ABSTRACT
Guanine nucleotide Exchange Factors (GEFs) are responsible for mediating GDP/GTP exchange for specific small G proteins, such as Rac. There has been substantial evidence for the involvement of Rac-GEFs in the control of cancer cell migration and metastatic progression. We have previously established that the Rac-GEF P-Rex1 is a mediator of actin cytoskeleton rearrangements and cell motility in breast cancer cells downstream of HER/ErbB receptors and the G-Protein Coupled Receptor (GPCR) CXCR4. P-Rex1 is highly expressed in luminal A and B breast cancer compared to normal mammary tissue, whereas expression is very low in basal breast cancer, and its expression correlates with the appearance of metastasis in patients. Here, we discuss the involvement of P-Rex1 as an effector of oncogenic/metastatic receptors in breast cancer and underscore its relevance in the convergence of receptor-triggered motile signals. In addition, we provide an overview of our recent findings describing a cross-talk between HER/ErbB receptors and CXCR4, and how this impacts on the activation of P-Rex1/Rac1 signaling, as well as highlight challenges that lie ahead. We propose a model in which P-Rex1 acts as a crucial node for the integration of upstream inputs from HER/ErbB receptors and CXCR4 in luminal breast cancer cells.
P-Rex1: A Rac-GEF implicated in cancer progression
Rac GTPases are small G proteins that regulate essential cellular functions, including the maintenance of cell morphology, polarity, adhesion, and migration. This is achieved mostly through regulation of the actin cytoskeleton structure, with Rac being activated by a variety of stimuli downstream of different receptors including tyrosine-kinases and G-Protein Coupled Receptors (GPCRs).Citation1-3 As with other small G proteins, Rac activation is regulated as part of a cycle between a GDP-bound inactive form and its GTP-bound active form. The control of this GTPase cycle is tightly regulated by 3 families of proteins. Firstly, the Guanine-nucleotide Exchange Factors (GEFs), which catalyze nucleotide exchange to facilitate GTP binding. In contrast, the GTPase Activating Proteins (GAPs) act as negative regulators, by stimulating the intrinsic GTPase activity of Rac to hydrolyze GTP, and thus lead to its inactivation. The third family is the GDP Dissociation Inhibitors (GDIs), which sequester the inactive GDP-bound form in the cytoplasm, preventing Rac translocation and activation.Citation4,5
Dysregulation of normal Rac signaling has been known to have a role in many different diseases. For example, hyperactivation of the Rac pathway in cancer results in enhanced migration and invasiveness.Citation6,7 Such alterations in Rac signaling can involve up-regulation of Rac itself,Citation8 expression of the active spliced variant Rac1bCitation8,9 or, very rarely, mutations in RAC genes.Citation10,11 However, the most common mechanism that accounts for Rac hyperactivation in breast cancer is the aberrant action of its direct regulators, such as overexpression and/or hyperactivation of Rac-GEFs. Relevant examples in breast cancer include the overexpression of Vav3, which together with Vav2, is implicated in breast tumor growth and lung-specific metastasis,Citation12,13 and the upregulation of P-Rex1 which was shown to have a prominent role in disease progression.Citation14,15 Down-regulation of Rac-GAPs in breast cancer has also been shown.Citation16,17
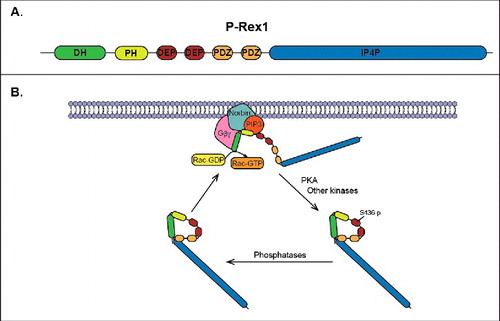
P-Rex1 was first identified in neutrophils as an exchange factor specific for Rac, where it was shown to regulate ROS production, motility, and polarity in response to activation by PIP3 and Gβγ.Citation18 This GEF is a large multidomain protein containing an N-terminal DH-PH domain tandem that is responsible for mediating its GEF activity toward Rac (). It also contains a pair of DEP and PDZ domains, as well as a C-terminal domain with homology to inositol polyphosphate 4-phosphatase (IP4P). The DH-PH tandem is known to be required for the interaction of P-Rex1 with PIP3 and Gβγ proteins, which have been shown to synergistically regulate its catalytic activity and localization.Citation18,19 Recent studies have successfully crystalized this DH-PH tandem of P-Rex1 in complex with Rac,Citation20,21 thus increasing our understanding of the structural mechanism behind these interactions and GEF activity. Post-translational modifications, specifically phosphorylations, as well as protein-protein interactions (such as the recently described association with norbin)Citation22 have been shown to regulate P-Rex1 activity. Of particular note is the phosphorylation by PKA, which inhibits PIP3- and Gβγ-stimulated P-Rex1 guanine nucleotide exchange activity on Rac.Citation23 PKA phosphorylates P-Rex1 in the first DEP domain, which was recently shown to mediate an autoinhibitory intramolecular interaction with the DH-PH tandem.Citation24 In contrast, the dephosphorylation of at least one site within the IP4P-like domain by PP1α positively regulates P-Rex1 activity.Citation25 The phosphorylation of P-Rex1 at other sites has also been implicated in the regulation of its GEF activity.Citation15,26,27 The regulation and function of P-Rex family members has been described in great detail in a recent review.Citation28
Figure 1. Regulation of P-Rex1 activity. (A) Domain structure of P-Rex1 showing the DH-PH tandem, PDZ domains, DEP domains, and IP4P-like domain. (B) Proposed cycle of activation/deactivation of P-Rex1. In response to receptor activation, inactive P-Rex1 in the cytoplasm is recruited to the plasma membrane by direct interactions with PIP3, Gβγ subunits and norbin. The interactions with norbin and PIP3 occur via the PH domain, while Gβγ subunits bind directly to the DH domain. At the plasma membrane P-Rex1 GEF-activity toward Rac is stimulated by PIP3, Gβγ and norbin, thus resulting in the release of GDP from Rac and the binding of GTP. PKA phosphorylates P-Rex1 at the plasma membrane in Ser436 located in the first DEP domain, which results in intramolecular autoinhibitory interactions between the DH-PH tandem and the first DEP domain.
Since the initial discovery of P-Rex1, this protein has emerged as an important factor in the progression of multiple cancers. For example, P-Rex1 overexpression has been reported in a high proportion of primary metastatic melanomas, as well as some prostate cancers, where it was shown to contribute to the metastatic phenotype.Citation29,30 Interestingly, another P-Rex family member, P-Rex2, has also been demonstrated as having oncogenic potential, with overexpression identified in a small sample of gastric cancersCitation31 as well as mutations found in metastatic melanomas.Citation32 Previous studies by our lab and others have identified P-Rex1 as a mediator of actin reorganization and cell motility in luminal breast cancer cells.Citation14,15 We showed that overexpression of the P-Rex1 protein occurs in luminal A and B breast cancer, and observed a significant correlation with estrogen receptor expression. On the other hand, P-Rex1 is not expressed in basal/triple negative breast cancer, where other GEFs may control Rac1 activation. P-Rex1 up-regulation in luminal breast cancer involves the demethylation of the PREX1 gene promoter, unlike Vav3, which does not seem to be epigenetically regulated.Citation33 It became clear that P-Rex1 is an effector of both tyrosine-kinase and G-protein-coupled receptors (GPCRs), and that this Rac-GEF has a primary role as a mediator of motile responses driven by receptor stimulation.Citation14
P-Rex1 as a node for HER/ErbB receptor tyrosine-kinase and GPCR signals
One of the hallmarks of breast cancer is the hyperactivation of HER/ErbB receptor signaling. The HER/ErbB family of proteins consists of the type I transmembrane growth factor receptors EGFR/HER1/ErbB1, HER2/ErbB2, HER3/ErbB3, and HER4/ErbB4. These receptors contain an extracellular region that binds a ligand (except for the ligandless receptor HER2), a transmembrane domain, and an intracellular catalytic domain. Ligand binding induces a conformational change in the extracellular domain thus promoting HER/ErbB protein homo- or heterodimerization and consequently transphosphorylation of intracellular domains.Citation34 The HER2/HER3 complex is a major oncogenic unit in mammary tumors, with overexpression of HER2 identified in 20–25% of invasive breast cancers.Citation35 This results in enhanced proliferation and survival signaling, and is functionally translated into epithelial-mesenchymal transition, loss of polarity, loss of adhesion, cell invasiveness, genome instability, metabolic changes, and angiogenesis.Citation36 Arguably the most important oncogenic signaling cascades activated by the HER2/HER3 complex are the phosphatidylinositol 3-kinase (PI3K)/Akt, extracellular-signal regulated kinase (ERK), and protein kinase C (PKC) pathways.Citation37 We previously established that activation of HER/ErbB receptors in luminal breast cancer cells leads to a migratory response that is mediated by P-Rex1/Rac1. HER ligands induce the relocalization of P-Rex1 to the plasma membrane, a site where it promotes GTP loading onto Rac1, with the subsequent activation of motility pathways.Citation14
In addition to the role of HER/ErbB receptors in breast cancer, GPCRs have also been described as essential components of the tumorigenic process. CXCR4, a GPCR that selectively binds the chemokine Stromal cell-Derived Factor-1 (SDF-1/CXCL12), is expressed at high levels in breast cancer when compared to adjacent normal tissue.Citation38 CXCR4 overexpression has been associated with a significant reduction of disease free survival and overall survival in breast cancer patients, as well as increased metastasis to lymph nodes.Citation39-41 Furthermore, aberrant expression of this chemokine receptor is known to cause changes in invasion, transendothelial migration at the primary tumor site, proliferation, angiogenesis, and the recruitment of immune cells, thus contributing to cancer progression.Citation42 CXCR4 controls these relevant functions through the activation of signaling cascades that include PI3K/Akt, NF-κB, Erk, and STAT3 pathways.Citation43-46 Interestingly, a correlation between CXCR4 and HER2 expression in human breast tumors has been reported, with a significant number of patients exhibiting co-expression of these receptors.Citation47 It has also been demonstrated that CXCR4 is required for HER2-mediated invasion in vitro as well as metastasis in vivo, thus establishing a functional link between the HER2 and CXCR4 signaling pathways.Citation48
In a previous study,Citation14 we reported that CXCR4 stimulation with SDF-1 in breast cancer cells leads to Rac1 activation and a motile response, effects that are mediated by P-Rex1. Moreover, we found that CXCR4 was required for the activation of P-Rex1 and Rac1 by the HER/ErbB receptors. Indeed, P-Rex1/Rac1 activation by the HER3 ligand heregulin (HRG) was greatly reduced upon CXCR4 RNAi silencing. A detailed analysis revealed that stimulation of luminal breast cancer cells with HRG caused transactivation of CXCR4, an effect that was not affected by a CXCR4 antagonist and therefore was independent of the CXCR4 ligand SDF-1. HRG treatment led to a significant elevation in Ser324/325- and Ser330-CXCR4 phosphorylation, residues that typically regulate CXCR4 signaling and trafficking, as well as promote arrestin-2 binding to the GPCR. This not only showed that CXCR4 becomes indirectly activated in response to HER3 ligands but also established a role for CXCR4 in the activation of P-Rex1/Rac1 by HER/ErbB receptors. CXCR4 couples to Gαi, therefore promoting the release of Gβγ subunits upon stimulation. A reasonable hypothesis is that this transactivation represents a major contributor to the activation of the P-Rex1/Rac1-dependent motility, since P-Rex1 is a PIP3- and Gβγ-dependent Rac-GEF. Not surprisingly, HER3-driven breast cancer cell migration was found to be sensitive to Gαi inhibition with pertussis toxin.Citation14 Furthermore, studies by other groups have confirmed the emergence of P-Rex1 as a key regulator in the metastatic response of cancer cells to the stimulation of HER/ErbB and CXCR4 receptors.Citation15,30
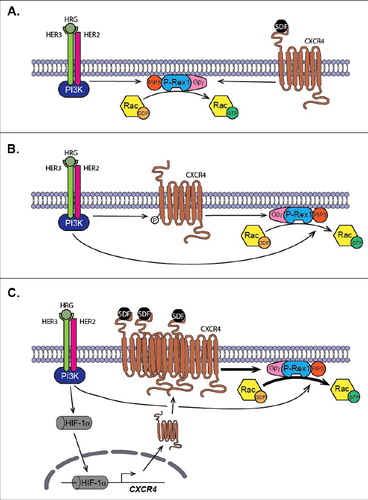
In our recent work,Citation49 we demonstrated that, in addition to receptor transactivation, another mechanism of synergism between HER/ErbB receptors and CXCR4 signaling exists that ultimately impacts on P-Rex1/Rac1-driven cell motility. In this study, we observed that sustained exposure to HRG (a scenario that mimics the ligand overexpression that occurs in a significant proportion of breast tumors) sensitizes breast cancer cells through the HER3 receptor to SDF-1-induced Rac1 activation and motility, effects largely mediated by P-Rex1. Knocking down P-Rex1 essentially abolished this sensitizing mechanism. It was found that enhanced Rac1 activation by SDF-1 is due to increased surface expression of CXCR4. It has previously been established that hypoxia stress promotes the expression of CXCR4 through activation of the hypoxia inducible factor-1 α (HIF-1α) transcription factor.Citation50-52 Like hypoxia, many growth factors and cytokines also up-regulate HIF-1α protein levels under normoxic conditions.Citation53-55 Remarkably, after continuous stimulation of HER3 receptors with HRG, the levels of HIF-1α protein increased.Citation49,55 We further demonstrated, through the use of mutagenesis and chromatin immunoprecipitation, that this transcription factor activates the CXCR4 gene via binding to a hypoxia-responsive element (HRE) located in positions -1376 to -1372 in the CXCR4 promoter. The functional consequence was the elevation of CXCR4 surface expression by HRG in luminal breast cancer cells, the augmentation of Rac1 activation via P-Rex1, and the consequent increase in cell motility by SDF-1.Citation49 The model we propose is that P-Rex1 is a key integrator of upstream inputs from HER/ErbB receptors and CXCR4 in breast cancer cells. Signals from oncogenic receptors converge on P-Rex1, therefore leading to Rac1 activation, and this occurs by at least 3 different mechanisms (). The first is the direct activation of P-Rex1 by HER/ErbB receptors and CXCR4. The second involves the transactivation of CXCR4 by HER/ErbB receptors in an SDF-1-independent manner, a step that provides the required Gβγ component for P-Rex1 activation. Finally, the P-Rex1/Rac1 motility signaling is enhanced as a consequence of CXCR4 upregulation caused by sustained HER receptor activation. It is likely that other tyrosine-kinase receptors and GPCRs utilize similar mechanisms to signal via P-Rex1 to promote motile and metastatic responses.
Figure 2. P-Rex1 acts as a convergence point for pro-oncogenic/metastatic pathways. (A) Activation of P-Rex1 by ligand stimulation of HER/ErbB receptors (for example with HRG) and CXCR4 (with SDF-1). PIP3 and Gβγ subunits generated upon receptor stimulation activate P-Rex1 GEF-activity toward Rac. (B) HER/ErbB receptors transactivate CXCR4 independently of SDF-1, leading to P-Rex1/Rac activation. (C) Sustained stimulation of HER3/ErbB3 stabilizes the expression of the HIF-1α, leading to enhanced transcriptional activation of the CXCR4 gene. This results in up-regulation of CXCR4 surface expression and augmented P-Rex1/Rac1 motility signaling.
Future perspectives
The convergence of multiple tumorigenic signaling inputs on P-Rex1 makes this Rac-GEF an interesting target for anti-cancer therapy. The prospect of a P-Rex1 inhibitor, which would prevent Rac activation and therefore reduce the impact of CXCR4 or HER oncogenic signaling, is particularly thrilling. Despite the obvious difficulties in generating cell permeable P-Rex1 small molecule inhibitors capable of interfering with the P-Rex1-Rac interactions at suitably low concentrations, the recent identification of leading P-Rex1 inhibitor compoundsCitation56 and the availability of P-Rex1 3-D structuresCitation20,21 are encouraging indicators for near future success. This endeavor would be greatly facilitated by a better understanding of the mechanisms of P-Rex1 regulation, such as phosphorylation modifications and the identification of protein partners, which should provide us with alternative approaches for functionally inhibiting this Rac-GEF.
There are many questions surrounding the role of P-Rex1 in cancer that remain to be answered. For example, the role of P-Rex1 in leukocytes has been well characterized,Citation28 but its role in the interaction between these cells and cancer cells during tumorigenesis is yet to be investigated. Furthermore, most of our current understanding centers on P-Rex1 GEF activity via the DH-PH tandem, while the role of other protein domains or potential GEF-independent functions in cancer are yet to be identified. Novel regulatory mechanisms of GEF activity are also beginning to emerge, with the recent identification of the binding partner norbin,Citation22 as well as insights into how this large multidomain Rac-GEF is regulated by kinases.Citation24,57 Also, the role of any other Rac-GEFs that may provide redundancy for P-Rex1 has yet to be investigated. Despite the questions that remain unanswered, P-Rex1 has undoubtedly become a prominent pro-oncogenic and pro-metastatic protein in multiple cancers, and further insights into its regulation and function will be of particular interest.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70:389-99; PMID:1643657; https://doi.org/10.1016/0092-8674(92)90163-7
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70:401-10; PMID:1643658; https://doi.org/10.1016/0092-8674(92)90164-8
- Stephens L, Milne L, Hawkins P. Moving towards a better understanding of chemotaxis. Curr Biol 2008; 18:R485-94; PMID:18522824; https://doi.org/10.1016/j.cub.2008.04.048
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247-69; PMID:16212495; https://doi.org/10.1146/annurev.cellbio.21.020604.150721
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol 2005; 6:167-80; PMID:15688002; https://doi.org/10.1038/nrm1587
- Bustelo XR. Intratumoral stages of metastatic cells: a synthesis of ontogeny, Rho/Rac GTPases, epithelial-mesenchymal transitions, and more. Bioessays 2012; 34:748-59; PMID:22706877; https://doi.org/10.1002/bies.201200041
- Wang SE, Shin I, Wu FY, Friedman DB, Arteaga CL. HER2/Neu (ErbB2) signaling to Rac1-Pak1 is temporally and spatially modulated by transforming growth factor beta. Cancer Res 2006; 66:9591-600; PMID:17018616; https://doi.org/10.1158/0008-5472.CAN-06-2071
- Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, Graeff H, Harbeck N, Schmitt M, Lengyel E. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene 2000; 19:3013-20; PMID:10871853; https://doi.org/10.1038/sj.onc.1203621
- Singh A, Karnoub AE, Palmby TR, Lengyel E, Sondek J, Der CJ. Rac1b, a tumor associated, constitutively active Rac1 splice variant, promotes cellular transformation. Oncogene 2004; 23:9369-80; PMID:15516977; https://doi.org/10.1038/sj.onc.1208182
- Hwang SL, Lieu AS, Chang JH, Cheng TS, Cheng CY, Lee KS, Lin CL, Howng SL, Hong YR. Rac2 expression and mutation in human brain tumors. Acta Neurochir (Wien) 2005; 147:551-4; discussion 4; PMID:15812594; https://doi.org/10.1007/s00701-005-0515-5
- Krauthammer M, Kong Y, Ha BH, Evans P, Bacchiocchi A, McCusker JP, Cheng E, Davis MJ, Goh G, Choi M, et al. Exome sequencing identifies recurrent somatic RAC1 mutations in melanoma. Nat Genet 2012; 44:1006-14; PMID:22842228; https://doi.org/10.1038/ng.2359
- Barrio-Real L, Kazanietz MG. Rho GEFs and cancer: linking gene expression and metastatic dissemination. Sci Signal 2012; 5:pe43; PMID:23033535; https://doi.org/10.1126/scisignal.2003543
- Citterio C, Menacho-Marquez M, Garcia-Escudero R, Larive RM, Barreiro O, Sanchez-Madrid F, Paramio JM, Bustelo XR. The rho exchange factors vav2 and vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Signal 2012; 5:ra71; PMID:23033540; https://doi.org/10.1126/scisignal.2002962
- Sosa MS, Lopez-Haber C, Yang C, Wang H, Lemmon MA, Busillo JM, Luo J, Benovic JL, Klein-Szanto A, Yagi H, et al. Identification of the Rac-GEF P-Rex1 as an essential mediator of ErbB signaling in breast cancer. Mol Cell 2010; 40:877-92; PMID:21172654; https://doi.org/10.1016/j.molcel.2010.11.029
- Montero JC, Seoane S, Ocana A, Pandiella A. P-Rex1 participates in Neuregulin-ErbB signal transduction and its expression correlates with patient outcome in breast cancer. Oncogene 2011; 30:1059-71; PMID:21042280; https://doi.org/10.1038/onc.2010.489
- Casado-Medrano V, Barrio-Real L, Garcia-Rostan G, Baumann M, Rocks O, Caloca MJ. A new role of the Rac-GAP beta2-chimaerin in cell adhesion reveals opposite functions in breast cancer initiation and tumor progression. Oncotarget 2016; PMID:27058424
- Yang C, Liu Y, Leskow FC, Weaver VM, Kazanietz MG. Rac-GAP-dependent inhibition of breast cancer cell proliferation by {beta}2-chimerin. J Biol Chem 2005; 280:24363-70; PMID:15863513; https://doi.org/10.1074/jbc.M411629200
- Welch HC, Coadwell WJ, Ellson CD, Ferguson GJ, Andrews SR, Erdjument-Bromage H, Tempst P, Hawkins PT, Stephens LR. P-Rex1, a PtdIns(3,4,5)P3- and Gbetagamma-regulated guanine-nucleotide exchange factor for Rac. Cell 2002; 108:809-21; PMID:11955434; https://doi.org/10.1016/S0092-8674(02)00663-3
- Hill K, Krugmann S, Andrews SR, Coadwell WJ, Finan P, Welch HC, Hawkins PT, Stephens LR. Regulation of P-Rex1 by phosphatidylinositol (3,4,5)-trisphosphate and Gbetagamma subunits. J Biol Chem 2005; 280:4166-73; PMID:15545267; https://doi.org/10.1074/jbc.M411262200
- Lucato CM, Halls ML, Ooms LM, Liu HJ, Mitchell CA, Whisstock JC, Ellisdon AM. The Phosphatidylinositol (3,4,5)-Trisphosphate-dependent Rac Exchanger 1. Ras-related C3 Botulinum Toxin Substrate 1 (P-Rex1.Rac1) Complex Reveals the Basis of Rac1 Activation in Breast Cancer Cells. J Biol Chem 2015; 290:20827-40; PMID:26112412; https://doi.org/10.1074/jbc.M115.660456
- Cash JN, Davis EM, Tesmer JJ. Structural and biochemical characterization of the catalytic core of the metastatic factor P-Rex1 and its regulation by PtdIns(3,4,5)P3. Structure 2016; 24:730-40; PMID:27150042; https://doi.org/10.1016/j.str.2016.02.022
- Pan D, Barber MA, Hornigold K, Baker MJ, Toth JM, Oxley D, Welch HC. Norbin stimulates the catalytic activity and plasma membrane localization of the guanine-nucleotide exchange factor P-Rex1. J Biol Chem 2016; 291:6359-75; PMID:26792863; https://doi.org/10.1074/jbc.M115.686592
- Mayeenuddin LH, Garrison JC. Phosphorylation of P-Rex1 by the cyclic AMP-dependent protein kinase inhibits the phosphatidylinositiol (3,4,5)-trisphosphate and Gbetagamma-mediated regulation of its activity. J Biol Chem 2006; 281:1921-8; PMID:16301320; https://doi.org/10.1074/jbc.M506035200
- Chavez-Vargas L, Adame-Garcia SR, Cervantes-Villagrana RD, Castillo-Kauil A, Bruystens JG, Fukuhara S, Taylor SS, Mochizuki N, Reyes-Cruz G, Vázquez-Prado J. Protein kinase A (PKA) type I interacts with P-Rex1, a Rac guanine nucleotide exchange factor: EFFECT ON PKA LOCALIZATION AND P-Rex1 SIGNALING. J Biol Chem 2016; 291:6182-99; PMID:26797121; https://doi.org/10.1074/jbc.M115.712216
- Barber MA, Hendrickx A, Beullens M, Ceulemans H, Oxley D, Thelen S, Thelen M, Bollen M, Welch HC. The guanine-nucleotide-exchange factor P-Rex1 is activated by protein phosphatase 1alpha. Biochem J 2012; 443:173-83; PMID:22242915; https://doi.org/10.1042/BJ20112078
- Montero JC, Seoane S, Pandiella A. Phosphorylation of P-Rex1 at serine 1169 participates in IGF-1R signaling in breast cancer cells. Cell Signal 2013; 25:2281-9; PMID:23899556; https://doi.org/10.1016/j.cellsig.2013.07.018
- Nie B, Cheng N, Dinauer MC, Ye RD. Characterization of P-Rex1 for its role in fMet-Leu-Phe-induced superoxide production in reconstituted COS(phox) cells. Cell Signal 2010; 22:770-82; PMID:20074642; https://doi.org/10.1016/j.cellsig.2010.01.001
- Welch HC. Regulation and function of P-Rex family Rac-GEFs. Small GTPases 2015; 6:49-70; PMID:25961466; https://doi.org/10.4161/21541248.2014.973770
- Lindsay CR, Lawn S, Campbell AD, Faller WJ, Rambow F, Mort RL, Timpson P, Li A, Cammareri P, Ridgway RA, et al. P-Rex1 is required for efficient melanoblast migration and melanoma metastasis. Nat Commun 2011; 2:555; PMID:22109529; https://doi.org/10.1038/ncomms1560
- Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, Scofield MA, Dowd FJ, Lin MF, Tu Y. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene 2009; 28:1853-63; PMID:19305425; https://doi.org/10.1038/onc.2009.30
- Guo B, Liu L, Yao J, Ma R, Chang D, Li Z, Song T, Huang C. miR-338-3p Suppresses Gastric Cancer Progression through a PTEN-AKT Axis by Targeting P-REX2a. Am Associat Cancer Res 2014; 12:313-21; PMID:24375644
- Berger MF, Hodis E, Heffernan TP, Deribe YL, Lawrence MS, Protopopov A, Ivanova E, Watson IR, Nickerson E, Ghosh P, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature 2012; 485:502-6; PMID:22622578
- Barrio-Real L, Benedetti LG, Engel N, Tu Y, Cho S, Sukumar S, Kazanietz MG. Subtype-specific overexpression of the Rac-GEF P-REX1 in breast cancer is associated with promoter hypomethylation. Breast Cancer Res 2014; 16:441; PMID:25248717; https://doi.org/10.1186/s13058-014-0441-7
- Hendriks BS, Opresko LK, Wiley HS, Lauffenburger D. Quantitative analysis of HER2-mediated effects on HER2 and epidermal growth factor receptor endocytosis: distribution of homo- and heterodimers depends on relative HER2 levels. J Biol Chem 2003; 278:23343-51; PMID:12686539; https://doi.org/10.1074/jbc.M300477200
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244:707-12; PMID:2470152; https://doi.org/10.1126/science.2470152
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001; 2:127-37; PMID:11252954; https://doi.org/10.1038/35052073
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007; 26:6469-87; PMID:17471238; https://doi.org/10.1038/sj.onc.1210477
- Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, Bianchi R, Basik M. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat 2006; 97:275-83; PMID:16344916; https://doi.org/10.1007/s10549-005-9121-8
- Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res 2005; 7:R402-10; PMID:15987445; https://doi.org/10.1186/bcr1022
- Mukherjee D, Zhao J. The Role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res 2013; 3:46-57; PMID:23359227
- Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res 2003; 5:R144-50; PMID:12927045; https://doi.org/10.1186/bcr627
- Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res 2014; 124:31-82; PMID:25287686; https://doi.org/10.1016/B978-0-12-411638-2.00002-1
- Peng SB, Peek V, Zhai Y, Paul DC, Lou Q, Xia X, Eessalu T, Kohn W, Tang S. Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells. Mol Cancer Res 2005; 3:227-36; PMID:15831676
- Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 2003; 278:21631-8; PMID:12690099; https://doi.org/10.1074/jbc.M300609200
- Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res 2005; 65:9891-8; PMID:16267013; https://doi.org/10.1158/0008-5472.CAN-05-1293
- Vila-Coro AJ, Rodriguez-Frade JM, Martin De Ana A, Moreno-Ortiz MC, Martinez AC, Mellado M. The chemokine SDF-1alpha triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J 1999; 13:1699-710; PMID:10506573
- Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, Wang J, Li J, Du Q, Sun B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res 2010; 29:16; PMID:20181250; https://doi.org/10.1186/1756-9966-29-16
- Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell 2004; 6:459-69; PMID:15542430; https://doi.org/10.1016/j.ccr.2004.09.027
- Lopez-Haber C, Barrio-Real L, Casado-Medrano V, Kazanietz MG. Heregulin/ErbB3 signaling enhances cxcr4-driven Rac1 activation and breast cancer cell motility via HIF-1alpha. Mol Cell Biol 2016; PMID:27185877
- Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med 2003; 198:1391-402; PMID:14597738; https://doi.org/10.1084/jem.20030267
- Ishikawa T, Nakashiro K, Klosek SK, Goda H, Hara S, Uchida D, Hamakawa H. Hypoxia enhances CXCR4 expression by activating HIF-1 in oral squamous cell carcinoma. Oncol Rep 2009; 21:707-12; PMID:19212630
- Zagzag D, Lukyanov Y, Lan L, Ali MA, Esencay M, Mendez O, Yee H, Voura EB, Newcomb EW. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest 2006; 86:1221-32; PMID:17075581; https://doi.org/10.1038/labinvest.3700482
- Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol 1999; 154:1125-35; PMID:10233851; https://doi.org/10.1016/S0002-9440(10)65365-5
- Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM. Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem 2005; 280:22473-81; PMID:15802268; https://doi.org/10.1074/jbc.M500963200
- Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 2001; 21:3995-4004; PMID:11359907; https://doi.org/10.1128/MCB.21.12.3995-4004.2001
- Cardama GA, Comin MJ, Hornos L, Gonzalez N, Defelipe L, Turjanski AG, Alonso DF, Gomez DE, Menna PL. Preclinical development of novel Rac1-GEF signaling inhibitors using a rational design approach in highly aggressive breast cancer cell lines. Anticancer Agents Med Chem 2014; 14:840-51; PMID:24066799; https://doi.org/10.2174/18715206113136660334
- Urano D, Nakata A, Mizuno N, Tago K, Itoh H. Domain-domain interaction of P-Rex1 is essential for the activation and inhibition by G protein betagamma subunits and PKA. Cell Signal 2008; 20:1545-54; PMID:18514484; https://doi.org/10.1016/j.cellsig.2008.04.009
