug Resist. 2019;12:2853–2863. doi: 10.2147/IDR.S221212
- Merchel Piovesan Pereira B, Wang X, Tagkopoulos I. Biocide-induced emergence of antibiotic resistance in Escherichia coli. Front Microbiol. 2021;12:640923. doi: 10.3389/fmicb.2021.640923
- Alekshun MN, Kim YS, Levy SB. Mutational analysis of MarR, the negative regulator of marRAB expression in Escherichia coli, suggests the presence of two regions required for DNA binding. Mol Microbiol. 2002;35(6):1394–1404. doi: 10.1046/j.1365-2958.2000.01802.x
- Ruiz C, Levy SB. Many chromosomal genes modulate MarA-mediated multidrug resistance in Escherichia coli. Antimicrob Agents Chemother. 2010;54(5):2125–2134. doi: 10.1128/AAC.01420-09
- Sharma P, Haycocks JRJ, Middlemiss AD, et al. The multiple antibiotic resistance operon of enteric bacteria controls DNA repair and outer membrane integrity. Nat Commun. 2017;8(1). doi: 10.1038/s41467-017-01405-7
- Chubiz LM, Rao CV. Role of the mar-sox-rob regulon in regulating outer membrane porin expression. J Bacteriol. 2011;193(9):2252–2260. doi: 10.1128/JB.01382-10
- McOsker CC, Fitzpatrick PM. Nitrofurantoin: mechanism of action and implications for resistance development in common uropathogens. J Antimicrob Chemother. 1994;33(suppl A):23–30. doi: 10.1093/jac/33.suppl_A.23
