ABSTRACT
The intracellular movement of membrane-bound vesicles is closely tied to their formation, maturation and ultimate function within the cell. Motor proteins and their associated cytoskeletal networks are critical for vesicle transport, but whether these factors play a more direct role in vesicle biogenesis is unclear. In recent work, we found that the Drosophila kinesin proteins Khc and Klp98A are both required for the normal anterograde movement of autophagosomes and autolysosomes during starvation-induced autophagy. In addition, Klp98A has a transport-independent function of promoting autophagosome-lysosome fusion, a key step in the maturation of autophagic vesicles. This function correlates with the association of Klp98A with the autophagosomal protein Atg8 and with the endolysosomal protein Rab14, suggesting that Klp98A may promote vesicle fusion by physically linking these vesicle surface proteins. These findings demonstrate how the delivery of vesicles to their proper destination can be coordinated with additional steps in their life cycle through molecular motor-based interactions.
Intracellular homeostasis is maintained by the orchestration of an incredibly high number of events within one cell. Among them, vesicle fusion is essential for cell defense and regulation of intracellular processes through its effects on vesicle maturation, signaling and cargo degradation. Interestingly, fusion of vesicles is closely linked to their transport, as for 2 vesicles to fuse, they must first find each other within the cell. How these 2 processes are coordinated, and whether they share any machinery, is poorly understood.
Two families of motor proteins, the kinesin and the dynein complexes, are involved in vesicle motility by driving their cargo along the microtubule cytoskeleton.Citation1,2 The majority of identified kinesins act as plus-end-directed motors, although some transport their cargo toward the cell center. Kinesins are involved in many forms of cellular transport, and can be classified into several broad families.Citation3 Among these, members of the kinesin-3 family are notable for their high processivity and possession of a lipid binding domain, making them especially suited to long-range movement of vesicles.Citation4
Rab proteins are master regulators of multiple stages of intracellular trafficking processes in eukaryotic cells.Citation5 Many kinesins and dynein proteins are known Rab effectors,Citation6 however the partnership between Rabs and motor proteins remains to be extensively characterized. Motor proteins have been shown to interact with Rabs either directly or through adaptor proteins that bind both the motor and the small GTPase. For example, KIF20A directly interacts with Rab6A to promote cytokinesis,Citation7 and KIF16B binds directly to Rab14 to promote Golgi-to-endosome trafficking during embryonic development.Citation8 In contrast, Rab3A associates through an adaptor protein with KIF1Bβ/KIF1A (kinesin-3 subunits) during axonal transport,Citation9 and Rab11A regulates endosomal trafficking events by associating with KIF3B via Rip11.Citation10
Autophagy is a central pathway, conserved in metazoans, that helps to maintain cellular homeostasis by mediating the turnover of cellular structures through lysosomal degradation.Citation11 Double-membrane vesicles, called autophagosomes, form in the cytoplasm and engulf bulk cytosol or specifically-targeted organelles. Once the autophagosome is fully closed, the outer autophagosomal membrane fuses with late endosomes and lysosomes to form a single-membrane vesicle called the autolysosome. By this fusion event, the autophagic cargo becomes exposed to degradative lysosomal enzymes, leading to the breakdown and recycling of cellular components.
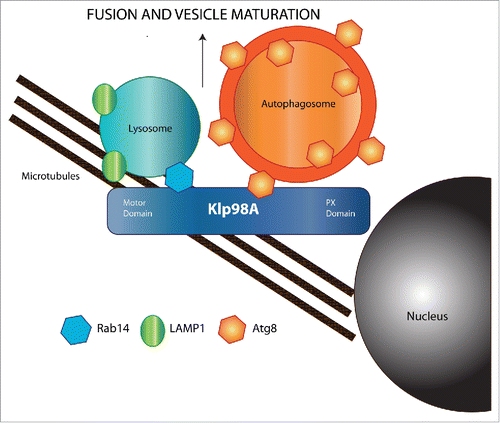
In recent work, we identified the Drosophila kinesin-3 family member Klp98A as a novel regulator of autophagic vesicle intracellular localization.Citation12 Klp98A is an ortholog of mammalian KIF16B and contains both an N-terminal motor domain as well as a C-terminal PI3P-binding PX domain. We found that Klp98A gain- or loss-of-function modified the localization of vesicles marked by the autophagosomal membrane protein Atg8/LC3 and of lysosomes marked by LAMP1.Citation13 Klp98A depletion significantly disrupted the localization of the 3 populations of vesicles involved in autophagy, i.e., autophagosomes, lysosomes, and autolysosomes. Each of these vesicles was aberrantly localized to the perinuclear area upon Klp98A depletion, and to the cortical region of cells overexpressing Klp98A. Depletion of the conventional Kinesin heavy chain (Khc) gene also led to a statistically significant shift of autophagosomes and autolysosomes toward the cell center. Interestingly, genetic rescue experiments showed that the vesicle localization defects caused by depletion of either protein could be rescued by overexpression of the other, suggesting that Khc and Klp98A likely function together to promote anterograde vesicle movement.
In addition to its effects on vesicle localization, we found that depletion of Klp98A also disrupted the fusion of autophagosomes and lysosomes. Accordingly, acidification of autolysosomes and degradation of autophagic cargo were also defective in these cells, indicating that Klp98A is required for the whole autophagic cascade. Interestingly, although depletion of Khc or Klp98A led to a similar disruption of vesicle transport, vesicle fusion was normal in Khc-depleted cells. These results suggest that the fusion function of Klp98A is separable from its effects on vesicle transport. Moreover, the similar mislocalization of autophagosomes and lysosomes in Klp98A depleted cells further suggests that the role of Klp98A in vesicle fusion extends beyond merely moving autophagosomes and lysosomes to the same area of the cell.
If the ability of Klp98A to mediate vesicle fusion is independent of its effects on transport, how might this protein promote fusion? One potential clue may be found in the previously reported interaction of the Klp98A human ortholog KIF16B with Rab14.Citation8 We found that in Drosophila fat body cells, Rab14 strongly localizes to both lysosomes and autolysosomes, and that the interaction between Rab14 and Klp98A/KIF16B is conserved in Drosophila. This interaction is likely a critical aspect of Rab14 function, as depletion of Rab14 led to a defect in autophagosome fusion and acidification markedly similar to that of Klp98A depleted cells. In addition, using GST pulldown assays we found that Klp98A also bound to the autophagosomal membrane protein Atg8. Thus, by binding to Atg8 and Rab14, Klp98A is uniquely suited to act as a physical bridge between autophagosomes and lysosomes. Through these interactions, Klp98A may bring the autophagosomal and lysosomal membranes into close proximity, helping to promote autophagosome-lysosome fusion.
Together, our data suggest that in addition to functioning as motors, kinesins may also affect some functions by acting as a scaffold (). This model provides a satisfying explanation for how one protein can promote both vesicle movement and fusion. To strengthen and extend this model, it will be important to characterize mutations in the motor domain and to precisely define the Atg8/LC3- and Rab14-interacting regions of Klp98A. A number of putative LC3 interacting regions (LIRs) can be identified in the Klp98A peptide sequence using prediction software,Citation14 and mutation of these motifs should help to define the role of this interaction. In mammalian cells, the interaction between KIF16B and Rab14 is influenced by GTP/GDP binding, providing a means to regulate cargo-motor association.Citation8 It is tempting to speculate that autophagic vesicle transport and/or fusion may be regulated by a similar mechanism. In addition, the rate of vesicle fusion can have an important impact on vesicle size and may therefore influence the type of cargo within autophagosomes. In this regard, it is notable that Klp98A depletion leads to a reduction in number and size of both autophagosomes and autolysosomes. Whether regulation of Rab14/Klp98A docking plays a role in these aspects of vesicle maturation would be interesting to further investigate. The direct impact of Klp98A on induction of autophagosome formation and their size will need further experiments to fully understand the mechanisms beneath these additional functions of Klp98A.
Figure 1. Trafficking and maturation of autophagic vesicles along microtubule networks. Molecular motors of the dynein and kinesin families promote retrograde and anterograde movement of autophagic vesicles, respectively. During their intracellular trafficking, autophagosomes fuse with lysosomes, leading to the formation of autolysosomes and subsequent degradation of autophagic cargo by lysosomal hydrolases. Association of Klp98A with lysosomes via Rab14 and with autophagosomes via Atg8 may provide a molecular bridge between these vesicles that physically supports the fusion event.
Klp98A shares similarities with FYCO1, another Rab effector protein mediating microtubule plus-end directed vesicle transport.Citation15 Like Klp98A, FYCO1 binds to PI(3)P, to Atg8/LC3 and to an endosomal Rab GTPase (Rab7), and depletion and overexpression of FYCO1 in HeLa cells causes phenotypes similar to Klp98A loss- and gain-of-function. FYCO1 promotes vesicle transport through an interaction with a motor protein, while Klp98A encompasses a motor domain directly within its N-terminal region. Interestingly, no FYCO1 ortholog has been identified in Drosophila, suggesting that the greater complexity in higher organisms may have driven the evolution of additional proteins to fulfill roles performed by Klp98A in Drosophila. Identification of the motor protein(s) that interact with FYCO1 would help to clarify this hypothesis.
Our results provide the first example of an Atg8 binding protein promoting vesicle fusion. It is interesting to consider whether other Atg8/LC3 interacting proteins such as p62, NBR1, NDP52, or OptineurinCitation16 might also serve a similar function. Further research toward this hypothesis may highlight novel features of known proteins and/or describe new regulators of autophagosome-lysosome fusion. This is of importance for the field as still little is known about the regulation of the event of autophagosome-lysosome fusion. Is this process constitutive, or can it be turned up or down in response to cellular stresses or other cues?
Lysosome positioning was recently shown to be regulated by Rab7-RILP-dependent retrograde transport.Citation17 The spatial and temporal control of membrane dynamics is essential for cellular processes and may underly pathologies, as for example lysosomes are distributed toward the plasma membrane to promote invasion and metastasis in some cancer cells.Citation18 Our findings provide insight into Klp98A as a novel player in autophagic vesicle movement and maturation, which acts as a scaffold protein though its interdependent domains. Klp98A may act as a focal point for multiple regulatory signals.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Vale RD. The molecular motor toolbox for intracellular transport. Cell 2003; 112(4):467-80; PMID:12600311; http://dx.doi.org/10.1016/S0092-8674(03)00111-9
- Bryantseva SA, Zhapparova ON. Bidirectional transport of organelles: unity and struggle of opposing motors. Cell Biol Int 2012; 36(1):1-6; PMID:22142363; http://dx.doi.org/10.1042/CBI20110413
- Hirokawa N, Tanaka Y. Kinesin superfamily proteins (KIFs): Various functions and their relevance for important phenomena in life and diseases. Exp Cell Res 2015; 334(1):16-25; http://dx.doi.org/10.1016/j.yexcr.2015.02.016
- Soppina V, Norris SR, Dizaji AS, Kortus M, Veatch S, Peckham M, Verhey KJ. Dimerization of mammalian kinesin-3 motors results in superprocessive motion. Proc Natl Acad Sci U S A 2014; 111(15):5562-7; PMID:24706892; http://dx.doi.org/10.1073/pnas.1400759111
- Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128(17):3171-6; PMID:26272922; http://dx.doi.org/10.1242/jcs.166074
- Horgan CP, McCaffrey MW. Rab GTPases and microtubule motors. Biochem Soc Trans 2011; 39(5):1202-6; PMID:21936789; http://dx.doi.org/10.1042/BST0391202
- Echard A, Jollivet F, Martinez O, Lacapère JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science 1998; 279(5350):580-5; PMID:9438855; http://dx.doi.org/10.1126/science.279.5350.580
- Ueno H, Huang X, Tanaka Y, Hirokawa N. KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev Cell 2011; 20(1):60-71; PMID:21238925; http://dx.doi.org/10.1016/j.devcel.2010.11.008
- Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol 2008; 10(11):1269-79; PMID:18849981; http://dx.doi.org/10.1038/ncb1785
- Schonteich E, Wilson GM, Burden J, Hopkins CR, Anderson K, Goldenring JR, Prekeris R. The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J Cell Sci 2008; 121(Pt 22):3824-33; PMID:18957512; http://dx.doi.org/10.1242/jcs.032441
- Noda NN, Inagaki F. Mechanisms of Autophagy. Annu Rev Biophys 2015; 44:101-22; PMID:25747593; http://dx.doi.org/10.1146/annurev-biophys-060414-034248
- Mauvezin C, Neisch AL, Ayala CI, Kim J, Beltrame A, Braden CR, Gardner MK, Hays TS, Neufeld TP. Coordination of autophagosome-lysosome fusion and transport by a Klp98A-Rab14 complex in Drosophila. J Cell Sci 2016; 129(5):971-82; PMID:26763909; http://dx.doi.org/10.1242/jcs.175224
- Mauvezin C, Ayala C, Braden CR, Kim J, Neufeld TP. Assays to monitor autophagy in Drosophila. Methods 2014; 68(1):134-9; PMID:24667416; http://dx.doi.org/10.1016/j.ymeth.2014.03.014
- Kalvari I, Tsompanis S, Mulakkal NC, Osgood R, Johansen T, Nezis IP, Promponas VJ. iLIR: a web resource for prediction of Atg8-family interacting proteins. Autophagy 2014; 10(5):913-25; PMID:24589857; http://dx.doi.org/10.4161/auto.28260
- Pankiv S, Alemu EA, Brech A, Bruun JA, Lamark T, Overvatn A, Bjørkøy G, Johansen T. FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J Cell Biol 2010; 188(2):253-69; PMID:20100911; http://dx.doi.org/10.1083/jcb.200907015
- Lippai M, Low P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. Biomed Res Int 2014; 2014:832704; PMID:25013806; http://dx.doi.org/10.1155/2014/832704
- Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol 2016; 18(4):404-17; PMID:26950892; http://dx.doi.org/10.1038/ncb3324
- Machado E, White-Gilbertson S, van de Vlekkert D, Janke L, Moshiach S, Campos Y, Finkelstein D, Gomero E, Mosca R, Qiu X, et al. Regulated lysosomal exocytosis mediates cancer progression. Sci Adv 2015; 1(11):e1500603; PMID:26824057; http://dx.doi.org/10.1126/sciadv.1500603
