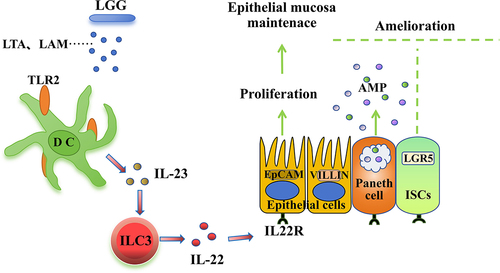
ABSTRACT
Salmonella is a foodborne pathogen that causes disruption of intestinal mucosal immunity, leading to acute gastroenteritis in the host. In this study, we found that Salmonella Typhimurium (STM) infection of the intestinal tract of mice led to a significant increase in the proportion of Lacticaseibacillus, while the secretion of IL-22 from type 3 innate lymphoid cells (ILC3) increased significantly. Feeding Lacticaseibacillus rhamnosus GG (LGG) effectively alleviated the infection of STM in the mouse intestines. TLR2−/− mice experiments found that TLR2-expressing dendritic cells (DCs) are crucial for LGG‘s activation of ILC3. Subsequent in vitro experiments showed that heat-killed LGG (HK-LGG) could promote DCs to secrete IL-23, which in turn further promotes the activation of ILC3 and the secretion of IL-22. Finally, organoid experiments further verified that IL-22 secreted by ILC3 can enhance the intestinal mucosal immune barrier and inhibit STM infection. This study demonstrates that oral administration of LGG is a potential method for inhibiting STM infection.
Introduction
The symbiotic relationship between intestinal probiotics and the host contributes to the maturation of intestinal function and the development of the immune system. Among probiotics, Lacticaseibacillus rhamnosus GG (LGG) is one of the most extensively studied. LGG is a Gram-positive beneficial bacterium present in the human gut, exhibiting strong adhesion to intestinal cells [Citation1]. LGG can enhance the proliferation and differentiation of mouse intestinal epithelial cells, thereby strengthening the intestinal immune barrier [Citation2]. Studies have shown that LGG can promote intestinal B cell development in piglets by the secreted p40 protein [Citation3]. Studies have shown that indole substances in LGG metabolites can promote AhR receptor response to enhance intestinal immunity [Citation4]. Moreover, LGG can inhibit pathogen infection by reducing intestinal pH, competing with pathogens for nutrients, and producing antimicrobial substances [Citation5,Citation6]. LGG can also activate macrophage activation by releasing lipoteichoic acid, promote the migration of mesenchymal stem cells, and ultimately protect the intestinal epithelium from radiation damage [Citation7]. These studies found that LGG itself, secreted proteins and metabolites may have an important role in promoting intestinal immune development and disease prevention.
Salmonella Typhimurium (STM) is one of the primary pathogens responsible for acute gastroenteritis in hosts, capable of infecting various poultry and mammals, as well as humans [Citation8]. STM infections can afflict the ileum and colon, and even the entire gastrointestinal tract of humans, posing serious health risks. In the early stages of STM infection, it can induce intestinal inflammation through direct infection of the intestinal barrier and destruction of intestinal epithelial cells [Citation9]. Some studies have reported that LGG can inhibit Salmonella infection by regulating macrophages [Citation10]. In this study, we further explored the pathways by which LGG activates intestinal immunity to inhibit STM infection.
Innate Lymphoid Cells (ILCs) are a newly discovered class of lymphocytes involved in innate immunity, playing a crucial role in protecting tissue health and combating infections. Among them, ILC3 plays a key role in maintaining homoeostasis within the intestine. They respond to various dietary components and microbes both inside and outside the body, can sense changes in the gut microbiome, thereby regulating intestinal immune responses and maintaining the stability of the gut microbiota [Citation11,Citation12]. ILC3s can secrete cytokines such as IL-22 and IL-17 to regulate intestinal mucosal immunity, not only interacting with intestinal stem cells to regulate their differentiation and function but also playing a crucial role in tissue repair processes [Citation13–15]. A study elucidated the mechanism behind ILC3-driven intestinal tissue repair, revealing that ILC3 can stimulate epithelial cell proliferation and tissue regeneration by activating the Yap1 signalling pathway in intestinal crypt cells [Citation16]. ILC3s can regulate epithelial cells through IL-22 signalling, including the expression of tight junction proteins, the expression of Major Histocompatibility Complex II (MHC-II), and the production of antimicrobial peptides [Citation17,Citation18]. ILC3s are also capable of inhibiting intestinal epithelial cell death and maintaining barrier integrity under the regulation of chemokine CCL3 and T cells [Citation19]. Recent research findings have clarified that innate immune cells, such as dendritic cells (DCs) and macrophages, can regulate ILC3 through the production of IL-1β and IL-23 [Citation20]. Most importantly, recent studies have found that ILC3 can inhibit Salmonella infection [Citation21]. In summary, we mainly studied whether feeding LGG could regulate the gut microbiota and enhance intestinal mucosal immunity by activating ILC3, ultimately inhibiting STM infection.
Toll-like receptors (TLRs) are a class of pattern recognition receptors (PRRs) in the innate immune system responsible for the early detection of invading pathogens [Citation22]. Among them, TLR2 is expressed on a variety of cell types, including immune cells, endothelial cells, and epithelial cells [Citation23]. The expression of TLR2 on DCs can regulate T cells’ response to Th2, induce the proliferation of CD4+CD25+Foxp3+ T cells, and promote the production of IL-10 and TGF-β by T cells [Citation24]. TLR2 has been shown to play a protective role during infections by triggering a strong pro-inflammatory response [Citation25]. Studies have demonstrated that in a mouse model of Mycobacterium tuberculosis infection, activation of TLR2 on CD4+ T cells leads to an increase in the protective IFN-γ secretion by T cells [Citation26]. Moreover, administering TLR2 agonists can enhance the phagocytic action and bactericidal activity of neutrophils, thereby protecting mice from infection with Methicillin-resistant Staphylococcus aureus (MRSA) [Citation27]. In summary, TLR2 is closely related to the recognition of the gut microbiota, and whether DC expressing TLR2 plays a role in inhibiting STM infection deserves further exploration.
In this study, we found that feeding mice with LGG significantly inhibited infection by STM. Subsequent experiments with TLR2−/− mice and in vitro cell studies revealed that heat-killed LGG (HK-LGG) activates ILC3 through DCs. It is conjectured that IL-22 secreted by ILC3 plays a crucial role in maintaining intestinal antibacterial functions and development, which can enhance the intestinal mucosal immune barrier and promote organoid development.
Materials and methods
Animals, and ethical statement
Wildtype mice (C57BL/6) used in this experiment were purchased from HFK Bioscience Co., Beijing, China. TLR2−/− mice (C57BL/6) purchased from Cyagen Biotechnology Co., Suzhou, China. For the duration of the experiments, all animals were accommodated in SPFgrade animal houses within a specific pathogen-free facility. The housing conditions included a 12-hour light/dark cycle and maintained appropriate ambient temperature and humidity levels. The entire animal experiment complied with the requirements of the Animal Management and Ethics Committee of Jilin Agricultural University and followed the National Guiding Principles for the Welfare of Laboratory Animals strictly. If the animal developed dyspnoea, haemorrhagic diarrhoea, or showed signs of mortality, they were euthanized immediately by CO2 inhalation.
Bacterial strains
STM was provided by Jilin Agricultural University. For the STM used to infect mice, it was first cultured overnight in LB medium, then passaged in fresh LB medium containing 0.3 M sodium chloride until the OD600 value reached, followed by two washes with PBS buffer. The bacterial sediment was resuspended in PBS, and the final concentration of the STM suspension was adjusted to 1 × 107 CFU/mL and stored for later use.
LGG (ATCC 53,103) was grown in De Man, Rogosa, and Sharpe (MRS) broth for 12 h at 37°C. After culturing overnight, the bacteria were inoculated 1:100 in fresh MRS broth and grown under anaerobic conditions until reaching the mid-log phase. Then, the colonies were counted, and the cell density was adjusted to 1 × 108 CFU/mL.
STM Infection Experiment and Sample Collection
Six-week-old mice were randomly divided into two groups, each consisting of 10 mice. The PBS group was fed 200 μL of PBS for 8 consecutive days. The STM group was fed 100 μL (1 × 107 CFU/mL) of STM suspension daily for 8 consecutive days. Then, mouse weight changes and survival rates were recorded. Following this, an STM infection experiment was repeated with another 20 six-week-old mice, and the mice were euthanized four days later. The small intestine and colon were collected for length measurements and formalin fixation.
Pathological sections and indirect immunofluorescence
Histopathological analysis was carried out on small intestine, colon, and spleen samples collected after infection. All samples were fixed with 4% paraformaldehyde, and sections were stained with haematoxylin and eosin to examine pathological changes. For immunofluorescence, diluted primary antibodies VILLIN (Abcam, ab130751), EpCAM (Abcam, ab213500), LGR5 (Abcam, ab75850) were added and incubated overnight at 4°C in the dark, followed by washing. Secondary antibodies AF594 anti-rabbit (Abcam, ab150080) was incubated for 1 hour at 4°C in the dark. After washing, nuclear staining was performed using PBS diluted DAPI at 1:5000 at room temperature in the dark for 10 minutes, allowing for nuclear staining. Following another wash, slides were mounted for microscope examination.
Cell separation
Cell samples obtained from the the mouse intestine were subjected to subsequent flow assay and in vitro cell culture and qPCR experiment. Firstly, after euthanasia of mice, the small intestine and Colon were dissected longitudinally, rinsed with PBS and divided into 1 cm sized intestinal fragments, which were then transferred to the separation solution (15 mL of RPMI-1640, 1% penicillin and streptomycin (Sigma, V900929), 1% HEPES (Sigma, H3375), 2.5 mM EDTA (Sigma, E8008), 1 mM DTT (Sigma, 3 December 3483), and 1% heat-inactivated FBS (Sigma, F8318)) and incubated for 28 minutes in a shaking incubator at 37°C and 200 rpm, and then removed. After incubation for 18 minutes in a shaking incubator at 37°C and 180 rpm, the intestinal fragments were obtained rinsed and continued into the enzyme digestion solution (8 mL RPMI-1640 medium, 1% penicillin and streptomycin, 1% HEPES, 20 mg collagenase IV (Sigma, V900893-1 G), 0.5 mg DNase I (Sigma 10,104,159,001), and 1% FBS), and incubated for 25 minutes in a shaking incubator at 37°C and 220 rpm before being removed, and then filtered through a 70-μm cell strainer to get the LPL cells in the mouse intestine. Finally, percoll (GE Healthcare 17,089,101) was used for density gradient centrifugation to obtain lymphocytes for subsequent experiments.
Flow cytometry and antibody information
First, antibodies were added to tubes containing 1 × 106 cells, mixed thoroughly and stained for 30 min at 4°C under dark conditions. Then, add 1 mL of PBS, centrifuge at 2000 r pm and 4°C for 5 min and discard the supernatant. The cells can then optionally be fixed and permeabilized, and after permeabilization the antibody can be used to continue the staining. The staining is completed and detected using a flow cytometric analyser (BD).
BD Pharmingen: Fixable Viability Stain 780 (L/D) (565388), purified rat anti-mouse CD16/CD32 (Mouse BD Fc Block) (553142), γδ T (Biotin) (553176), CD19 (Biotin) (553784), CD11b (Biotin) (557395), TCRβ (Biotin) (553168), Ly6G/C (Biotin) (553124), TER-119 (Biotin) (553672), streptavidin protein (APC-cy7) (554063), CD45 (FITC) (551874), CD127 (PE-cy7) (560733), RORγt (PE) (562607), and GATA3 (BV421) (563349), IL-22 (Alexa Fluor 647) (567160), MHCII (PE) (558593), CD11c (FITC) (553801), IL-23 (Alexa Fluor 647) (565317).
LGG inhibited the STM infection
We divided the 5 Weeks old mice into three groups of 10 animals each. The PBS group were fed PBS for 14 days as the control group. Then the PBS+STM group was first fed PBS for 7 days, and then received STM infection. The LGG+STM group continued to be fed with LGG for 7 days, each mouse is fed 100 μL (1 × 107CFU) LGG per day, and then received STM infection. Next, mouse weight changes and survival rates were recorded for 8 days. The PBS+STM group and LGG+STM group experiments were repeated, and the mice were euthanized on the fourth day of infection with STM, and then intestinal tissues and cells were collected for subsequent experiments.
ELISA and qPCR experiments
Cytokine protein and total RNA were extracted from the mouse intestine and secretion of IL-22 was detected using the ELISA kit (MEIMIAN, MM-0892 M2). Next, total RNA was extracted, and 1 mg of RNA was reversed into cDNA by reverse transcriptase (Promega) which reverse transcribed Moloney mouse leukaemia virus (M-MLV). In the real-time qPCR system of Biological System 7500, qPCR was performed using SYBR green mixture (Takara). The average mRNA fold changes were calculated by 2-ΔΔCT method and compared with the control group.
Primer design: IL-22 (NM_016971.2),
CCTGCTTCTCATTGCCCTGTGG,
AAGGTGCGGTTGACGATGTATGG.
AGCCAACTCCTCCAGCCAGAG,
CGCTGCCACTGCTGACTAGAAC.
16S rRNA-seq experiment
We performed a 16S rRNA sequencing of the intestinal contents of the mice. Novogene Co., for providing technical services such as detecting and analysing of 16S rRNA-seq raw data.
Flow sorting of ILC3 and DC
Single-cell suspensions were incubated with antibodies including Lin (γδ T, CD19, CD11b, TCR-β, Ly6G/C, TER-119), L/D, MHC-II, CD11c, CD45, etc. DCs were sorted as L/D−CD11c+MHCII+ cells, and ILC3 were obtained through Lin−L/D−CD45+ sorting, noting that Lin−L/D−CD45++ indicates ILC2.
In vitro stimulation culture of primary cells
ILC3s were seeded in a 24-well plate at a density of 5 × 106 cells per well. HK-LGG (50 μL), LGG supernatant (50 μL), and DCs (5 × 105) were added to the culture medium with ILC3 and incubated for 8 hours before being analysed by flow cytometry and qPCR.
Organoid extraction and culture
After euthanizing the mouse, the small intestine was removed, mesentery and fat were discarded, and the intestinal segment was longitudinally opened and washed with cold PBS until the supernatant was clear. The intestinal segments were cut into 2 mm pieces and gently washed with cold PBS, then added to 15 mL of crypt isolation solution (1 mM EDTA in PBS). Incubated at room temperature for 30 minutes.
The crypt isolation solution was discarded, and 10 mL of DPBS was added to repeatedly pipette the fragments. After the fragments settled, the supernatant was collected through a 70 μm cell strainer into a 50 mL centrifuge tube, labelled as 1, and this step was repeated four times. The 3rd and 4th filtrates were centrifuged at 300×g for 5 minutes, and the supernatant was discarded. The pellet was resuspended in 1 mL of DME/F12 + 1% P/S and transferred to a 1.5 mL centrifuge tube, then centrifuged at 200×g for 3 minutes, and the supernatant was discarded.
The pellet was mixed with 250 μL of complete medium and 250 μL of Matrigel (operation on ice), mixed well by pipetting. 50 μL was pipetted into the centre of a well in a 24-well plate and incubated in a culture incubator for 30 minutes. Then, 500 μL of complete culture medium (STEMCELL #6000) was added to each well, and 500 μL of PBS was added to the remaining wells.
When organoids begin to bud, they should be passaged. First, the old culture medium is removed, and 2 mL of DME/F12 is added for pipetting up and down before collection into a centrifuge tube. After centrifugation, the supernatant is discarded, and the pellet is resuspended in complete culture medium and Matrigel for further cultivation.
Co-culture Model of ILC3 and intestinal organoids
HK-LGG, DCs, and ILC3 are added to the organoid culture medium to observe their effects on the growth and development of organoids. Medium 1 is the organoid culture medium. Medium 2 consists of RPMI-1640 (1% penicillin and streptomycin, 1% HEPES, 10% FBS). HK-LGG, DCs, and ILC3 can be added to Medium 2.
Statistical analysis of data
Flow cytometry results were analysed using FlowJo version 10.8.1. Graphs were plotted using GraphPad Prism version 8.0.2 software. Data analysis was carried out using one-way ANOVA to compare differences between control and experimental groups. (p < 0.05 is denoted by *; p < 0.01 by **; p < 0.001 by ***).
Results
STM Infection Causes Mortality and Intestinal Lesions in Mice
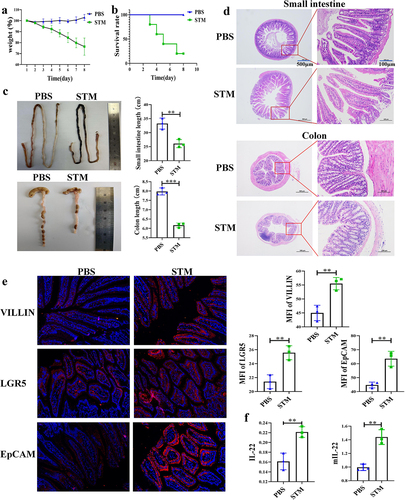
This study found that mice infected with STM exhibited weight loss and even death. The body weight of mice in the PBS group increased, while that of mice in the STM group significantly decreased, with mortality observed on the third day and a survival rate of only 25% by the eighth day (). STM infection also led to the atrophy of the small intestine and colon in mice. The length of the small intestine in the PBS group was about 33 cm, and the colon was about 8 cm. In contrast, the small intestine in the STM group was about 26 cm, and the colon was about 6 cm (). In order to explore the immune changes in the mouse gut during this process, we repeated the infection experiment and euthanized the mice on the fourth day. Pathological sections revealed tissue damage in the small intestine and colon of mice in the STM group, including villi fracture, thinning of the intestinal wall, and extensive infiltration of red blood cells and lymphocytes (). Immunofluorescence experiments identified significant expression of intestinal villin protein, stem cell differentiation protein LGR5, and epithelial cell marker protein EpCAM in the intestines of STM-infected mice (). ELISA and qPCR analyses showed that the secretion of IL-22 and the transcription level of the mIL-22 gene in the intestines of mice in the STM group were significantly higher than in the PBS group (). The experimental results indicate that STM infection in the mouse intestine causes severe intestinal damage and endangers the lives of the mice, with higher levels of IL-22 being secreted in the intestine.
Figure 1. Fig. 1 | STM infection caused death and intestinal lesions in the mice a: STM infection leads to decreased host weight in mice (n = 5). b: STM infection results in mortality of mice (n = 5). c: STM infection causes the shrinking of the small intestine and colon in mice. d: STM infection induces pathological changes in the small intestine and colon of mice. e: after STM infection, mice exhibit elevated expression of VILLIN, LGR5, and EpCAM proteins in the small intestine. f: following STM infection, there is a significant increase in the secretion of IL-22 protein and transcription of mIL-22 in the intestinal tract of mice.
STM Infection Leads to Significant Changes in Mouse gut microbiota and IL-22 Secretion by ILC3
The results of phylum level analysis from 16s-RNA sequencing found that infection with STM resulted in increased proportion of Firmicutes, Proteobacteria and Actinobacteriota. The genus level analysis found that the proportion of Lacticaseibacillus in the gut increased significantly (). Box plots of α-diversity analysis indicated that the abundance and diversity of intestinal microbiota significantly decreased after STM infection (). β-diversity analysis showed significant differences in species diversity between the PBS and STM groups (). PCA analysis Also shows a difference in the PBS and STM groups ().
Figure 2. STM infection leads to significant changes in the gut microbiota and IL-22 secretion by ILC3 in mice.a: Changes in Lactobacillus Genus in the intestinal tract of piglets (n=5). b: Box plot of alpha diversity analysis (Chao1, Simpson index). c: Heatmap of beta diversity analysis. d: Inter-group PCA analysis. e: Differences in IL-22 secretion by CD45+ immune cells between the PBS and STM groups. f: Flow cytometry analysis of the number of ILC3 cells and the level of IL-22 secretion in the lamina propria of the mouse small intestine. g: Flow cytometry analysis of the number of TH17 cells and the level of IL-22 secretion in the lamina propria of the mouse small intestine.
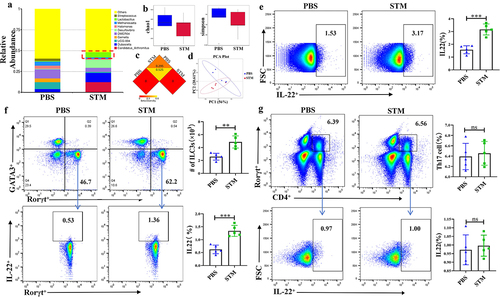
Flow cytometry detection revealed that IL-22 expression mainly originated from CD45+ immune cells (), with gating strategy shown in Fig. s1A. Lymphocytes known to secrete IL-22 primarily include ILC3 and CD4+ T cells. To determine the source of IL-22, we separately measured the levels of IL-22 secreted by ILC3 and CD4+ T cells. Results showed a significant increase in both the number of ILC3 cells and the IL-22 they secreted (), with gating strategy shown in Fig. s1B. In every million lymphocytes, the absolute number of ILC3 in the PBS group was 47,800, compared to 25,600 in the STM group. Meanwhile, the secretion of IL-22 by CD4+ T cells showed almost no change (), with gating strategy shown in Fig. s1C. The significant increase of Lacticaseibacillus in the early stage of STM infection, as well as the secretion of IL-22 by ILC3, led us to speculate that Lacticaseibacillus may play a key role in inhibiting STM infection
Feeding mice with LGG can inhibit STM infection
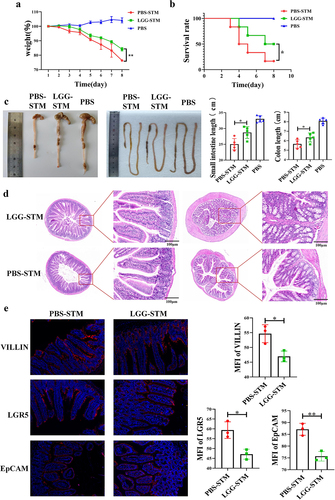
To explore the specific role of Lacticaseibacillus in the intestinal STM infection, we orally administered LGG to mice and then infected them with STM after 7 days. By analysing the mortality and body weight changes of the mice, it was found that compared to the LGG-STM group, mice in the PBS-STM group experienced more severe weight loss () and had a lower survival rate (). The lengths of the small intestine and colon in the LGG-STM group were also found to be closer to those of the PBS group (). Analysis of the STM load in faeces revealed a significantly lower number of STM in the faeces of mice in the LGG-STM group compared to the PBS-STM group (Fig. s2A). The degree of pathological changes in the intestines of mice in the LGG-STM group was also significantly lower than that in the PBS-STM group, with the villi in the jejunum of the LGG+STM group mice showing shortening and atrophy, and epithelial cells showing mild lesions. The villi in the jejunum of PBS+STM group mice exhibited shortening, fragmentation, and breaking, with vacuolization, necrosis, and shedding of the intestinal epithelial cells, among other histopathological changes (). Immunofluorescence experiments further revealed that the expression levels of VILLIN and LGR5 proteins in the small intestine of mice in the LGG+STM group were lower than in the PBS-STM group (). These results suggest that feeding LGG can significantly reduce the mortality and intestinal lesions caused by STM infection in mice.
Figure 3. LGG can help mice resist STM infection.a: Feeding LGG significantly alleviates weight loss in mice after STM infection. b: Feeding LGG significantly reduces mortality in mice after STM infection. c: Feeding LGG significantly alleviates the shrinking of the small intestine and colon in mice after STM infection. d: Feeding LGG can significantly alleviate the pathological changes in the small intestine and colon in mice after STMinfection. e: Feeding LGG significantly reduces the expression of VILLIN, LGR5, and EpCAM proteins in the small intestine of mice after STM infection.
Feeding LGG Promotes the Development of ILC3 and Secretion of IL-22 in the Mouse Intestine
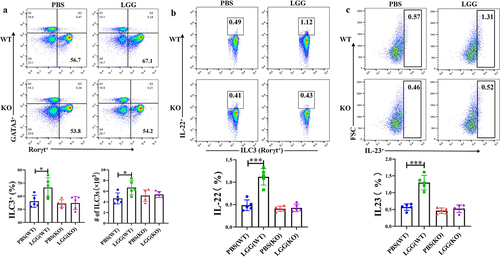
We further investigated the effect of feeding LGG on the activation of intestinal ILC3 in mice and used TLR2−/− mice to verify the importance of TLR2 in this process. Flow cytometry results showed that feeding LGG increased the number of ILC3 in the mouse intestine and promoted the secretion of IL-22. However, after feeding LGG to TLR2−/− mice, the activation effect of LGG on ILC3 was absent (), indicating a key role of TLR2 in the activation of ILC3 by LGG. It is known that TLRs are mainly expressed on the surface of DCs in the mouse intestine. We also conducted flow cytometry analysis on DCs (gating strategy shown in Fig. s2B), and results showed significant differences in the expression of IL-23 by DCs in the lamina propria of TLR2−/− mice and wild-type mice after feeding LGG (). In summary, feeding LGG to wild-type mice significantly promoted the secretion of IL-23 by DCs in the lamina propria, and concurrently, the number of ILC3 and the secretion of IL-22 were also significantly increased, while feeding LGG to TLR2−/− mice did not induce these changes. These results indicate that LGG may interact with DCs and promote the secretion of IL-23. Next, we conducted in vitro experiments to verify whether IL-23 secreted by DCs stimulated by LGG could promote the activation of ILC3 and the secretion of IL-22.
Figure 4. LGG promotes activation of ILC3 cells and is associated with IL-23 expression by TLR2 and DCs.a: Flow cytometry analysis of changes in ILC3 in the lamina propria of WT and KO mice after LGG feeding. b: Flow cytometry analysis of the level of IL-22 secretion by ILC3 in the lamina propria of WT and KO mice after LGG feeding. c: Flow cytometry analysis of the level of IL-23 expression by DCs in the lamina propria of WT and KO mice after LGG feeding.
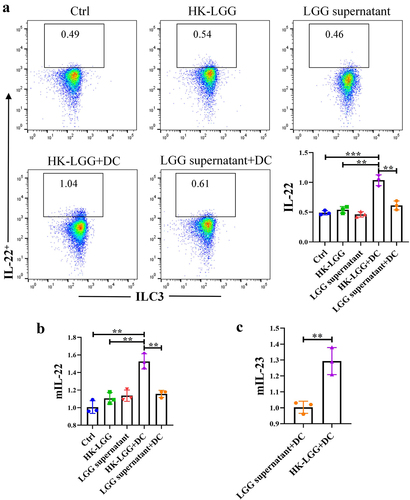
HK-LGG promotes IL-22 secretion by ILC3 through DCs
Initially, we obtained DCs and ILC3, and the flow cytometry sorting strategy is shown in Fig. s3A/B. Subsequently, an in vitro co-culture model was constructed (Fig. s3C), followed by control experiments using LGG culture supernatant and HK-LGG. In vitro studies found that without DCs, neither HK-LGG nor LGG supernatant could promote IL-22 secretion by ILC3. However, when DCs were added, the HK-LGG could promote IL-22 secretion by ILC3 (). Next, we measured the transcription levels of mIL-22 in co-cultured ILC3 cells and mIL-23 in DCs via qPCR experiments. It was found that the transcription of mIL-22 in the culture medium of the HK-LGG+DC group was significantly higher than in other groups (), while the transcription level of mIL-23 in the LGG supernatant+DC group was significantly lower than in the HK-LGG+DC group (). These results indicate that HK-LGG can promote the secretion of IL-23 by DCs, and the IL-23 secreted by DCs can further promote the secretion of IL-22 by ILC3. To explore whether IL-22 could further enhance the function of the intestinal mucosal immune barrier in this process, we conducted further experimental studies using intestinal organoids.
Figure 5. HK-LGG activates ILC3 by promoting IL-23 secretion from DCs.a: Flow cytometry analysis of the activating effect of HK-LGG, LGG supernatant, and DCs on IL-22 secretion by mouse ILC3. b: Transcription levels of the IL-22 gene in ILC3 under stimulation by different groups. c: Differential levels of IL-23 secretion promoted by HK-LGG and LGG supernatant from DCs.
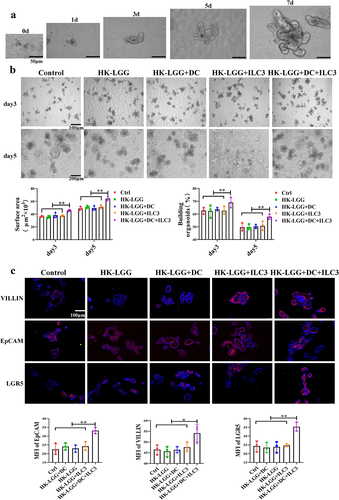
IL-22 Regulates the Immune Barrier Function of Intestinal Epithelium
Firstly, we successfully established an in vitro culture model of mouse intestinal organoids (Fig. s3D), with isolated intestinal crypts approximately 10 μm in size, cultured in a matrix gel. Budding began in large numbers on day 3, and by day 7, they had grown into mature entities approximately 100 μm in diameter (). Next, we co-cultured HK-LGG, DCs, ILC3, and organoids. The results showed no significant developmental changes in the organoids in the Ctrl group, HK-LGG group, HK-LGG+DC group, and HK-LGG+ILC3 group; however, the intestinal organoids in the HK-LGG+DC+ILC3 group growed faster. On days 3 and 5, we assessed the volume and budding of the organoids, finding that budding and growth in the HK-LGG+DC+ILC3 group were significantly higher than in the other groups (). Immunofluorescence experiments revealed that the expression of villin, epithelial protein, and LGR5 protein in the organoids of the HK-LGG+DC+ILC3 group was also higher than in the ILC3 group and the DC+ILC group (). These results demonstrate that HK-LGG can promote DCs to secrete IL-23, which then encourages ILC3 to secrete IL-22, and IL-22 ultimately promotes the development of intestinal organoids.
Figure 6. HK-LGG can promote the development of mice intestinal organoids through activating ILC3s to secrete IL-22.a: Crypts isolated from mice intestines were cultured in vitro, and the images depict the changes in organoid size over an 7-day period. b: Effects of HK-LGG; HK-LGG+DC; HK-LGG+ILC3 and HK-LGG+DC+ILC3 on the growth of intestinal organoids. Measure the size (surface area) and budding rate (building organoids) of the organs on the third and fifth days respectively. c: Immunofluorescence experiment demonstrating the expression of proteins such as Villin, EpCAM, LGR5 between different groups.
Studies have reported that components of the LGG cell wall can be recognized by TLR2, stimulating DCs to secrete IL-237. Based on existing research reports and our experimental results, a regulatory pathway diagram was created: LTA and LAM from HK-LGG can be recognized by TLR2-expressing DCs leading to the secretion of IL-23, which acts on ILC3 to promote the secretion of IL-22. IL-22 can perform multiple functions, including promoting the development of organoids and activating epithelial cells and Paneth cells ().
Discussion
STM is a facultative intracellular pathogen that can actively invade and replicate in host cells [Citation28]. STM has a wide range of hosts, including humans, various livestock, wild animals, etc., and is mainly transmitted through contaminated food or water sources [Citation29,Citation30]. Studies have found that intestinal mucosal immunity can trigger local inflammation when inhibiting STM infection [Citation31]. Recent studies have shown that the host immune system will produce specific cytokines to rapidly inhibit STM infection, among which IL-22 is one of the most upregulated cytokines in the intestine [Citation32]. This study found that mice infected with STM have increased abundance of Lacticaseibacillus in the intestine and intestinal lesions, while there was a significant increase in ILC3 and the secretion of IL-22 in the intestinal lamina propria. These results suggest that Lacticaseibacillus in the mouse intestine may play an important role during STM infection, potentially regulating ILC3 to secrete IL-22. Thus, the regulatory relationship between the microbiota and ILC3—whether it is a positive or negative feedback mechanism – warrants further exploration.
Intestinal microbiota play a crucial role in the development and maintenance of the host’s immune system, especially in regulating the development and differentiation of lymphocytes within the intestinal lamina propria [Citation33,Citation34]. In addition, the gut microbiota promotes the development of early B lymphocytes in the lamina propria of the mouse small intestine [Citation33,Citation34]. Preclinical studies and clinical practice have shown that the use of probiotics can limit the overgrowth of pathogenic bacteria and control the host’s pathological processes [Citation35]. Among them, LGG is one of the most widely used probiotic strains and has long been recognized for its beneficial effects on human health [Citation36,Citation37]. To explore the role of Lacticaseibacillus in STM infection, we orally administered the model probiotic LGG to mice before subjecting them to STM infection. The results showed that feeding LGG significantly alleviated the symptoms of STM infection in mice.
TLR2 recognizes and responds to threats early in bacterial infections and can influence the downstream immune response to the host’s benefit or detriment [Citation38]. Research has reported that DCs expressing TLR2 play an important role in the regulation of intestinal microbiota [Citation39], particularly lipoarabinomannan (LAM) and lipoteichoic acid (LTA) from LGG, which can activate DCs to secrete IL-23 through TLR2 [Citation40,Citation41]. Subsequently, ILC3 can produce the cytokine interleukin IL-22 in response to IL-23 signalling [Citation42]. To further explore the activating effect of LGG on the intestinal immune barrier, we constructed an in vitro cell co-culture model for validation. Our results found that HK-LGG can activate DCs via TLR2 and promote the secretion of IL-23, which in turn can enhance the proliferation of ILC3 cells and the secretion of IL-22.
Some studies have reported that the intestinal microbiota is important for IL-22 production in the intestine, but the underlying regulatory mechanism remains unclear [Citation43]. In the mouse intestine, research has documented the crucial role of the IL-22-IL-22 R signalling axis in immune responses and mucosal surface barrier functions [Citation44]. Studies also report that IL-22 can promote epithelial cell activation and the expression of antimicrobial peptides through the activation of the STAT3 signalling pathway [Citation45]. Multiple studies have underscored the importance of IL-22 produced by ILC3 in maintaining intestinal homoeostasis [Citation46]. We constructed a mouse intestinal organoid model to further validate the impact of IL-22 on organoid development, showing that IL-22 can also act on Paneth cells, stem cells, and epithelial cells in organoids to promote their growth and development.
Salmonella infection has caused serious health problems for people in developing countries. Studies have found that the use of yeast probiotics protects against Salmonella infections [Citation47]. Studies have also found the role of Sphingolipids on Innate Immunity to Intestinal Salmonella Infection [Citation48]. In this study, we discovered that LAM and LTA from LGG can activate DCs and secrete IL-23 through TLR2, and IL-23 can further activate ILC3 to secrete IL-22, maintaining intestinal immune homoeostasis. While this work provides a theoretical basis and experimental foundation for the development of intestinal health regulatory products and treatment strategies, the complexity of the microbial species in the intestine leaves unanswered whether LAM and LTA from other microbial sources can also exert similar immunomodulatory effects.
In recent years, research into the interactions between microbiota and the immune system has garnered considerable attention. On one hand, the immune system can regulate and shape the microbial flora [Citation49]. On the other hand, the colonized microbial flora can promote the development of the host’s immune system and provide signals for subsequent immune responses [Citation50]. However, to date, our understanding of the interactions between microbiota and the immune system remains significantly limited, and unravelling these mysteries requires coordinated innovation across multiple disciplines. Our work is just the beginning, and in the future, we will delve deeper into exploring the mechanisms of interaction between LGG and intestinal immune cells.
Author contributions
Cell isolation, J.H.W.; data analysis, M.G.; manuscript preparation and writing, J.H.W.; Information collection, J.R.W.; supervision and project administration, C.F.W., Y.Z., and X.C. Preparation of experimental reagent materials, J.H.W., M.G. All authors contributed to the article and approved the submitted version.
Ethics approval
All experiments in this study were conducted according to the regulations of the Administration of Affairs Concerning Experimental Animals in China. The animal management procedures and all laboratory procedures abided by the regulations of the Animal Care and Ethics Committees of Jilin Agriculture University. The ethical review acceptance number is 20,220,302,006.
Fig._s1.jpg
Download JPEG Image (1.3 MB)Fig._s3.jpg
Download JPEG Image (1.4 MB)Fig._s2.jpg
Download JPEG Image (1.1 MB)Acknowledgements
We thank Novogene Co., for providing technical services such as detecting and analyzing of 16S rRNA sequencing.
Disclosure statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Data availability statement
The data that support the findings of this study are openly available in https://figshare.com/s/641045c425176601b3b6, DOI: 10.6084/m9.figshare.25826965. And 16S rRNA-seq data in https://www.ncbi.nlm.nih.gov/bioproject, reference number is [PRJNA1073044].
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21505594.2024.2384553
Additional information
Funding
References
- Forestier C, De Champs C, Vatoux C, et al. Probiotic activities of lactobacillus casei rhamnosus: in vitro adherence to intestinal cells and antimicrobial properties. Res Microbiol. 2001;152(2):167–14. doi: 10.1016/S0923-2508(01)01188-3
- Ravi MP, Loren SM, Ashish RK, et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am J Pathol. 2011;180(2):626–635. doi: 10.1016/j.ajpath.2011.10.025
- Yu-Bei J, Xin C, Chun-Wei S, et al. Lactobacillus rhamnosus GG promotes Early B lineage development and IgA production in the Lamina Propria in piglets. J Immunol. 2021;207(8):2179–2191. doi: 10.4049/jimmunol.2100102
- Wang J, Zhao Y, Cui T, et al. AhR ligands from LGG metabolites promote piglet intestinal ILC3 activation and IL-22 secretion to inhibit PEDV infection. bioRxiv 2023:2023.12.05.570065. J Virol. 2024. doi: 10.1128/jvi.01039-24
- Seria Masole S, Bo F, Wentao Y, et al. The regulatory effect of lactobacillus rhamnosus GG on T lymphocyte and the development of intestinal villi in piglets of different periods. AMB Express. 2020;10(1):10. doi: 10.1186/s13568-020-00980-1
- Yongsong B, Kaidi M, Jibo L, et al. Deoxynivalenol exposure induces liver damage in mice: inflammation and immune responses, oxidative stress, and protective effects of lactobacillus rhamnosus GG. Food Chem Toxicol. 2021;156:156. doi: 10.1016/j.fct.2021.112514
- Riehl TE, David A, Xueping E, et al. Lactobacillus rhamnosus GG protects the intestinal epithelium from radiation injury through release of lipoteichoic acid, macrophage activation and the migration of mesenchymal stem cells. Gut. 2018;68(6):1003–1013. doi: 10.1136/gutjnl-2018-316226
- Uzzau S, Brown DJ, Wallis T, et al. Host adapted serotypes of Salmonella enterica. Epidemiol Infect. 2000;125(2):229–255. doi: 10.1017/S0950268899004379
- Shannon EM, Jennie M, Elaine S, et al. The global burden of nontyphoidal salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–889. doi: 10.1086/650733
- Bingjie D, Lina S, Ruihan L, et al. Lactobacillus rhamnosus GG defense against Salmonella enterica serovar typhimurium infection through modulation of M1 macrophage polarization. Microb Pathog. 2021;156:156. doi: 10.1016/j.micpath.2021.104939
- Michael HS, Nobuhiko K, Yun-Gi K, et al. Microbiota-induced IL-1β, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209(2):251–258. doi: 10.1084/jem.20111703
- Sonnenberg GF, Monticelli LA, Alenghat T, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi: 10.1126/science.1222551
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001
- Maarten C, Anna R, Stefanie Katharina G, et al. Innate lymphoid cell type 3–derived interleukin-22 boosts lipocalin-2 production in intestinal epithelial cells via synergy between STAT3 and nf-κB. J Biol Chem. 2019;294(15):6027–6041. doi: 10.1074/jbc.RA118.007290
- Zindl CL, Witte SJ, Laufer VA, et al. A nonredundant role for T cell-derived interleukin 22 in antibacterial defense of colonic crypts. Immunity. 2022;55(3):494–511.e11. doi: 10.1016/j.immuni.2022.02.003
- Mónica R-H, Patricia A-D, Natalie P, et al. Yap1-driven intestinal repair is controlled by group 3 innate lymphoid cells. Cell Rep. 2020;30(1):37–45.e3. doi: 10.1016/j.celrep.2019.11.115
- Xiaohuan G, Ju Q, Tony T, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40(1):25–39. doi: 10.1016/j.immuni.2013.10.021
- Hepworth MR, Fung TC, Masur SH, et al. Immune tolerance. Group 3 innate lymphoid cells mediate intestinal selection of commensal bacteria-specific CD4+ T cells. Science. 2015;348(6238):1031–1035. doi: 10.1126/science.aaa4812
- Angélique J, Zacarias G, Solenne M, et al. Inflammation triggers ILC3 patrolling of the intestinal barrier. Nat Immunol. 2022;23(9):1317–1323. doi: 10.1038/s41590-022-01284-1
- Baomei W, Jong-Hyung L, Tetsuhiro K, et al. Macrophage β2-integrins regulate IL-22 by ILC3s and protect from lethal citrobacter rodentium-induced colitis. Cell Rep. 2019;26(6):1614–1626.e5. doi: 10.1016/j.celrep.2019.01.054
- Ann MJ, Gregory FS. ILC3 pyroptosis limits salmonella infection. Nat Microbiol. 2022;7(7):933–934. doi: 10.1038/s41564-022-01165-1
- Dolasia K, Bisht M, Pradhan G, et al. TLRs/Nlrs: shaping the landscape of host immunity. Int Rev Immunol. 2018;37(1):3–19. doi: 10.1080/08830185.2017.1397656
- Laura O-N, Paola M, Lee MW. The role of TLR2 in infection and immunity. Front Immunol. 2012;3:3. doi: 10.3389/fimmu.2012.00079
- Jelena C, Nataša I, Alisa G-M, et al. DC-SIGN signalling induced by Trichinella spiralis products contributes to the tolerogenic signatures of human dendritic cells. Sci Rep. 2020;10(1):10. doi: 10.1038/s41598-020-77497-x
- Anna T, Evelyn G, Esther J, et al. Determinant role for Toll-like receptor signalling in acute mycobacterial infection in the respiratory tract. Microbes Infect. 2006;8(7):1790–1800. doi: 10.1016/j.micinf.2006.02.017
- Reba SM, Li Q, Onwuzulike S, et al. TLR2 engagement on CD4 + T cells enhances effector functions and protective responses to mycobacterium tuberculosis. Eur J Immunol. 2014;44(5):1410–1421. doi: 10.1002/eji.201344100
- Yi-Guo C, Yong Z, Lin-Qiang D, et al. Control of methicillin-resistant staphylococcus aureus pneumonia utilizing TLR2 agonist Pam3CSK4. PLoS One. 2016;11(3):11. doi: 10.1371/journal.pone.0149233
- Audrey C, Tregei S, Ciaran EF, et al. A role for the salmonella type III secretion system 1 in bacterial adaptation to the cytosol of epithelial cells. Mol Microbiol. 2019;112(4):1270–1283. doi: 10.1111/mmi.14361
- Ohad G-M, Erin CB, Guntram AG. Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front Microbiol. 2014;5. doi: 10.3389/fmicb.2014.00391
- Rafaela GF, Denes KAR, Adelino C-N, et al. Worldwide epidemiology of salmonella serovars in animal-based foods: a meta-analysis. Appl Environ Microbiol. 2019;85(14). doi: 10.1128/AEM.00591-19
- Taher A, Maryam Z, Mahmood Alizadeh S, et al. Molecular mechanisms of salmonella effector proteins: a comprehensive review. Infect Drug Resist. 2020;13:11–26. doi: 10.2147/IDR.S230604
- Behnsen J, Jellbauer S, Wong CP, et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity. 2014;40(2):262–273. doi: 10.1016/j.immuni.2014.01.003
- Zegarra-Ruiz DF, Kim DV, Norwood K, et al. Thymic development of gut-microbiota-specific T cells. Nature. 2021;594(7863):413–417. doi: 10.1038/s41586-021-03531-1
- Michelle GR, Wendy SG. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42
- Pitocco D, Di Leo M, Tartaglione L, et al. The role of gut microbiota in mediating obesity and diabetes mellitus. Eur Rev Med Pharmacol Sci. 2020;24(3):1548–1562. doi: 10.26355/eurrev_202002_20213
- Marijke ES, Sarah L. Towards a better understanding of Lactobacillus rhamnosus gg–host interactions. Microb Cell Fact. 2014;29(13 Suppl 1):S7. doi:10.1186/1475-2859-13-S1-S7.
- Thomas L, Adam B. Molecular mechanisms of Lacticaseibacillus rhamnosus, LGG® probiotic function. Microorganisms. 2024;12(4):12. doi: 10.3390/microorganisms12040794
- Simpson M, Petri W. TLR2 as a therapeutic target in bacterial infection. Trends Mol Med. 2020;26(8):715–717. doi: 10.1016/j.molmed.2020.05.006
- Chenfeng J, Ziyi Z, Jinrui C, et al. Immune-enhancing effects of a novel Glucan from purple sweet potato ipomoea batatas (L.) Lam on RAW264.7 macrophage cells via TLR2- and TLR4-mediated pathways. J Agric Food Chem. 2021;69(32):9313–9325. doi: 10.1021/acs.jafc.1c03850
- Margarida C-N, Jérôme N, Zaynab M, et al. Immunological hyporesponsiveness in tuberculosis: the role of mycobacterial glycolipids. Front Immunol. 2022;13. doi: 10.3389/fimmu.2022.1035122
- Weaver Jr DJ, Reis ES, Pandey MK, et al. C5a receptor-deficient dendritic cells promote induction of treg and Th17 cells. Eur J Immunol. 2009;40(3):710–721. doi: 10.1002/eji.200939333
- Bielecki P, Riesenfeld SJ, Hütter JC, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature. 2021;592(7852):128–132. doi: 10.1038/s41586-021-03188-w
- Wenjing Y, Tianming Y, Xiangsheng H, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat Commun. 2020;11(1):11. doi: 10.1038/s41467-020-18262-6
- Makowski L, Chaib M, Rathmell J. Immunometabolism: from basic mechanisms to translation. Immunol Rev. 2020;295(1):5–14. doi: 10.1111/imr.12858
- Hou Q, Ye L, Liu H, et al. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death Differ. 2018;25(9):1657–1670. doi: 10.1038/s41418-018-0070-2
- Zenewicz L, Yancopoulos G, Valenzuela D, et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–957. doi: 10.1016/j.immuni.2008.11.003
- Abraham Majak G, Todor V, Thomas Y, et al. Salmonella infection – prevention and treatment by antibiotics and probiotic yeasts: a review. Microbiol (Read). 2018;164(11):1327–1344. doi: 10.1099/mic.0.000709
- Fu-Chen H. The role of sphingolipids on innate immunity to intestinal salmonella infection. IJMS. 2017;18(8):18. doi: 10.3390/ijms18081720
- Rooks M, Garrett W. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–352. doi: 10.1038/nri.2016.42
- Ansaldo E, Farley T, Belkaid Y. Control of immunity by the microbiota. Annu Rev Immunol. 2021;39(1):449–479. doi: 10.1146/annurev-immunol-093019-112348.