ABSTRACT
This commentary addresses the role of P-cadherin in collective cell migration (CCM), a cooperative and coordinated migration mode, used by cells during normal and pathological migration processes. We discuss how cadherin-mediated cell-cell junctions (CCJs) play a critical role in CCM through their ability to regulate Rho GTPase-dependent pathways and how this leads to the generation and orientation of mechanical forces. We will also highlight the key function of P-cadherin (a poor prognostic marker in several tumors) in promoting collective cell movement in epithelial and mesenchymal cells.
Introduction
Tissue modeling, repair, wound healing, cell invasion and metastasis formation require CCM.Citation14 This process, whereby each single cell within a group communicates with its neighbors, allows the orchestrated movement of cells toward specific locations. To achieve this, cells need to form a cohesive tissue through physical interactions and force transmission between cells and the surrounding substrates. Adhesion and force transmission between cells is mediated by proteins of the cadherin superfamily, whereas adhesion and force transmission to the extracellular matrix components is mediated via integrin receptors.
Cancers of epithelial (carcinomas) and mesenchymal (sarcomas) origin use CCM to invade surrounding tissues. Indeed, rhabdomyosarcoma (a tumor of muscle origin), breast cancer, colorectal carcinoma and melanoma show collective cell behavior.Citation13,17,24 Most of the mortality associated with solid tumors is the final result of CCM (i.e., tumor cell invasion and metastasis formation). Therefore, targeting the biomechanical pathways leading to CCM might be an efficient therapeutic strategy to block cancer progression.
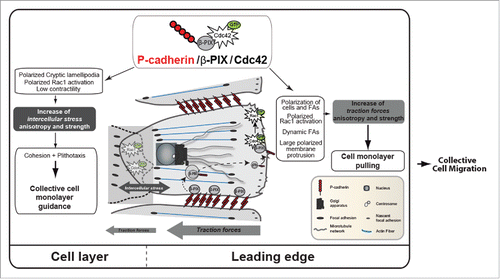
During the last decade, efforts have been made to understand the molecular and physical mechanisms involved in CCM. However, to fully capture CCM biomechanical mechanisms is challenging due to the complexity of the biological systems. Indeed, collectively migrating cells during embryo development or tumor invasion are heterogeneous and complex structures that are the result of the crosstalk between different cell types and their surrounding environment. To efficiently tackle all the complex biological questions concerning CCM, robust and reproducible in vivo and in vitro experimental models have been developed. For instance, lateral line migration during zebrafish development, neural crest migration during chicken neurogenesis and border cell migration in the fly egg chamber are good in vivo models for understanding CCM mechanism. On the other hand, in vitro models (i.e., organotypic culture systems, wound healing-like and 3-dimensional invasion assays) allow the fast study of specific aspects of the underlying molecular processes and the high resolution imaging of specific cellular events. We took advantage of a reproducible in vitro directional migration assay in which cells are allowed to migrate in the direction perpendicular to the free edges after removal of a physical barrier to study the biomechanical pathways leading to CCM upon P-cadherin expression. We performed quantitative analysis of cell movement, cell organization and mechanical parameters using time-lapse, confocal and Föster resonance energy transfer imaging and traction force microscopy. Our study shows that P-cadherin specifically induces CCM when expressed in myoblasts. We then demonstrated that P-cadherin recruits the guanine exchange factor (GEF) β-PIX that allows Cdc42 activation. This signaling cascade leads to massive reorganizations, from the polarization of cells, membrane protrusions and focal adhesions (FA) to the global collective movement of the entire cell monolayer. Mechanically, the P-cadherin/β-PIX/Cdc42 axis drives CCM by increasing the intercellular stress through a physical process called plithotaxis and promotes the strength and orientation of traction forces in the migration directionCitation39,44 ().
Figure 1. P-cadherin expression induces CCM. P-cadherin expression promotes a mechanical tug-of-war. Indeed, P-cadherin expression is associated with increased intercellular stress anisotropy and strength that promote collective cell guidance, called plithotaxis. P-cadherin expression also increases traction-force anisotropy (by increasing the Tx/Ty ratio that is the ratio between the traction forces parallel to the direction of migration (Tx) and the traction forces perpendicular to the direction of migration (Ty)) and strength that pull the cell layer. P-cadherin expression activates CDC42 through the GEF β-PIX. This generates biological responses, such as polarization of the cell layer, of RAC1 activity, cryptic lamellipodia and FAs in the migration direction, polarized membrane protrusions and FA dynamics, thereby controlling mechanical force anisotropy and strength.
Cadherins in CCM of epithelial and mesenchymal cells
Cadherins are a central CCJ component and major CCM drivers.Citation16 There are 5 main type-1 classical cadherins in mammals: E, M, N, P and R-cadherin. E, N and P-cadherin have been involved in CCM in different models, whereas R-cadherin and M-cadherin do not seem to contribute to CCM. However, we can easily imagine that depending on the cell system, the cadherin type involved in CCM could be different. The tissue anatomy and peripheral microenvironment geometry also could influence the cadherin type involved in CCM. For instance, N-cadherin regulates CCM of MDCK cells in 3D, but not in 2D environments.Citation37 CCM is observed in both epithelial and mesenchymal cells, but the involved cadherins are different. Specifically, E-cadherin plays a role exclusively in CCM of epithelial cells, while N-cadherin regulates CCM of mesenchymal cells. On the other hand, P-cadherin is involved in CCM of both cell types.Citation2,25,33
CCM of epithelial cells
A specific feature of epithelial cells is that they maintain stable CCJ during CCM, as observed during carcinoma ductal invasion through E- and P-cadherin,Citation12,39 or during angiogenesis and tubular ramification through VE-cadherin (a type-2 cadherin).Citation32 In most of these CCM models, epithelial cells show highly directional movement and E-cadherin inhibition increases randomness. For instance, in vivo studies on D. melanogaster border cell migration in the ovary have demonstrated that collective movement, cell cohesion, directionality and mechanical sensing are controlled through E-cadherin engagement.Citation5,34 For years, E-cadherin was considered to be the main cadherin involved in CCM of epithelial cells. However, recently P-cadherin emerged as an additional key player. P-cadherin depletion in epithelial cells impairs drastically CCM in both 2D and 3D in vitro culture systems.Citation25,26 Our collaborators Bazellières and colleagues showed that during CCM of epithelial cells, “while P-cadherin predict the level of intercellular force, E-cadherin predicts the rate at which intercellular force builds up,” suggesting, for the first time, a real mechanical function of P-cadherin in force transmission within the cell monolayer.Citation2
CCM of mesenchymal cells
CCM of mesenchymal cells also requires cadherin-mediated CCJs.Citation41 Mesenchymal cells that originate from epithelial to mesenchymal transitions during development or tumor progression undergo cadherin switching from E- to N-cadherin or to other cadherin types.Citation42,48 This cadherin switch is associated with increased cell migration and invasion. P-cadherin is up-regulated in invasive alveolar rhabdomyosarcoma (a tumor of mesenchymal origin). When expressed in myoblasts, P-cadherin promotes a cadherin switch, inducing their transformation, migration and invasion.Citation43 By examining in details the migration parameters of P-cadherin-expressing myoblasts we found that P-cadherin promotes CCM very efficiently.Citation33
CCM of mesenchymal cells is associated with highly dynamic CCJs, as recently shown for N-cadherin during astrocyte collective migration.Citation31 In P-cadherin-expressing myoblasts, cells within the monolayer maintain low cell-cell interaction by forming cryptic lamellipodia, membrane extensions seen in front of cells that migrate collectively and extending underneath the cell behind. Similarly, some studies showed that cryptic lamellipodia formation is essential for collective movement of epithelial cells.Citation8,11 This means that in epithelial and mesenchymal cells, dynamic CCJs are required for efficient CCM. Indeed, cells migrating as a collective tissue move more continuously and persistently.Citation49 Moreover, CCM is associated with increased polarity, which is a key process in CCM.Citation7,30 Cadherins are considered to be important regulators of polarity. In astrocytes, N-cadherin-mediated CCJs directly regulate the polarity pathways, leading to FA organization at the leading edge of migrating cells and strengthening the persistence and orientation of the migrating cell monolayer.Citation6,10 We found that P-cadherin (but not E- or R-cadherin) expression in myoblasts induces a strong polarization of cells and their trajectories, of their membrane protrusions and cryptic lamellipodia at the front of migrating cells across the layer, and of their FAs at the leading edge and in the monolayer.Citation33
Cadherin-dependent mechanical coupling
CCM occurs through the generation and transmission of physical forces that drive cellular movement. Two different force types are important in CCM: traction forces that are exerted on the cell substrate through FA modulesCitation9,45 and intercellular forces transmitted between neighboring cells through CCJs.Citation21,22,36,39,45,46 In the last years, several groups have been working on the identification of the molecular mechanisms that govern force transmission between cells, together with the activation of contractility and the crosstalk to cell substrate adhesion molecules.Citation2,5,25,47 These studies highlight the key function of different proteins, particularly cadherins and integrins, in force transmission.
Cadherin role in inter-cellular force generation and transmission at CCJs
Cadherins, particularly E- and N-cadherin, regulate the physical properties of cell monolayers by transducing mechanical forces between the actomyosin cytoskeleton and the plasma membrane of neighboring cells.Citation3,5,20 Bazellières et al have shown that during CCM of epithelial cells, P-cadherin allows force transmission through the entire cell monolayer.Citation2 By using magnetic tweezers, they could confirm that E-cadherin and P-cadherin, when co-expressed, play different mechanical roles. E-cadherin strengthens cell adhesion and P-cadherin regulates the amount of tension that a FA can transmit. Furthermore, they demonstrated that when E-cadherin is knocked down, P-cadherin can take over its role as a tension regulator. In myoblasts, only P-cadherin expression increases the intercellular stresses within the cell monolayer, while E- and R-cadherin co-expression does not.Citation33 All these data clearly indicate that E-cadherin is not the only cadherin involved in the regulation of intercellular tension, and that P-cadherin also should be taken into account to understand how forces are generated and transmitted within a cell monolayer.
Polarization of the cell cytoskeleton, organelles and forces is a crucial step for directed and efficient CCM. CCJs play a crucial role in inducing coordinated migration by allowing the alignment of intercellular forces with their velocity, a phenomenon referred to as plithotaxis.Citation8,36,39,44 However, the identity of the proteins that lead to this mechanical polarized behavior is still under investigation. As explained above, P-cadherin expression in myoblasts strongly affects cell polarity. P-cadherin also increases the anisotropy of intercellular forces across cell-cell junctions as well as plithotaxis, which allows the efficient translocation of the entire cell layer.Citation33 Thus, P-cadherin expression not only polarizes cells toward the migration direction, but also extensively polarizes intercellular stresses.
Cross-talk between intercellular and cell matrix forces and generation of traction forces
The architecture and polarity of a cell monolayer are maintained thanks to the crosstalk and mutual inhibition of CCJs and FAs. Interestingly, Mertz el al., using an in vitro model, demonstrated that E-cadherin has a key role in the reorganization and relocalization of traction forces around the cell cluster, and highlighted the existence of a bi-directional feedback loop between intercellular and cell-matrix forces.Citation23
Theoretical studies suggest that single cells within the monolayer tend to align their traction forces and that cell-substrate traction may play a role in polarizing neighboring cells in the same direction.Citation1,15 Furthermore, Zaritsky et al. demonstrated that a subpopulation of cells within the monolayer transmit mechanical cues by inducing normal stress (i.e., traction/compression forces) on follower rear cells and shear stress (i.e., parallel stress) on neighboring cells on their side.Citation51 Propagation of this cell-cell mechanical communication over time and space results in group of cells that migrate and exert forces in a coordinated and polarized manner. Taken together these theoretical studies strongly support the notion that cell-cell crosstalk can feedback with cell-substrate signaling to promote polarized CCM.
In our study, we used an in vitro system to study P-cadherin contribution to CCM physical properties. We demonstrated that P-cadherin expression in myoblasts induces a highly oriented and directed CCM, a phenotype that was never observed upon expression of other cadherins, such as E- and R-cadherin. The reorientation of FAs and cell protrusions in the first row of P-cadherin-expressing cells in the monolayer led us to investigate the mechanical properties of these cell monolayers using traction force microscopy and monolayer stress microscopy, a technique based on the principle that, according to Newton's laws, traction forces applied at the cell-gel interface must be balanced by intra- and intercellular forces.Citation22,45 We found that traction forces and intercellular stress anisotropy were increased in P-cadherin-expressing cells. Specifically, cells at the leading edge could generate more traction thanks to FA remodeling, leading to an increase in the pulling forces on the follower cells. P-cadherin, which is localized at CCJs, then propagates the tension toward the entire monolayer, leading to an increase of intercellular tension. In our model, progression of P-cadherin expressing cells is achieved through the development of traction forces that counterbalance the intercellular stresses and drive the CCM of the cell layer (). Our data confirm the predictive theory of soft active matter according to which high polarization and high viscosity/friction (i.e., traction exerted on the cell substrate) would lead to rapid and cohesive migration in the presence of high intercellular stresses. Our study demonstrates that P-cadherin has a major role in intercellular force generation and polarization to promote cell guidance, and also in traction force generation at the extracellular matrix to drag the cell layer.
Cadherins signal to Rho GTPases during CCM
Cell-cell adhesion molecules of the cadherin family are important for CCM, but insights into how they regulate multicellular migration and into the involved signaling pathways are only beginning to emerge. GTPases of the Rho family, which are well-known regulators of adhesion and migration dynamics, appear to be key actors of cadherin-mediated CCM. In mammals there are 21 Rho family members, but so far, only RhoA, Rac1, Cdc42 and RhoE have been identified as key regulators of CCM, as reviewed in ref. Citation52. However, it is not precisely known how cadherin-mediated cell-cell adhesion affects Rho GTPase activity. Our recent work brings some clues.
We found that in myoblasts (cells of mesenchymal origin), P-cadherin (but not E-cadherin or R-cadherin) expression specifically activates Cdc42 during CCM.Citation33 P-cadherin-mediated Cdc42 activation requires cadherin homodimer formation and promotes the polarized cell organization (i.e., polarization of cells, membrane protrusions and FA in the migration direction) and the polarization of mechanical forces to allow CCM. Our work in myoblasts not only confirmed CDC42 main role in CCM, which was first identified by Alan Hall and Sandrine Etienne-Maneville and collaborators using several cell systems, but also identified a new P-cadherin/Cdc42 connection. Using a scratch-induced fibroblast migration assay, Cau et al reported that Cdc42 controls the polarization of both membrane protrusions and the Golgi/centrosome.Citation7 In astrocytes, N-cadherin-mediated cell-cell adhesion controls cell polarization. Reduced N-cadherin expression in tumor glial cells is associated with loss of Cdc42 and cell polarity and increased cell velocity.Citation6,10 Finally, our work shows that P-cadherin-mediated Cdc42 activation coordinately controls at least 2 major pathways during CCM: cell polarity and the actin cytoskeleton dynamics.
Cdc42 not only activates Rac1 through a mechanism that remains to be identified, but it is also responsible for the spatial localization of Rac1 activity, a process observed also in vivo during CCM of anterior visceral endoderm cells.Citation28 Rac1 activation is required for CCM, as demonstrated both in vitro and in vivo (reviewed in ref. Citation52). However, its activity must be polarized to promote oriented protrusion activity and migration, because isotropic Rac1 activation is inefficient. Moreover, Arf6-dependent membrane trafficking is required for the polarized recruitment of Rac1 and of the PAR6-aPKC polarity complex at the front edge of migrating cells.Citation29 Recently, using epithelial MDCK cells undergoing CCM, Das et al demonstrated that leading cells pull the membrane of follower cells, leading to redistribution of the cytoskeletal protein Merlin from CCJs to the cytoplasm, thus allowing the activation of polarized Rac1.Citation8 Using Föster resonance energy transfer measurement of Cdc42 and Rac1 activities, we demonstrated that their spatio-temporal activity is dynamic during CCM. Specifically, these 2 GTPases are strongly activated at the cell front of migrating cells in the whole monolayer and also at the lateral cell-cell contact sites. This polarized Rac1 activation is required for the formation of protrusions and cryptic lamellipodia, which most probably contribute to the increase in traction forces.Citation35,38,40
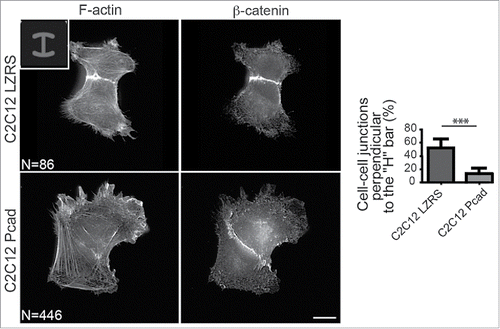
Activation of most Rho GTPases is controlled by guanine exchange factors (GEF) that promote GTP-loading in response to external cues. We identified the GEF β-PIX as a P-cadherin partner required for P-cadherin-mediated Cdc42 activation and polarization, mechanical force generation and polarization and CCM. β-PIX also controls CCM of anterior visceral endoderm cells during early mouse embryogenesis.Citation28 During CCM of astrocytes, β-PIX is detected at CCJs, the cell front and in intracellular vesicles.Citation6,29,30 At the cell front, β-PIX negatively regulates FA maturation and promotes protrusion formation and FA turnover to allow cell migration.Citation19 We detected β-PIX at the cell front and in intracellular vesicles of P-, E- or R-cadherin-expressing cells. Conversely, β-PIX was localized at CCJs only in P-cadherin-positive cells and during CCM. Because tension generated at cadherin adhesion sites is an important process for protein recruitment at CCJs,Citation4,50 the higher intercellular stress generated by P-cadherin during CCM, compared with E- or R-cadherin,Citation2,33 might be responsible of β-PIX recruitment. Finally, in P-cadherin-expressing cells we did not detect any global change in RhoA or ROCK activities.Citation33 Conversely we could show using cell doublets on an H-shaped micropatternCitation46 that P-cadherin locally decreases cell contractility (). This is in agreement with previous studies showing that RhoA/ROCK-mediated contractility decreases to allow efficient CCM.Citation18,27
Figure 2. P-cadherin expression decreases cell contractility as indicated by intercellular junction positioning. Immunostaining of β-catenin and F-actin in control (C2C12 LZRS: C2C12 cells expressing only the empty vector) and P-cadherin-expressing cell doublets plated on H-shaped micropatterns. Most of control C2C12 myoblasts (82%) have intercellular junctions that are perpendicular to the H bar. Conversely, the intercellular junction position and orientation are strongly perturbed upon P-cadherin expression. Indeed, junctions that are perpendicular to the H bar, like in control cells, are observed only in 11% of P-cadherin-expressing cells. For all panels, the mean ± SEM is shown; *** P < 0.0005.
Concluding remarks
CCM provides an effective strategy for transporting group of cells to a new location for colonization, for instance during embryo development, but also during tumor cell invasion, thus facilitating metastasis formation.Citation12 Elucidating CCM mechanisms, particularly the role of cell-cell adhesion molecules that play key roles in multicellular migration, will not only improve our understanding of normal development, but will also provide insights on how to target and slow down pathological cell migration, thereby improving current strategies for suppressing tumor cell invasion and metastasis formation.
By comparing the behavior of myoblasts that express different cadherin types, we found that P-cadherin is a very efficient pro-migratory molecule, compared with E- or R-cadherin. P-cadherin is expressed in rhabdomyosarcoma, an invasive sarcoma of skeletal muscle origin, and in some invasive carcinomas. Targeting P-cadherin is probably an interesting strategy to control metastasis formation in patients with cancers in which P-cadherin expression is upregulated. A human monoclonal antibody against P-cadherin showed anti-tumor and anti-metastatic activity in different P-cadherin overexpressing tumor models.Citation53
Abbreviations
| CCJ | = | cell-cell junction |
| CCM | = | collective cell migration |
| FA | = | focal adhesion |
| GEF | = | guanine exchange factor |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by CNRS and INSERM institutional grants and contracts from the Ligue Nationale contre le Cancer (LNCC) (“Equipe labellisée”), the Institut National du Cancer (INCa) and from the Ligue Régionale contre la Cancer (comité “Hérault”).
References
- Basan M, Elgeti J, Hannezo E, Rappel WJ, Levine H. Alignment of cellular motility forces with tissue flow as a mechanism for efficient wound healing. Proc Natl Acad Sci U S A 2013; 110:2452-9; PMID:23345440; http://dx.doi.org/10.1073/pnas.1219937110
- Bazellieres E, Conte V, Elosegui-Artola A, Serra-Picamal X, Bintanel-Morcillo M, Roca-Cusachs P, Munoz JJ, Sales-Pardo M, Guimera R, Trepat X. Control of cell-cell forces and collective cell dynamics by the intercellular adhesome. Nat Cell Biol 2015; 17:409-20; PMID:25812522; http://dx.doi.org/10.1038/ncb3135
- Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci U S A 2010; 107:13324-9; PMID:20566866; http://dx.doi.org/10.1073/pnas.1002662107
- Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 2014; 346:1254211; PMID:25359979; http://dx.doi.org/10.1126/science.1254211
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014; 157:1146-59; PMID:24855950; http://dx.doi.org/10.1016/j.cell.2014.03.045
- Camand E, Peglion F, Osmani N, Sanson M, Etienne-Manneville S. N-cadherin expression level modulates integrin-mediated polarity and strongly impacts on the speed and directionality of glial cell migration. J Cell Sci 2012; 125:844-57; PMID:22275437; http://dx.doi.org/10.1242/jcs.087668
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci 2005; 118:2579-87; PMID:15928049; http://dx.doi.org/10.1242/jcs.02385
- Das T, Safferling K, Rausch S, Grabe N, Boehm H, Spatz JP. A molecular mechanotransduction pathway regulates collective migration of epithelial cells. Nat Cell Biol 2015; 17:276-87; PMID:25706233; http://dx.doi.org/10.1038/ncb3115
- du Roure O, Saez A, Buguin A, Austin RH, Chavrier P, Silberzan P, Ladoux B. Force mapping in epithelial cell migration. Proc Natl Acad Sci U S A 2005; 102:2390-5; PMID:15695588; http://dx.doi.org/10.1073/pnas.0408482102
- Dupin I, Camand E, Etienne-Manneville S. Classical cadherins control nucleus and centrosome position and cell polarity. J Cell Biol 2009; 185:779-86; PMID:19487453; http://dx.doi.org/10.1083/jcb.200812034
- Farooqui R, Fenteany G. Multiple rows of cells behind an epithelial wound edge extend cryptic lamellipodia to collectively drive cell-sheet movement. J Cell Sci 2005; 118:51-63; PMID:15585576; http://dx.doi.org/10.1242/jcs.01577
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10:445-57; PMID:19546857; http://dx.doi.org/10.1038/nrm2720
- Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, Black M, Zanker KS. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res 1995; 55:4557-60; PMID:7553628.
- Friedl P, Wolf K. Plasticity of cell migration: a multiscale tuning model. J Cell Biol 2010; 188:11-19; PMID:19951899; http://dx.doi.org/10.1083/jcb.200909003
- Gov NS. Traction forces during collective cell motion. HFSP journal 2009; 3:223-227; PMID:20119479; http://dx.doi.org/10.2976/1.3185785
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 2006; 20:3199-214; PMID:17158740; http://dx.doi.org/10.1101/gad.1486806
- Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res 2002; 62:2125-30; PMID:11929834.
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol 2011; 13:49-58; PMID:21170030; http://dx.doi.org/10.1038/ncb2133
- Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 2011; 13:383-93; PMID:21423176; http://dx.doi.org/10.1038/ncb2216
- Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mege RM. Strength dependence of cadherin-mediated adhesions. Biophys J 2010; 98:534-42; PMID:20159149; http://dx.doi.org/10.1016/j.bpj.2009.10.044
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A 2010; 107:9944-9; PMID:20463286; http://dx.doi.org/10.1073/pnas.0914547107
- Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci U S A 2011; 108:4708-13; PMID:21383129; http://dx.doi.org/10.1073/pnas.1011123108
- Mertz AF, Che Y, Banerjee S, Goldstein JM, Rosowski KA, Revilla SF, Niessen CM, Marchetti MC, Dufresne ER, Horsley V. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proc Natl Acad Sci U S A 2013; 110:842-7; PMID:23277553; http://dx.doi.org/10.1073/pnas.1217279110
- Nabeshima K, Inoue T, Shimao Y, Kataoka H, Koono M. Cohort migration of carcinoma cells: differentiated colorectal carcinoma cells move as coherent cell clusters or sheets. Histol Histopathol 1999; 14:1183-97; PMID:10506935.
- Ng MR, Besser A, Danuser G, Brugge JS. Substrate stiffness regulates cadherin-dependent collective migration through myosin-II contractility. J Cell Biol 2012; 199:545-63; PMID:23091067; http://dx.doi.org/10.1083/jcb.201207148
- Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, Yaswen P, Werb Z, Ewald AJ. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci U S A 2012; 109:E2595-2604; PMID:22923691; http://dx.doi.org/10.1073/pnas.1212834109
- Omelchenko T. Regulation of collective cell migration by RhoGAP myosin IXA. Small GTPases 2012; 3:213-8; PMID:22735295; http://dx.doi.org/10.4161/sgtp.20495
- Omelchenko T, Rabadan MA, Hernandez-Martinez R, Grego-Bessa J, Anderson KV, Hall A. beta-Pix directs collective migration of anterior visceral endoderm cells in the early mouse embryo. Genes Dev 2014; 28:2764-77; PMID:25512563; http://dx.doi.org/10.1101/gad.251371.114
- Osmani N, Peglion F, Chavrier P, Etienne-Manneville S. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol 2010; 191:1261-9; PMID:21173111; http://dx.doi.org/10.1083/jcb.201003091
- Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 2006; 16:2395-405; PMID:17081755; http://dx.doi.org/10.1016/j.cub.2006.10.026
- Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol 2014; 16:639-51; PMID:24929360; http://dx.doi.org/10.1038/ncb2985
- Perryn ED, Czirok A, Little CD. Vascular sprout formation entails tissue deformations and VE-cadherin-dependent cell-autonomous motility. Dev Biol 2008; 313:545-55; PMID:18062955; http://dx.doi.org/10.1016/j.ydbio.2007.10.036
- Plutoni C, Bazellieres E, Le Borgne-Rochet M, Comunale F, Brugues A, Seveno M, Planchon D, Thuault S, Morin N, Bodin S, Trepat X, Gauthier-Rouviere C. P-cadherin promotes collective cell migration via a Cdc42-mediated increase in mechanical forces. J Cell Biol 2016; 212:199-217; PMID:26783302; http://dx.doi.org/10.1083/jcb.201505105
- Pocha SM, Montell DJ. Cellular and molecular mechanisms of single and collective cell migrations in Drosophila: themes and variations. Annual review of genetics 2014; 48:295-318; PMID:25421599; http://dx.doi.org/10.1146/annurev-genet-120213-092218
- Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir 2003; 19:1573-9; http://dx.doi.org/10.1021/la026142j
- Serra-Picamal X, Conte V, Vincent R, Anon E, Tambe DT, Bazellieres E, Butler JP, Fredberg JJ, Trepat X. Mechanical waves during tissue expansion. Nat Phys 2012; 8:628-34; http://dx.doi.org/10.1038/nphys2355
- Shih W, Yamada S. N-cadherin as a key regulator of collective cell migration in a 3D environment. Cell Adh Migr 2012; 6:513-7; PMID:23076138; http://dx.doi.org/10.4161/cam.21766
- Stricker J, Aratyn-Schaus Y, Oakes PW, Gardel ML. Spatiotemporal constraints on the force-dependent growth of focal adhesions. Biophys J 2011; 100:2883-93; PMID:21689521; http://dx.doi.org/10.1016/j.bpj.2011.05.023
- Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. Collective cell guidance by cooperative intercellular forces. Nat Mater 2011; 10:469-75; PMID:21602808; http://dx.doi.org/10.1038/nmat3025
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A 2003; 100:1484-9; PMID:12552122; http://dx.doi.org/10.1073/pnas.0235407100
- Theveneau E, Mayor R. Cadherins in collective cell migration of mesenchymal cells. Curr Opin Cell Biol 2012; 24:677-84; PMID:22944726; http://dx.doi.org/10.1016/j.ceb.2012.08.002
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139:871-90; PMID:19945376; http://dx.doi.org/10.1016/j.cell.2009.11.007
- Thuault S, Hayashi S, Lagirand-Cantaloube J, Plutoni C, Comunale F, Delattre O, Relaix F, Gauthier-Rouviere C. P-cadherin is a direct PAX3-FOXO1A target involved in alveolar rhabdomyosarcoma aggressiveness. Oncogene 2013; 32:1876-87; PMID:22710718; http://dx.doi.org/10.1038/onc.2012.217
- Trepat X, Fredberg JJ. Plithotaxis and emergent dynamics in collective cellular migration. Trends Cell Biol 2011; 21:638-46; PMID:21784638; http://dx.doi.org/10.1016/j.tcb.2011.06.006
- Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nat Phys 5:426-30; http://dx.doi.org/10.1038/nphys1269
- Tseng Q, Duchemin-Pelletier E, Deshiere A, Balland M, Guillou H, Filhol O, Thery M. Spatial organization of the extracellular matrix regulates cell-cell junction positioning. Proc Natl Acad Sci U S A 2012; 109:1506-11; PMID:22307605; http://dx.doi.org/10.1073/pnas.1106377109
- Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 2012; 22:104-15; PMID:22169071; http://dx.doi.org/10.1016/j.devcel.2011.10.013
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci 2008; 121:727-35; PMID:18322269; http://dx.doi.org/10.1242/jcs.000455
- Winklbauer R, Selchow A, Nagel M, Angres B. Cell interaction and its role in mesoderm cell migration during Xenopus gastrulation. Dev Dyn 1992; 195:290-302; PMID:1304824; http://dx.doi.org/10.1002/aja.1001950407
- Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mege RM, et al. Force-dependent conformational switch of alpha-catenin controls vinculin binding. Nat Commun 2014; 5:4525; PMID:25077739.
- Zaritsky A, Welf ES, Tseng YY, Angeles Rabadan M, Serra-Picamal X, Trepat X, Danuser G. Seeds of Locally Aligned Motion and Stress Coordinate a Collective Cell Migration. Biophys J 2015; 109:2492-500; PMID:26682808; http://dx.doi.org/10.1016/j.bpj.2015.11.001
- Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small GTPases 2014; 5:e28997; PMID:25054920; http://dx.doi.org/10.4161/sgtp.28997
- Zhang CC, Yan Z, Zhang Q, Kuszpit K, Zasadny K, Qiu M, Painter CL, Wong A, Kraynov E, Arango ME, et al. PF-03732010: a fully human monoclonal antibody against P-cadherin with antitumor and antimetastatic activity. Clin Cancer Res 2010; 16:5177-88; PMID:20829331; http://dx.doi.org/10.1158/1078-0432.CCR-10-1343
