ABSTRACT
More than 60 Rab GTPases exist in the human genome to regulate vesicle trafficking between organelles. Rab GTPases are members of the Ras GTPase superfamily that broadly control budding, uncoating, motility and fusion of vesicles in most cell types. Rab proteins interconvert between active, GTP-bound form and inactive, GDP-bound form. In their active conformation, they interact with various effector molecules to carry out diverse functions. Rab GTPases are usually small containing only a GTPase domain with a C-terminal prenylation site for membrane anchoring. Recently, we identified a large G protein, CRACR2A (CRAC channel regulator 2A), which uncovers novel functions of Rab GTPases. First, CRACR2A encodes a large Rab GTPase containing multiple functional domains contrary to small Rab GTPases. Second, CRACR2A plays an unexpected role in regulating intracellular signaling pathways important for T cell activation, unlike the canonical role of small Rab GTPases. Vesicles containing CRACR2A bud out from the proximal Golgi area and translocate into the immunological synapse to activate these signaling pathways. Third, instead of recycling, CRACR2A is consumed by a unidirectional pathway. These events are sequentially regulated by prenylation, GTP binding, protein interaction with a signaling adaptor Vav1, and degradation. Together, our findings reveal a novel function of a large Rab GTPase in intracellular signaling pathways, which may be shared by other large Rab GTPases, Rab44 and Rab45.
T cells can be categorized into 2 main classes of CD4+ helper T cells and CD8+ cytolytic T cells. The main function of helper T cells is production of cytokines, which control activation and recruitment of other cell types while cytolytic T cells remove cells displaying abnormal properties caused by infection or cancer. Activation of helper T cells requires signals from direct contact between T cell receptor (TCR)-CD3 complex and antigen presented by major histocompatibility complex (MHC) class II molecules expressed on the surface of antigen-presenting cells (e.g. dendritic cells). The binding of TCRs to cognate peptide-MHCs induces clustering of the TCRs and recruitment of the kinases Lck (lymphocyte-specific protein tyrosine kinase) and ZAP70 (ζ-chain-associated protein kinase) by CD4 and CD3ζ chain, respectively. Activated ZAP70 temporarily binds to and phosphorylates a signaling adaptor Lat that forms a signalosome, containing phospholipase C-γ1 (PLCγ1) and Vav1.Citation1-3 PLCγ1 produces a second messenger inositol 1,4,5-triphosphate (InsP3) that binds to the InsP3 receptor on the endoplasmic reticulum (ER) and triggers depletion of the ER Ca2+ stores. By sensing ER Ca2+ depletion, stromal interaction molecule 1 (STIM1), an ER-resident Ca2+-binding protein oligomerizes and translocates to the plasma membrane (PM)-proximal regions, and activates Orai1, the pore subunit of the CRAC (Ca2+ release-activated Ca2+) channels.Citation4-6 The increased cytoplasmic Ca2+ ions bind to calmodulin-calcineurin complex, which dephosphorylates NFAT (nuclear factor of activated T cells) transcription factor to expose its nuclear localization signal and induce transcriptional activation. Vav1, a guanine nucleotide exchange factor (GEF) and adaptor molecule, accumulates at the immunological synapse (IS) via interaction with Lat and recruits small G proteins such as Rac1 and CDC42 (cell division control protein 42 homolog) to activate the c-Jun N-terminal kinase (Jnk) and p38 MAPK (mitogen-activated protein kinase) pathways, leading into activation of AP1 transcription factors.Citation7 Activation of both the Ca2+ and MAPK signaling pathways are essential for differentiation and cytokine production of helper T cells and dysregulation of these pathways result in immune deficiency or autoimmune disorders in humans and mice.Citation8-10
It was thought that these TCR signaling events occur only at the level of the plasma membrane. However, recent studies on Lat molecules argue that vesicles recruited from the intracellular pool can be also important for transmitting TCR signals to downstream events. In addition to its localization at the plasma membrane, Lat also exists in subsynaptic vesicles that translocate into the plasma membrane-proximal regions of the immunological synapse, an interface between T cells and antigen-presenting cells, after TCR stimulation.Citation11-13 Recruitment of this pool of Lat is important for its phosphorylation and further activation of downstream signaling. A v-SNARE (soluble N-ethylmaleimide-sensitive protein-attached protein receptor) protein VAMP7 guides these vesicles into the plasma membrane potentially by docking to the t-SNARE proteins, surprisingly, in a mechanism that does not involve actual membrane fusion.Citation14 These results suggest that components of the molecular machinery utilized in trafficking of synaptic vesicles in the neuronal synapse such as SNAREs and small Rab GTPases play an important role in the trafficking of subsynaptic vesicles in T cells to activate intracellular signaling pathways. However, the importance of vesicles in TCR signaling has been uncovered only recently and the identity and functions of these subsynaptic vesicles in T cell activation needs further investigation.
Various small G proteins of the Ras superfamily are known to play an important role in proximal TCR signaling. Most G proteins cycle between an inactive GDP-bound state and an active GTP-bound effector state. The exchange of GDP for GTP is mediated by GEFs and the intrinsic GTPase activity of the G proteins is enhanced by GTPase activating proteins (GAPs). The G proteins contain a C-terminal prenylation motif, which can anchor them onto cellular membranes. Guanine nucleotide dissociation inhibitors (GDIs) can bind to the C-terminal prenylation lipids, thereby inhibiting membrane association and blocking the effector function of the small G proteins. Upon TCR stimulation, Vav1, gets tyrosine phosphorylated, which activates its GEF activity toward RhoA, Rac1 and CDC42. Various studies have identified important role of RhoA, Rac1 and CDC42 in regulating vital functions in T cells including thymocyte development, cytoskeleton dynamics, gene transcription and cell cycle progression.Citation15,16 Interestingly there are families of GTPases that are in a constitutively GTP bound state, like members of the RhoH, RhoU, Rnd (Rho-related GTP-binding protein family) and RhoV family of GTPases, that are not regulated by GEFs or GAPs and are termed as atypical GTPases. These proteins are thought to be regulated by their phosphorylation, localization or stability.Citation16 The Rab family of GTPases are predominantly involved in vesicle formation, motility, docking and fusion, thereby mediating cellular protein trafficking as well as secretion. In T cells it was identified that distinct Rab proteins mediate secretion of cytokines either directionally toward the immunological synapse or multidirectionally. Rab3d and Rab19 were implicated in directional secretion of IL-2 and IFN-γ cytokines at the immunological synapse, whereas Rab37 was shown to mediate multidirectional secretion of TNF and IL-4.Citation17 Most of the G proteins are small in size of ∼20 KDa and comprise primarily of a GTPase domain, without additional protein interaction/signaling motifs. Recently, we identified a large Rab GTPase, CRACR2A-a, which is ∼85 KDa in size, contains multiple domains in addition to the C-terminal Rab GTPase domain, and plays an important role in intracellular signaling pathways (). Our recent work (detailed below) emphasizes the role of CRACR2A contained in vesicles, which are independent from Lat-containing vesicles, in activation of the Ca2+-NFAT and Jnk-AP1signaling pathways.Citation18 Together, the results from studies on the roles of Lat and CRACR2A suggest that TCR signaling events are not restricted to the plasma membrane, but they also involve additional steps of vesicle trafficking that carries signaling molecules from the intracellular pool to the immunological synapse.
Figure 1. CRACR2A encodes a large Rab GTPase. (a) Schematic showing the predicted domain structure of human CRACR2A-a. CRACR2A-a and CRACR2A-c share EF-hand motifs, coiled-coil domains (CC1 and CC2) and leucine-rich region (LR), which interact with the Orai1-STIM1 complex to regulate Ca2+ entry. CRACR2A-a contains additional proline-rich domain (PRD) and a predicted Rab GTPase domain with a prenylation site at the C terminus. (b) Homology modeling of CRACR2A-a GTPase domain (yellow) with Rab3a (red). Sequence alignment between GTPase domain of CRACR2A-a and Rab3a gave a continuous alignment with sequence identity of 46% and similarity of 65% (Clustal Omega) (left). MODELLERCitation30 was used for homology modeling of CRACR2A-a GTPase domain to a high-resolution structure of a GPPNHP-bound Rab3a (PDB ID: 3RAB). A zoomed-in view of the GPPNHP binding site (right). GPPNHP and side-chains of residues important for GTP binding and hydrolysis, Thr559, Gln604 and Asn658 are shown in stick representation. A loop consisting of residues 561-570 was removed for clarity. Panels were generated using PyMOL (Version 1.5.0.4 Schrödinger, LLC). From Srikanth et al.Citation18 Reprinted with permission from AAAS.
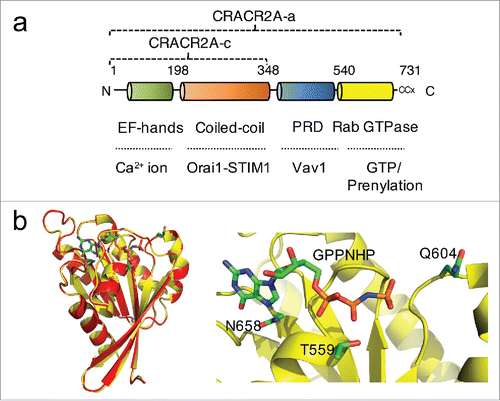
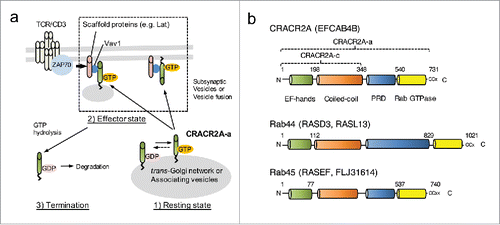
Human CRACR2A (alternatively EFCAB4B) gene encodes 2 transcriptional isoforms, CRACR2A-a (herein CRACR2A, unless specified) and CRACR2A-c, but the current mouse genome database shows presence of only CRACR2A-c. The short isoform CRACR2A-c, which commonly exists in both human and mouse genome was previously identified to facilitate CRAC channel function by stabilizing Orai1-STIM1 interaction ().Citation19 Recent work validated presence of the long isoform CRACR2A-a in both human and murine tissues (and cells), particularly, in lymphoid organs.Citation18,20 Both these isoforms share conserved function in activation of the Ca2+-NFAT pathway, but only CRACR2A-a has a unique role in activation of the Jnk MAPK pathway, emphasizing the importance of the additional proline-rich and Rab GTPase domains in these events. CRACR2A-a contains N-terminal EF hands and a coiled coil domain, which are conserved with CRACR2A-c. A proline-rich domain and C-terminal Rab GTPase domain are uniquely present in CRACR2A-a. The proline-rich domain of CRACR2A shows a strong interaction with Vav1 after TCR stimulation. Both, truncation of this proline-rich domain or deficiency of Vav1 decrease CRACR2A recruitment, suggesting that Vav1 interaction mediated by its proline-rich domain is important for the recruitment of CRACR2A-containing vesicles into the immunological synapse. The C-terminal Rab GTPase domain determines localization of CRACR2A-a in resting conditions. Localization of GTP/GDP binding defective mutants of CRACR2A-a showed that WT CRACR2A-a resides near the trans Golgi network in a constitutively GTP-bound form. Mutants impaired in GTP binding (T559N and N658I) showed a predominant cytoplasmic localization. These observation suggests that CRACR2A-a may have a very slow GTP hydrolysis rate and its function is mostly governed by its localization, interaction with Vav1 and degradation (see below). These data also suggest that only the signaling adaptor function of Vav1 is important for CRACR2A-a function, and not its GEF activity.
Although CRACR2A-a has many unusual features as a large Rab GTPase (compared to small Rab GTPases), the function of the Rab GTPase domain of CRACR2A-a is well conserved with other small Rab GTPases and 3-dimensional homology modeling to a high-resolution crystal structure of Rab3aCitation21 showed almost complete overlap (). Full length CRACR2A-a hydrolyzed GTP in a GTPase assay, validating presence of a functional GTPase domain. The mutants T559N, Q604L, and N658I showed a pronounced reduction in GTPase activity possibly due to defects in GTP binding (T559N and N658I) and hydrolysis (Q604L). The Rab GTPase domain of CRACR2A-a is also prenylated like other small GTPases. Ras GTPases are farnesylated by farnesyl transferase while Rac and Rho GTPases are geranylgeranylated by type-I geranylgeranyl transferase (GGT). Rab GTPases are also geranylgeranylated, but by the type-II enzyme. Statin family drugs are useful tools to investigate protein prenylation because they are inhibitors of 3-hydroxyl-3-methyl-glutaryl-CoA (HMG-CoA) reductase, the key rate-liming enzyme in the cholesterol synthesis pathway. Statins suppress generation of farnesyl and geranygeranyl pyrophosphate, substrates of prenyl transferases, and thus inhibit prenylation of small G proteins.Citation22 PrePS sequence analysis tool predicts attachment of a 20-carbon geranylgeranyl moiety onto the C-terminal di-Cys motif of CRACR2A-a with high confidence although the C-terminal CCG residues of CRACR2A do not constitute a conventional prenylation motif for Rab GTPases (CxC or CC, x - any amino acid). However, the prenylation of CRACR2A-a was confirmed by mutation of these residues as well as after treatment with a prenylation inhibitor, atorvastatin. Under both these conditions, cytoplasmic localization of CRACR2A-a was observed, indicating that CRACR2A is indeed prenylated.
Another unique feature of CRACR2A-a is that GDP binding (GTP hydrolysis) and prenylation are closely related with its degradation. Small GTPases bind to GDIs after GTP hydrolysis, that allows their stabilization in the cytoplasm and eventually re-insertion into the membrane.Citation23,24 Contrary to small GTPases, CRACR2A-a is degraded after re-localization into the cytoplasm upon GTP hydrolysis. Atorvastatin treatment also induced de-prenylation, cytoplasmic localization and degradation of CRACR2A-a. Degradation of CRACR2A-a within the cytoplasm could be due to lack of GDI molecules to stabilize the cytoplasmic CRACR2A-a molecules. Activation and inactivation of CRACR2A-a consists of resting, effector, and termination steps controlled by GTP binding, prenylation, protein interactions, and degradation (). In resting T cells CRACR2A-a predominantly exists in a GTP-bound form (resting state). After TCR stimulation, CRACR2A-a-containing vesicles are recruited into the immunological synapse via interaction with Vav1 to activate the downstream Ca2+-NFAT and Jnk-AP1 signaling pathways (effector state). At the current stage of understanding, it is not clear whether CRACR2A-a-containing vesicles are actually fused to the plasma membrane or remain independent like Lat-containing vesicles. Inactivation of CRACR2A-a occurs via GTP hydrolysis to induce its cytoplasmic localization and finally degradation (termination step). A similar termination step can be also induced by statin treatment. Currently, the composition and destiny of the CRACR2A-a-containing vesicles per se remain unknown due to a lack of additional marker molecules.
Figure 2. Role of CRACR2A-a in regulation of TCR signaling. (a) A proposed model showing the role of CRACR2A-a in T cell receptor signaling. In resting T cells, CRACR2A-a is predominantly localized near trans Golgi network in a GTP-bound form (Resting state). Upon T cell receptor stimulation, CRACR2A-a-containing vesicles accumulate into the immunological synapse; by interaction of its proline-rich domain with Vav1 to activate downstream signaling pathways (Effector state). Currently, it is not known whether these vesicles are fused to the plasma membrane or remain as independent vesicles. Inactivation mechanisms include cytoplasmic localization of CRACR2A-a by GTP hydrolysis (potentially by other signaling molecules) or possible de-prenylation (Termination state). The cytoplasmic state of CRACR2A-a is likely to be temporary due to rapid degradation. Statin treatment also can induce this termination step by triggering de-prenylation, cytoplasmic location and degradation of CRACR2A-a. (b) Large GTPase family defined by their overall structure, including CRACR2A-a (EFCAB4B), Rab44 (RASD3, RASL13) and Rab45 (RASEF). Unconventional C-terminal prenylation sites are indicated. The amino acids of the short isoform CRACR2A-c and the long isoform CRACR2A-a are also indicated. PRD; proline-rich domain.
These results establish a foundation to understand the function and the regulatory mechanisms of other large Rab GTPases including Rab44 and Rab45 that have a high similarity in their overall structure with CRACR2A-a (). Numerous gene linkage analyses of acute and chronic myeloid leukemia, and melanoma identified Rab45 (alternatively RASEF [RAS and EF-hand domain containing] or FLJ31614) as a potential tumor suppressor gene.Citation25,26 However, the molecular mechanism underlying its relationship with human diseases is not clearly understood. Therefore, studies of CRACR2A-a can uncover possible roles of these large Rab GTPases in intracellular signaling and vesicle trafficking in the future.
More than 60 members of the Rab GTPase family have been identified in humans. However, our knowledge of Rab proteins is limited within the scope of the role of small Rab GTPases of 20-25 kDa in size in membrane trafficking. Therefore, identification of CRACR2A proteins as an intracellular signaling module bridging 2 important proximal TCR signaling pathways, Ca2+-NFAT and Jnk, to affect T cell activation will provide a conceptual framework to advance our understanding of the potential role of large Rab GTPases in intracellular signaling and their pathological mechanisms related to human diseases. Statins including atorvastatin are widely prescribed for their cholesterol-lowering effects. Interestingly, statin treatment also decreases TCR signaling and these drugs are also used to suppress autoimmune diseases in clinics.Citation27-29 Our results suggest that CRACR2A-a can be an important target of statins due to its high sensitivity toward statin treatment and the unique degradation mechanism after de-prenylation. Therefore, studies on CRACR2A-a can impact translational studies targeting prenylation of G proteins in intracellular signaling for T cell activation. Finally, the identity and function of CRACR2A-a-containing vesicles is likely to be an exciting area for future investigation to explore the role of vesicles in regulation of intracellular signaling pathways.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institute of Health grant AI083432 (Y.G.) and the American Heart Association grant 12SDG12040188 from (S.S).
References
- Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013; 13:257-69; PMID:23524462; http://dx.doi.org/10.1038/nri3403
- Villalba M, Hernandez J, Deckert M, Tanaka Y, Altman A. Vav modulation of the Ras/MEK/ERK signaling pathway plays a role in NFAT activation and CD69 up-regulation. Eur J Immunol 2000; 30:1587-96; PMID:10898494; http://dx.doi.org/10.1002/1521-4141(200006)30:6%3c1587::AID-IMMU1587%3e3.0.CO;2-T
- Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, Weiss A. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol 2010; 2:a002279; PMID:20452964; http://dx.doi.org/10.1101/cshperspect.a002279
- Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat Cell Biol 2009; 11:669-77; PMID:19488056; http://dx.doi.org/10.1038/ncb0609-669
- Carrasco S, Meyer T. STIM Proteins and the Endoplasmic Reticulum-Plasma Membrane Junctions. Annu Rev Biochem 2011; 80:973-1000; PMID:21548779.
- Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol 2011; 3:a003970; PMID:21791698; http://dx.doi.org/10.1101/cshperspect.a003970
- Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol 2005; 17:267-74; PMID:15886116; http://dx.doi.org/10.1016/j.coi.2005.04.003
- Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol 1997; 15:297-322; PMID:9143690; http://dx.doi.org/10.1146/annurev.immunol.15.1.297
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28:445-89; PMID:20192806; http://dx.doi.org/10.1146/annurev-immunol-030409-101212
- Kyriakis JM, Avruch J. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiol Rev 2012; 92:689-737; PMID:22535895; http://dx.doi.org/10.1152/physrev.00028.2011
- Purbhoo MA, Liu H, Oddos S, Owen DM, Neil MA, Pageon SV, French PM, Rudd CE, Davis DM. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci Signal 2010; 3:ra36; PMID:20460647; http://dx.doi.org/10.1126/scisignal.2000645
- Williamson DJ, Owen DM, Rossy J, Magenau A, Wehrmann M, Gooding JJ, Gaus K. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat Immunol 2011; 12:655-62; PMID:21642986; http://dx.doi.org/10.1038/ni.2049
- Balagopalan L, Barr VA, Kortum RL, Park AK, Samelson LE. Cutting edge: cell surface linker for activation of T cells is recruited to microclusters and is active in signaling. J Immunol 2013; 190:3849-53; PMID:23487428; http://dx.doi.org/10.4049/jimmunol.1202760
- Larghi P, Williamson DJ, Carpier JM, Dogniaux S, Chemin K, Bohineust A, Danglot L, Gaus K, Galli T, Hivroz C. VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites. Nat Immunol 2013; 14:723-31; PMID:23666293; http://dx.doi.org/10.1038/ni.2609
- Saoudi A, Kassem S, Dejean A, Gaud G. Rho-GTPases as key regulators of T lymphocyte biology. Small GTPases 2014; 5:e28208; PMID:24825161; http://dx.doi.org/10.4161/sgtp.28208
- Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol 2009; 9:630-44; PMID:19696767; http://dx.doi.org/10.1038/nri2606
- Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol 2006; 7:247-55; PMID:16444260; http://dx.doi.org/10.1038/ni1304
- Srikanth S, Kim KD, Gao Y, Woo JS, Ghosh S, Calmettes G, Paz A, Abramson J, Jiang M, Gwack Y. A large Rab GTPase encoded by CRACR2A is a component of subsynaptic vesicles that transmit T cell activation signals. Sci Signal 2016; 9:ra31; PMID:27016526; http://dx.doi.org/10.1126/scisignal.aac9171
- Srikanth S, Jung HJ, Kim KD, Souda P, Whitelegge J, Gwack Y. A novel EF-hand protein, CRACR2A, is a cytosolic Ca2+ sensor that stabilizes CRAC channels in T cells. Nat Cell Biol 2010; 12:436-46; PMID:20418871; http://dx.doi.org/10.1038/ncb2045
- Wilson LA, McKeown L, Tumova S, Li J, Beech DJ. Expression of a long variant of CRACR2A that belongs to the Rab GTPase protein family in endothelial cells. Biochem Biophys Res Commun 2015; 456:398-402; PMID:25475730; http://dx.doi.org/10.1016/j.bbrc.2014.11.095
- Dumas JJ, Zhu Z, Connolly JL, Lambright DG. Structural basis of activation and GTP hydrolysis in Rab proteins. Structure 1999; 7:413-23; PMID:10196122; http://dx.doi.org/10.1016/S0969-2126(99)80054-9
- Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol 2006; 6:358-70; PMID:16639429; http://dx.doi.org/10.1038/nri1839
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/10.1152/physrev.00059.2009
- van der Bliek AM. A sixth sense for Rab5. Nat Cell Biol 2005; 7:548-50; PMID:15928700; http://dx.doi.org/10.1038/ncb0605-548
- Kaplon J, Homig-Holzel C, Gao L, Meissl K, Verdegaal EM, van der Burg SH, Peeper DS. Near-genomewide RNAi screening for regulators of BRAF(V600E) -induced senescence identifies RASEF, a gene epigenetically silenced in melanoma. Pigment Cell Melanoma Res 2014; 27:640-52; PMID:24703243; http://dx.doi.org/10.1111/pcmr.12248
- Sweetser DA, Peniket AJ, Haaland C, Blomberg AA, Zhang Y, Zaidi ST, Dayyani F, Zhao Z, Heerema NA, Boultwood J, et al. Delineation of the minimal commonly deleted segment and identification of candidate tumor-suppressor genes in del(9q) acute myeloid leukemia. Genes Chromosomes Cancer 2005; 44:279-91; PMID:16015647; http://dx.doi.org/10.1002/gcc.20236
- Youssef S, Stuve O, Patarroyo JC, Ruiz PJ, Radosevich JL, Hur EM, Bravo M, Mitchell DJ, Sobel RA, Steinman L, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 2002; 420:78-84; PMID:12422218; http://dx.doi.org/10.1038/nature01158
- Weber MS, Youssef S, Dunn SE, Prod'homme T, Neuhaus O, Stuve O, Greenwood J, Steinman L, Zamvil SS. Statins in the treatment of central nervous system autoimmune disease. J Neuroimmunol 2006; 178:140-8; PMID:16860400; http://dx.doi.org/10.1016/j.jneuroim.2006.06.006
- Dunn SE, Youssef S, Goldstein MJ, Prod'homme T, Weber MS, Zamvil SS, Steinman L. Isoprenoids determine Th1/Th2 fate in pathogenic T cells, providing a mechanism of modulation of autoimmunity by atorvastatin. J Exp Med 2006; 203:401-12; PMID:16476765; http://dx.doi.org/10.1084/jem.20051129
- Morreale A, Venkatesan M, Mott HR, Owen D, Nietlispach D, Lowe PN, Laue ED. Structure of Cdc42 bound to the GTPase binding domain of PAK. Nat Struct Biol 2000; 7:384-8; PMID:10802735; http://dx.doi.org/10.1038/75158
