Abstract
This study analysed the impact of the Malayan Uniform management System (MUS) on liana abundance and their relationship with trees in the Bukit Panchor Forest Reserve, Malaysia. Two types of MUS-treated forest, medium-term MUS (M-MUS) and long-term MUS (L-MUS), were identified based on the management time span (19 and 42 years, respectively). Trees with diameter at breast height ≥ 10 cm were identified and examined for liana presence (diameter ≥ 2 cm) in ten 40 m × 40 m plots within each forest. Tree seedling and sapling abundance were also determined in a randomly selected 20 m × 20 m subplot within each plot. Liana abundance and infestation rates were significantly higher in the untreated forest than under the M-MUS, although they did not differ from those under the L-MUS. Tree diameter related significantly with liana load and basal area in all the forests except the M-MUS, which contained relatively more uniform diameter trees. Reduction in liana abundance and infestation in the treated forests favoured natural regeneration of the trees. Liana cutting was effective in reducing liana abundance and infestation in the medium term but not in the long term. Therefore, further liana cutting in the long term may be needed to control lianas.
Introduction
Lianas play an important role in forest dynamics and functioning (Laurence et al. Citation2001). They can contribute up to 38% of plant species diversity in the tropics (Addo-Fordjour et al. Citation2008). Lianas provide food for many animals in the forest, especially in the dry season when most trees are unable to perform that function (Bongers et al. Citation2005). They also help to close the canopy after tree fall, thereby helping to stabilise the microclimate beneath the forest (Schnitzer and Bongers Citation2002). Although lianas are important in the forest ecosystem, they can have an adverse impact on growth and productivity of tropical forest trees (Laurence et al. Citation2001). They also reduce the value of trees as timbers by causing their bending (cf. Bongers et al. Citation2005). Lianas could, for instance, suppress natural regeneration of trees when they become highly abundant (Schnitzer et al. Citation2000). Consequently, lianas are usually cut in managed forests as a form of a silvicultural treatment (Pérez-Salicrup Citation2001; Alvira et al. Citation2004; Addo-Fordjour et al. Citation2009a).
Silvicultural practices incorporating logging and liana cutting tend to open the forest canopy to promote regeneration and growth of trees (cf. Foli and Pinard Citation2009). In so doing, lianas capitalise on the canopy opening and colonise the disturbed areas in large numbers, and then grow rapidly in the high-resource environment (Schnitzer et al. Citation2000; Rutishauser Citation2011; Schnitzer and Bongers Citation2011). For this reason, silvicultural interventions could affect liana abundance as well as their relationships with trees by causing changes in liana and tree abundance. For instance, it has been reported that lianas are able to resprout to increase in numbers to pre-treatment levels 40 years after post-logging liana cutting (cf. Alvira et al. Citation2004). Silvicultural interventions adopted in a tropical forest in Ghana were reported to have contributed to a lower liana infestation rate in treated stands compared with untreated stands 40 years after application (Foli and Pinard Citation2009). Despite the potential impact of silvicultural treatments in influencing liana dynamics in tropical forest ecosystems, only a limited number of studies have been conducted to investigate the impact of post-logging liana cutting on liana abundance and their relationships with trees. To date only one study has thoroughly dealt with this subject (Addo-Fordjour et al. Citation2009a). Consequently, information on the types of liana–tree relationships following logging and liana cutting activities is limited. Since most silvicultural interventions are aimed at reducing effects of lianas on trees, it is important for studies to be conducted to examine both the medium- and long-term effects of such interventions on liana–tree relationships. At present, only a few studies have considered the influence of time span of silvicultural interventions on post-logging liana communities (cf. Alvira et al. Citation2004; Foli and Pinard Citation2009). To fully understand the post-logging liana community dynamics, not only should the short-term impact of silvicultural treatments on lianas be studied but also the medium- to long-term impacts must be considered. Ecological information on how lianas relate with trees in different management regimes would not only be useful in assessing the impact of silvicultural treatments on lianas but also in examining the impact of lianas on trees.
The Bukit Panchor Forest Reserve has undergone silvicultural treatments (mainly involving logging and liana cutting) for many years. However, there is no information on the impacts of the silvicultural treatments on liana abundance and relationships with trees. Furthermore, there is no information on the medium- and long-term impacts of the operations on tree regeneration in the forest. This study, therefore, sought to determine the impacts of two management regimes (a post-logging liana cutting treatment, the Malayan Uniform System (MUS), and a protected primary forest) in the Bukit Panchor Forest Reserve on liana abundance and relationships with trees. A primary forest that was not treated was used as a control. The following research questions were addressed: (1) What changes occur in liana abundance and relationships with trees under different management regimes? (2) Does the time span of a silvicultural treatment influence liana abundance and relationships with trees? (3) Do the changes in liana communities in the different management regimes influence tree regeneration?
Methodology
Study area
This study was conducted in the Bukit Panchor Forest Reserve in the Penang State, Malaysia (N 5° 09.631′ E 100° 32.889′) () from December 2011 to February 2012. The reserve, which is a lowland dipterocarp forest, is covered with natural forest plants such as Shorea parvilora, S. curtisii, S. leprosula, Hopea spp., and many other dipterocarp species. Part of the forest reserve, about 8 ha, has been developed into a recreational park. The average temperature of the study region is 33°C, and the average annual rainfall is 2670 mm. Relative humidity for the area is 70–90%.
Site selection and sampling
Two different management regimes, namely the MUS and an untreated protected primary forest, were selected for this study. Management regime plots with more or less similar topography were selected for this study so as to eliminate its effects in this study. Two types of MUS-treated forest, medium-term MUS (M-MUS) and long-term MUS (L-MUS), were identified. Both types of forest underwent the same treatment, but they differ in time span (19 and 42 years for the M-MUS and L-MUS, respectively). In both forests, all trees with diameter at breast height (dbh) ≥45 cm were cut down. This was followed by poison girdling of all defective and non-commercial trees, climber cutting and enrichment planting 5 years later (Appanah and Weinland Citation1993). A second management regime, an untreated protected primary forest, which did not undergo any form of silvicultural treatment, was added in order to determine the extent of liana recovery since the application of the silvicultural treatments in the other regimes. Data were collected from two sites in each forest regime. Each of the sites in the M-MUS and L-MUS covered a total area of about 5 ha, whereas each of the untreated primary forest sites spanned an area of about 10 ha.
In each of the sites, five plots (40 m × 40 m) were randomly demarcated, given a total of 10 plots (1.6 ha) in each forest. Within each plot, lianas with diameter ≥2 cm, which were located on trees with diameter ≥10 cm, were identified and counted. The positions of lianas on trees were recorded. Tree species that hosted lianas were identified and enumerated. In addition, all trees (diameter ≥ 10 cm) that did not host lianas were counted. The bark characteristic of each tree was recorded and ranked as follows: smooth = 1, rough = 2 and very rough = 3. The degree of liana infestation was categorised by modifying the classification of Alder and Synott (Citation1992): lianas on trunk only = 1, lianas on crown only = 2, lianas on trunk and crown = 3 and lianas completely covering the entire tree = 4. Each plot was subdivided into four 20 m × 20 m subplots, out of which one was randomly selected for the determination of tree seedling and sapling abundance. Seedlings and saplings were categorised as follows (Saenz et al. Citation1999): seedlings <1.5 m with no measured dbh and sapling >1.5 m height and <10 cm dbh.
Plant identification was carried out on the field using plant features, manuals and Floras (Dransfield Citation1979; Keng and Keng Citation1990; Zhengyi et al. Citation2010), and with the assistance of plant taxonomists. Nomenclature followed King (Citation1902), Dransfield (Citation1979) and Keng and Keng (Citation1990). Voucher specimens were kept at the herbarium of the School of Biological Sciences, Universiti Sains Malaysia, Penang, Malaysia.
Data analyses
Differences in liana, seedling and sapling abundance between forests were analysed with a one-way ANOVA. Liana infestation rate per plot was also compared between the forests with ANOVA. Fisher's LSD pair-wise comparison tests were used to determine differences of means amongst forest types. The data used for the ANOVA met normality and homogeneity of variance assumptions. Spearman's correlation analysis was used to determine the relationships between the following tree and liana parameters in each forest: (1) tree diameter and degree of liana infestation of trees according to the modified classification of Alder and Synott (Citation1992), (2) tree diameter and liana load, (3) tree diameter and liana basal area and (4) bark characteristics and liana load. Spearman's correlation was used for the above analysis because the data were not normally distributed. All analyses were conducted with the XLSTAT 2011.2 version (Addinsoft SARL, Paris, France) at a significant level of 5%.
Results
Liana abundance
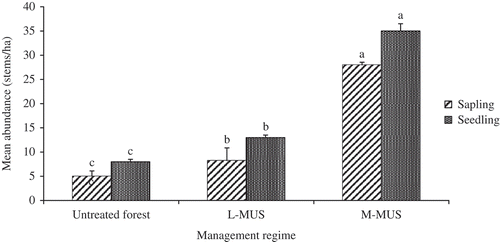
A total of 503 liana stems (105 stems/ha) belonging to 45 species, 27 genera and 15 families were identified in this study (). Liana abundance per plot was the same in the L-MUS and untreated forest, but both differed significantly from that of the M-MUS (; p < 0.001).
Table 1. Abundance of liana species in the medium-term Malayan Uniform System (M-MUS), long-term Malayan Uniform System (L-MUS) and untreated forest (UF)
Figure 2. Mean liana abundance per plot in the medium-term Malayan Uniform System (M-MUS), long-term Malayan Uniform System (L-MUS) and untreated forest.
Uncaria sclerophylla was the only species that was highly abundant in all the forests (). The five most abundant species in the M-MUS (U. sclerophylla, Spatholobus sp., Mitrella kentii, Tetracera indica and Artabotrys oblongus) constituted about 55% of the total liana stems. In the L-MUS, the five most abundant species (U. sclerophylla, A. oblongus, M. kentii, T. indica and A. macrophylla) accounted for 55% of the total liana stems. In the case of the untreated forest, the five most abundant species (U. sclerophylla, A. borneensis, T. petiolare, B. bidentata and D. rostrata) contributed 50% to the total number of liana stems.
Liana relationships with trees
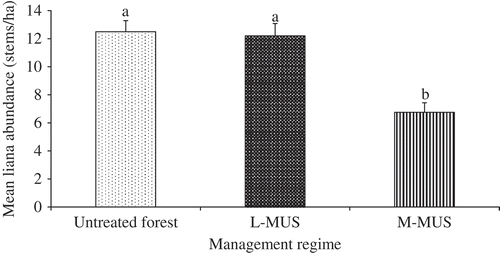
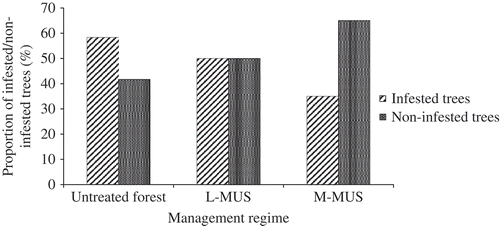
The rate of infestation in the untreated primary forest was higher than those of the M-MUS and L-MUS. Proportion of non-infested trees was lowest in the M-MUS (). There was no significant difference between liana infestation rate per plot in the L-MUS and untreated forest, although both differed significantly from that of the M-MUS (p < 0.001). There were more tree species in the untreated forest (34 species) that were highly infested by lianas (>0.5 infestation rate) compared to the M-MUS and L-MUS (24 and 20 species, respectively). The mean liana load per infested tree was comparable in the L-MUS and untreated forest, both of which were higher than that of the M-MUS ().
Table 2. Liana load and infestation on tree species in the medium-term Malayan Uniform System (M-MUS), long-term Malayan Uniform System (L-MUS) and untreated forest (UF) stages
Figure 3. Rate of liana infestation of trees in the medium-term Malayan Uniform System (M-MUS), long-term Malayan Uniform System (L-MUS) and untreated forest.
There was a significant positive relationship between tree diameter and liana load in the L-MUS (p < 0.001, r = 0.413) and untreated forest (p < 0.001, r = 0.429). However, there was no evidence to suggest such a relationship in the M-MUS (p = 0.346). Likewise, liana basal area correlated significantly with tree diameter in the L-MUS and untreated forest (p = 0.035, r = 0.201 and p < 0.001, r = 0.340, respectively), but there was no significant relationship between liana basal area and tree diameter in the M-MUS (p = 0.160). The bark characteristics of lianas did not bear significant relationship with liana load in all the forests (p = 0.533, p = 0.095 and p = 0.216 in M-MUS, L-MUS and untreated forest, respectively). There was a significant positive relationship between tree diameter and degree of liana colonisation in all the forests (p < 0.001, r = 0.42; p < 0.001, r = 0.303 and p < 0.001, r = 0.366 in M-MUS, L-MUS and untreated forest, respectively). Both tree seedling and sapling abundance differed significantly between the forests (; p < 0.001).
Discussion
Liana abundance
Generally, liana abundance in this study was relatively lower compared to other tropical forest ecosystems. For example, in the Amazonian forest, lianas were about 24 times more abundant (2471 stems/ha) than that recorded in this study (Pérez-Salicrup et al. Citation2001). In a Congolian forest (Ewango Citation2010) and neotropical forests (DeWalt and Chave Citation2004), liana abundance was six and five times (677 and 605 stems/ha, respectively) higher than that of this study.
The findings of this study indicated that liana abundance was significantly lower in the M-MUS compared to the untreated forest. This suggests that the impact of the MUS in the medium term was still noticeable. In spite of the fact that the removal of trees from the M-MUS created tree fall gaps favourable for liana multiplication, limited availability of supports probably imposed constraint on their initial regeneration and subsequent proliferation. This is buttressed by several studies which have cited the availability of support as a limiting factor in liana increase (DeWalt et al. Citation2000; Addo-Fordjour et al. Citation2008, Citation2009a, Citation2009b). This finding gives credence to the observation that forest disturbance may not necessarily cause an increase in liana abundance when it either removes lianas and/or host trees (Addo-Fordjour et al. Citation2008, Citation2009a; Rahman et al. Citation2010). However, lianas might increase in abundance in the M-MUS with time as they continue to recruit many trees. After 42 years of post-logging liana cutting treatment, liana abundance in the L-MUS was comparable with that of the untreated forest. Similar findings were reported elsewhere in which liana densities remained the same between silviculturally treated and untreated forests 40 years after post-logging liana cutting (cf. Alvira et al. Citation2004; Foli and Pinard Citation2009). This suggests that where treatment is not repeated after a short or medium term, liana numbers might increase to pre-treatment level in treated forests in the long term. The recovery ability of lianas in the L-MUS might have been facilitated by a balanced availability of support and light years after regeneration and growth of lianas (Madeira et al. Citation2009). Judging from the increase in tree abundance in the M-MUS, it is reasonable to state that, given enough time, lianas may recover to initial treatment level abundance as observed in the L-MUS in this study as well as in other studies (cf. Alvira et al. Citation2004; Foli and Pinard Citation2009). There is therefore the need for periodic monitoring of liana dynamics in the M-MUS to ensure that they do not increase to levels that might have an adverse impact on the forest.
Some liana species responded differently to the different management regimes with respect to abundance. Whereas, species such as A. borneensis and B. bidentata showed highest abundance in the M-MUS and lowest abundance in the untreated forest, Strophanthus sp. showed a reverse trend. There were also a number of species whose abundance peaked at the L-MUS, probably due to the presence of an optimal balance in supports (after years of recruitment and growth of trees) and light. The five most abundant species in all the forests accounted for at least half of the liana stems in this study, indicating that most of the liana species occurred in low numbers. Interestingly, the M-MUS and the untreated forest had four species in common with regards to the five most abundant species, suggesting that these species were able to proliferate in both open and closed forests.
Liana relationships with trees
The general rate of liana infestation in the Bukit Panchor Forest Reserve (48%) was relatively lower than what has been reported in other tropical forest ecosystems (Pérez-Salicrup et al. Citation2001; Addo-Fordjour et al. Citation2009a, Citation2009b). The rate of liana infestation of trees per plot was significantly less severe in the M-MUS in comparison with the untreated forest. Following post-logging liana cutting, the development of regenerating lianas in the M-MUS might have been limited by inadequate number of trees supporting them (Foli and Pinard Citation2009). At the species level, more trees were severely infested in the untreated forest in relation to the two treated forests. The removal of most trees and lianas in the treated forests could be responsible for this trend as reported in other studies (e.g. Addo-Fordjour et al. Citation2009a). The medium- and long-term impacts of MUS on the relationships between liana diameter and liana load and basal area differed. The significant relationship that existed between tree diameter and liana load in the L-MUS and the untreated forest was absent in the M-MUS. One of the silvicultural activities practiced was the removal of large diameter trees from the M-MUS, leaving trees of more or less uniform diameter. This obviously influenced the relationship between tree diameter and liana load in the M-MUS. The ability of the L-MUS to recruit many large diameter trees in the long term may explain why tree diameter correlated significantly with liana load despite undergoing the same treatment as the M-MUS. Similarly, tree diameter bore significant relationship with liana basal area in both the L-MUS and the untreated forest, but this was absent in the M-MUS. This relationship has been related to long exposure of large diameter trees to lianas than small diameter trees (Pérez-Salicrup et al. Citation2001; Pérez-Salicrup and de Meijere Citation2005). Therefore, the absence of significant relationship between tree diameter and liana basal area in the M-MUS may be linked to less exposure time of trees to lianas. Bark characteristics of trees did not influence liana load on trees in any of the forests. This is supported by the finding that bark characteristics may not play a significant role in liana invasion as the evolution of different climbing modes by lianas enable them to climb successfully on any kind of bark surface (cf. Kammesheidt Citation1999).
Implication for forest management and conservation
The application of the post-logging liana cutting in the medium term was effective as liana abundance and infestation rates in the M-MUS were significantly lower than those of the untreated forest. A similar silvicultural treatment practiced in the Bobiri Forest Reserve in Ghana yielded similar results (Foli and Pinard Citation2009). However, lianas were able to recover to numbers comparable with that of the untreated forest in the long term. Thus, the success of liana cutting may be limited in the long term as was also observed in other studies (cf. Alvira et al. Citation2004; Foli and Pinard Citation2009). This calls for regular monitoring of liana dynamics in the long term so as to control aggressive liana proliferation that may arise. That is, further liana cuttings should be implemented when necessary in order to prevent liana numbers from exploding.
One of the negative effects of lianas on the forest ecosystem is their ability to impede tree regeneration as reported in some studies (e.g. Schnitzer et al. Citation2004). The patterns of natural regeneration of trees observed in this study appear to support the above assertion. The abundance of both seedlings and saplings differed significantly between the forests, being highest in the M-MUS and lowest in the untreated forest. It must be noted that seedlings used to stock the M-MUS and the L-MUS did not form part of the regeneration flora enumerated in this study since they were recruited into the adult stage after many years. The superior natural regeneration of trees in the treated forests compared with the untreated forest was probably due to the lower liana abundance and infestation resulting in lower competition between trees and lianas (Schnitzer et al. Citation2005). Additionally, lower liana abundance probably reduced the number of seed predators of trees which depended on lianas in the treated forests (Kilgore et al. Citation2010), thereby favouring seed germination. The high infestation of trees in the untreated forest could have further impeded their ability to produce adequate number of seeds (cf. Bongers et al. Citation2005).
While the impacts of post-logging liana cutting on liana abundance and infestations and tree regeneration in this study are clear, their effects on animals in the forest are unknown. However, based on the current knowledge of importance of lianas to animals (cf. Bongers et al. Citation2005), it is reasonable to suggest that the silvicultural treatment probably affected animal diversity in the treated forests. The undesirable effects of post-logging liana cutting on forest community could be reduced by avoiding wholesale cutting of lianas in forest management operations. It is suggested that selective liana cutting be adopted so as to control liana numbers while at the same time preserving biodiversity of the forest.
Conclusion
Liana abundance and rate of infestation were significantly lower in the M-MUS compared with the untreated forest, indicating that the impact of post-logging liana cutting in the medium term was still evident. However, liana abundance and infestation were comparable in the L-MUS and the untreated forest, implying that lianas could recover to pre-treatment abundance and infestation levels in the long term. Tree diameter related significantly with liana load and basal area in all the forests, except for the M-MUS due to the removal of large diameter trees in it leaving tress of more or less uniform diameter. The degree of liana colonisation bore a significant positive relationship with tree diameter in all management regimes, because the rate of liana colonisation increases with tree diameter. Both tree seedling and sapling abundance were significantly higher in the treated forests, suggesting that liana cutting was an effective tool in not only controlling lianas but also enhancing natural regeneration. Generally, liana abundance and relationships with trees differed between the M-MUS and L-MUS, reflecting differences in medium- and long-term impacts of the silvicultural treatment on lianas. Post-logging liana cutting was effective in reducing liana abundance and infestation in the medium term but not in the long term. Therefore, further liana cutting in the long term may be needed to control lianas. Considering the significant role of lianas in forests, it is proposed that selective liana cutting be adopted to control liana numbers while at the same time preserving forest biodiversity.
Acknowledgements
This study was supported by TWAS-USM Postgraduate Fellowship and Research University Grant (RU) (1001/PBIOLOGI/815046). Our special thanks go to Mr. Abu Husin of the Forest Research Institute Malaysia, and Mr. Ntim Gyakari of the Forestry Commission of Ghana for the identification of plant species. We would like to express our heartfelt gratitude to Mr. S.M. Edzham of the School of Biological Sciences, USM, Malaysia, for his immense assistance on the field.
Notes
References
- Addo-Fordjour , P , Anning , AK , Atakora , EA and Agyei , PS. 2008 . Diversity and distribution of climbing plants in a semi-deciduous rain forest, KNUST Botanic Garden, Ghana . Int J Bot. , 4 ( 2 ) : 186 – 195 .
- Addo-Fordjour , P , Anning , AK , Larbi , JA and Akyeampong , S. 2009a . Liana species richness, abundance and relationship with trees in the Bobiri forest reserve, Ghana: impact of management systems . For Ecol Manage. , 157 ( 8 ) : 1822 – 1828 .
- Addo-Fordjour , P , Obeng , S , Addo , MG and Akyeampong , S. 2009b . Effects of human disturbances and plant invasion on liana community structure and relationship with trees in the Tinte Bepo forest reserve, Ghana . For Ecol Manage. , 258 ( 5 ) : 728 – 734 .
- Alder , D and Synott , JT. 1992 . Permanent sample plot techniques for mixed tropical forests , Oxford , , UK : Oxford Forestry Institute . Tropical Forestry Paper No. 25.
- Alvira , D , Putz , FE and Fredericksen , TS. 2004 . Liana loads and post-logging liana densities after liana cutting in a lowland forest in Bolivia . For Ecol Manage. , 190 ( 1 ) : 73 – 86 .
- Appanah , S and Weinland , G. 1993 . Will the management system for hill dipterocarp forests, stand up? . J Trop For Sci. , 3 ( 2 ) : 140 – 158 .
- Bongers , F , Parren , MPE , Swaine , MD and Traoré , D. 2005 . “ Forest climbing plants of West Africa: introduction ” . In Forest climbing plants of West Africa: diversity, ecology and management , Edited by: Bongers , F , Parren , MPE and Traoré , D . 5 – 18 . Oxfordshire , , UK : CAB International .
- DeWalt , SJ and Chave , J. 2004 . Structure and biomass of four lowland neotropical forests . Biotropica. , 36 ( 1 ) : 7 – 19 .
- DeWalt , SJ , Schnitzer , SA and Denslow , JS. 2000 . Density and diversity of lianas along a chronosequence in a central Panamanian lowland forest . J Trop Ecol. , 16 ( 1 ) : 1 – 19 .
- Dransfield , J. 1979 . A manual of the rattans of the Malay Peninsula , Kepong , , Malaysia : Forest Research Institute of Malaysia . Malaysian Forest Records. No. 29.
- Ewango , CEN . 2010 . The liana assemblage of a Congolian rainforest: diversity, structure and dynamics [PhD thesis] , Wageningen , , The Netherlands : Wageningen University .
- Foli , EG and Pinard , MA. 2009 . Liana distribution and abundance in moist tropical forest in Ghana 40 years following silvicultural interventions . Ghana J For. , 25 : 1 – 12 .
- Kammesheidt , L. 1999 . Liana infestation of trees: some observations in a neotropical lowland forest . Ecotropica. , 5 ( 1 ) : 217 – 220 .
- Keng , H and Keng , R-SL. 1990 . The concise flora of Singapore: gymnosperms and dicotyledons. Vol. 2 , Singapore : Singapore University Press, Kent Ridge .
- Kilgore , A , Lambert , TD and Adler , GH. 2010 . Lianas influence fruit and seed use by rodents in a tropical forest . Trop Ecol. , 51 ( 2 ) : 265 – 271 .
- King , G. 1902 . Materials for a flora of the Malayan Peninsula , Calcutta , West Bengal : Royal Botanic Garden .
- Laurence , WF , Pérez-Salicrup , D , Delamônica , P , Feamside , P , Sammya , DA , Jerozolinski , A , Pohl , L and Lovejoy , TE. 2001 . Rain forest fragmentation and the structure of Amazonian liana communities . Ecology. , 82 ( 1 ) : 105 – 116 .
- Madeira , BG , Espírito-Santo , MM , Neto , SD , Nunes , Y , Sánchez Azofeifa , GA , Fernandes , GW and Quesada , M. 2009 . Changes in tree and liana communities along successional gradient in a tropical dry forest in south-eastern Brazil . Plant Ecol. , 201 ( 1 ) : 291 – 304 .
- Pérez-Salicrup , DR. 2001 . Effect of liana cutting on tree regeneration in a liana forest in Amazonian Bolivia . Ecology. , 82 ( 2 ) : 389 – 396 .
- Pérez-Salicrup , DR and de Meijere , W. 2005 . Number of lianas per tree and number of trees climbed by lianas at Los Tuxtlas, Mexico . Biotropica. , 37 ( 1 ) : 153 – 156 .
- Pérez-Salicrup , DR , Sork , VL and Putz , FE. 2001 . Lianas and trees in a liana forest of Amazonian Bolivia . Biotropica. , 33 ( 1 ) : 34 – 47 .
- Rahman , MM , Begum , F , Nishat , A , Islam , KK and Vacik , H. 2010 . Species richness of climbers in natural and successional stands of Madhupur Sal (Shorea robusta C.F. Gaertn) forest, Bangladesh . Trop Subtrop Agroecosys. , 12 ( 1 ) : 117 – 122 .
- Rutishauser , SE . 2011 . Increasing liana abundance and biomass in tropical forests: testing mechanistic explanations [MS thesis] , Milwaukee , WI : University of Wisconsin – Milwaukee .
- Saenz , G , Finegan , B and Guariguata , M. 1999 . Sapling growth and mortality of seven tree species in an altered very moist tropical forest, Costa Rica . Rev Biol Trop. , 47 ( 1–2 ) : 45 – 57 .
- Schnitzer , SA and Bongers , F. 2002 . The ecology of lianas and their role in forests . Trends Ecol Evol. , 17 ( 5 ) : 223 – 230 .
- Schnitzer , SA and Bongers , F. 2011 . Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms . Ecol Lett. , 14 ( 4 ) : 397 – 406 .
- Schnitzer , SA , Dalling , JW and Carson , WP. 2000 . The impact of lianas on tree regeneration in tropical forest canopy gaps: evidence for an alternative pathway of gap-phase regeneration . J Ecol. , 88 ( 4 ) : 655 – 666 .
- Schnitzer , SA , Kuzee , ME and Bongers , F. 2005 . Disentangling above- and below-ground competition between lianas and trees in a tropical forest . J Ecol. , 93 ( 6 ) : 1115 – 1125 .
- Schnitzer , SA , Parren , MPE and Bongers , F. 2004 . Recruitment of lianas into logging gaps and the effects of pre-harvest climber cutting in a lowland forest in Cameroon . For Ecol Manage. , 190 ( 1 ) : 87 – 98 .
- Zhengyi , W , Raven , PH and Deyuan , H. 2010 . Flora of China. Vol. 10. Fabaceae , 121 St. Louis , MO : Botanical Garden Press .