ABSTRACT
The appearance of multicellularity implied the adaptation of signaling networks required for unicellular life to new functions arising in this remarkable evolutionary transition. A hallmark of multicellular organisms is the formation of cellular barriers that compartmentalize spaces and functions. Here we discuss recent findings concerning the role of RhoB in the negative control of Rac1 trafficking from endosomes to the cell border, in order to induce membrane extensions to restore endothelial barrier function after acute contraction. This role closely resembles that proposed for RhoB in controlling single cell migration through Rac1, which has also been observed in cancer cell invasion. We highlight these similarities as a signaling paradigm that shows that endothelial barrier integrity is controlled not only by the formation of cell-cell junctions, but also by a balance between ancestral mechanisms of cell spreading and contraction conserved from unicellular organisms and orchestrated by Rho GTPases.
Introduction
The formation of cellular barriers that compartmentalize cavities is one of the first requirements for the acquisition of multi cellularity.Citation1 The organization of cells into single layers has facilitated morphogenetic processes in complex organisms, starting from gastrulation, which ensures the precise organization of the embryo into cell sheets that differentiate into tissues. Many of these tissues conserve an organization into cellular barriers, namely parenchymal epithelia and vascular endothelia.Citation2,3 It is thus probable that the molecular mechanisms regulating the life of single cells, for example, chemotaxis and migration, had to be remodeled in order to adapt cells to the mechanical constriction and new duties related to barrier function. Indeed, central regulators of cell barrier function, such as cell-cell adhesion molecules or the actin cytoskeleton, probably played barrier-independent roles in unicellular organisms before multicellularity arose.Citation4-6 This commentary highlights the similarities between the roles of the RhoGTPases RhoB and Rac1 during the extension of lamellipodia in single amoeboid-type cell migration, and those that regulate endothelial barrier reformation after acute contraction has been induced by inflammatory mediators.Citation7
Rac1 endosomal trafficking mediates lamellipodial extension during single cell migration
The Rho GTPase family comprises 20 proteins, most of which cycle between GTP-loaded, active and a GDP-loaded, inactive state. Upon activation, Rho GTPases are translocated to membranes through the exposure of their C-terminus domain, which contains lipid modifications. Rho GTPases activate their effectors from the plasma membrane and also from internal cellular membranes such as those of the Golgi, or endosomes or even in the nucleus.Citation8,9 In addition, some Rho proteins, such as RhoB or Rac1, can localize in different compartments depending on the type and intensity of the stimulus.Citation7,9,10 In 2008, Palamidessi et al. showed that Rac1-mediated actin polymerization and membrane protrusion require spatially restricted endosomal trafficking, in which Rac1 is internalized in early endosomes, where it meets the Rho GEF Tiam1, becomes active and recycles back to the plasma membrane. Rac1 endosomal trafficking underlay the formation of actin structures such as dorsal ruffles in response to migration-promoting growth factors, namely HGF and VEGF.Citation11 Rac1 intracellular trafficking was detected not only in single motile mammalian tumor cells but also in primordial germinal cells from zebrafish, suggesting a general mechanism to locally restrict subcortical actin polymerization to regions where growth factor stimulus is detected.Citation11 In accordance, the endosomal accumulation of Rac and Rac GEFs has been observed in different eukaryotic organisms, from DictyosteliumCitation12 to humans.Citation11,13
Endosomal RhoB regulates actin polymerization and Rac1 activation in single cell migration
A few years before Palamidessi´s work, Margaret Frame´s laboratory reported that endosomal RhoB is required for translocation of the kinase Src from endosomes to the plasma membrane to mediate actin polymerization, which proved to be essential for lamellipodial extension in primary fibroblasts.Citation14 RhoB-positive endosomes harbored actin and scar1, a protein that is involved in actin polymerization. This was the first indication that endosomes could locally regulate actin polymerization, which was later confirmed when Dia1 and mDia2 were found in RhoB-positive intracellular vesicles. In these endosomes, RhoB recruits diaphanous formins, which locally polymerize actin, thereby regulating actin-dependent endosomal mobility.Citation15,16 These studies did not address the potential role of other GTPases in endosomal trafficking, actin polymerization and cell motility. In a more pathological context, RhoB has been found to be involved not only in cell migration but also in cell transformation and invasion. In bronchial cell lines, knockdown of RhoB promotes migration and invasiveness. Moreover, RhoB absence favors the aggressiveness of lung adenocarcinoma.Citation17 Interestingly, the role of RhoB constraining migration in bronchial cells is mediated through the negative regulation of Rac1 and Akt1 activity.Citation18,19 In smooth muscle cells and prostate cell lines, however, RhoB silencing has an opposing effect and decreases Rac1 activity and Rac-mediated lamellipodial protrusions, suggesting that RhoB activation positively regulates Rac1.Citation20 Together, these studies suggest that the effect of RhoB on Rac1 is cell and stimulus-specific.Citation21 In vascular and lymphatic endothelial beds, these opposing roles of RhoB in migration-related events are striking. Loss of RhoB reduces angiogenesis in the vascular endothelium and promotes it in lymphatic endothelium.Citation22
RhoB regulates Rac1 trafficking and activity thereby regulating endothelial barrier function during inflammation
In a search for new proteins essential to the inflammatory response, we have found that RhoB is upregulated in response to inflammatory cytokines such as IL-1β and TNF. Such upregulation is quite specific and the expression of most of the Rho family members is unaltered upon cytokine exposure.Citation7 RhoB can be considered as a stress protein that is upregulated in response to different stimuli. These include UV light,Citation23 hypoxiaCitation24 TGFβCitation25 and the aforementioned cytokines.Citation7,26 RhoB plays a role in cell survival and apoptosis, but its upregulation has an additional, often underestimated effect of increasing the total concentration and activity of the RhoA subfamily proteins on the stressed cell, which are the master promoters of actin polymerization. In human vascular endothelial cells exposed to TNF, quantification of the relative expression levels of upregulated RhoB with its closest relatives, RhoA and RhoC, revealed that RhoA, RhoB and RhoC are equally expressed and activated. Thus, upon RhoB upregulation, TNF increases the total protein expression in the RhoA subfamily, the main Rho kinase and actomyosin-regulators, between 40 and 50%.Citation7 Indeed, these 3 Rho proteins play redundant roles in the control of filamentous (F-) actin levels. Specific Rho silencing with siRNA induces the upregulation of the remaining Rho proteins, compensating the total RhoA subfamily protein levels in a remarkably quantitative way. In addition, single and double Rho silencing have a minor effect on endothelial F-actin, whereas triple Rho knockdown, reduces F-actin levels to those obtained upon treatment with the Rho family inhibitor, C3 transferase.Citation7,27 Therefore, in endothelial barriers RhoA, RhoB and RhoC compensate each other to maintain the homeostasis of F-actin.
However, in addition to these cooperative roles, specific Rho silencing led us to identify a specific role for RhoB in endothelial cells pretreated with TNF, which strikingly resembled that observed in single cell migration. Thrombin is a protease that activates by proteolysis the protease activated receptors (PARs), which are G protein-coupled receptors that activate actomyosin-mediated acute contraction. Thrombin and PARs are crucial in inflammation-related vascular pathologies, such as sepsis and acute respiratory distress syndrome. They contribute to the vascular tone but also induce vascular barrier disruption, often with fatal consequences.Citation28,29 Triple Rho knockdown or inhibition of common downstream effectors, such as ROCKs, inhibits thrombin-induced acute contraction in endothelial barriers.Citation7,30 However, reduction of RhoB expression, but not of RhoA or RhoC, accelerates the recovery of barrier function after this acute contraction. Interestingly, this increase in the ability of the endothelium to reform the cellular barrier appears to be independent of cell-cell junction integrity and is more closely related to the greater capacity of endothelial cells to form plasma membrane extensions in the absence of RhoB expression, which favors and enhances the establishment of new cell-cell contacts. The intensity of this effect is correlated with the physiological levels of RhoB expression: in the absence of TNF exposure, RhoB levels are low and the effect of siRNA RhoB on barrier repair is weaker than in TNF-pretreated cells, which express high levels of this GTPase. In addition, the effect of RhoB depletion occurs mostly during the time of barrier reformation, which is associated with the increase in trafficking of RhoB-positive vesicles during this particular period.Citation7
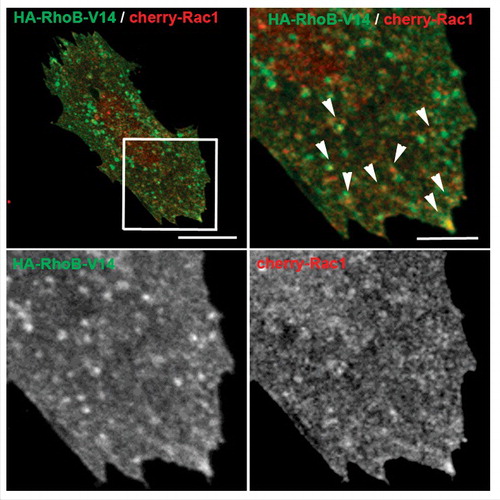
Taking into account the previous research on the role of RhoB in regulating Rac1 activity in tumor cell migration and the fact that Rac1 is the master regulator of lamellipodial extension, we investigated whether the effect of RhoB siRNA on barrier repair and plasma membrane extension after thrombin-induced contraction is mediated by Rac1 in endothelial cells. RhoB depletion increases Rac1 activation at the time period in which cells produce their lamellipodial-like extensions. Moreover, we observed that Rac1 is localized not only at the plasma membrane but also in intracellular vesicles (). During re-extension of the plasma membrane after acute contraction, Rac1-positive vesicle trafficking increases in a way similar to that previously observed for RhoB. In addition, pharmacological Rac1 inhibition during endothelial barrier reformation abrogates the increase in barrier recovery induced by RhoB depletion.Citation7 Collectively, these results suggest that RhoB controls endothelial barrier reformation by increasing Rac1 activation thereby forming plasma membrane extensions. A relative quantification of Rac1 subcellular localization revealed that high RhoB activity or expression retains Rac1 in a late endosomal compartment, whereas RhoB silencing promotes Rac1 translocation to the cell border. Although Rac1 and RhoB were found in the same vesicles (), super-resolution confocal microscopy and proximity assays, suggest that both GTPases are in proximity, but in different membrane domains.Citation7 Altogether, these findings indicate that RhoB regulates Rac1 dynamics between membrane early and late endosomal compartments. This Rac1 intracellular trafficking during endothelial barrier reformation closely resembles that found in single cell migration, although the effect of RhoB preventing Rac1 recycling to the cell border has not yet been addressed in migrating cells.
Figure 1. Rac1 co localizes with active RhoB in a vesicular compartment. The constitutively active mutant HA-RhoB-V14 and cherry-Rac1 were expressed in human microvascular endothelial cells. Cells were fixed, stained for HA and analyzed by confocal microscopy. Arrows point at vesicular structures containing active RhoB and Rac1. Top right and bottom images are enlargements of the squared area in the top left image. Bars, top left, 15 μm; top right, 4 μm.
A role for lamellipodia in endothelial barrier function has been suggested by other researchers.Citation31-33 Rac1 and the RacGEF Tiam1, a GEF that Rac1 finds in the endosomal compartment during its recycling toward the cell border during cell migration, are endothelial barrier enhancers.Citation34-37 In addition, soluble growth factors, namely HGF, that induce Rac1 endosomal trafficking in single cells, also preserve barrier function.Citation11,35,38 A close connection between lamellipodia and the maturation of cell-cell junctions has been described by Schnittler´s group.Citation33,39 At initial stages of endothelial barrier formation, cell-cell contact areas that are not yet occupied by adherens junctions emit circular lamellipodia that favor the formation of new VE-cadherin-mediated intercellular junctions. Membrane re-extension during endothelial barrier recovery may therefore concentrate the machinery required for adherens junction formation and maturation. Beyond intercellular contacts, but also in an inflammatory context, Rac1 promotes extension of ventral lamellipodia to repair microwounds caused by leukocytes undergoing paracellular and transcellular diapedesis.Citation40 However, the role of vesicular trafficking and Rac1 recycling has not yet been addressed for this membrane remodeling aimed to restore cell integrity.
Conclusions and future perspectives
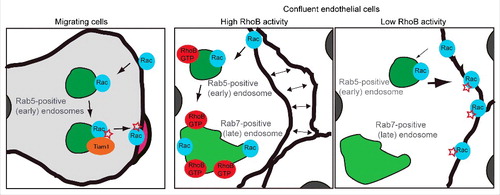
Our findings about the role of RhoB and Rac1 trafficking have similarities with those reported in previous studies of its role on Rac1 activity and enhanced single cell migration in different cell types, including cancer cells (). Lamellipodial-like extensions in confluent endothelial cells are required not to displace a cell out of the monolayer, but to reform the endothelial barrier after its disruption. Hence, it is easy to envisage endothelial monolayers as a colony of single cells coordinating ancient mechanisms of contraction and spreading to form a barrier that compartmentalizes important functions in complex organisms, such as oxygen, nutrient and immune cell transport by the circulatory system. Exposure to inflammatory cytokines would diminish the ability of cells to form cellular protrusions and re-establish cell-cell contacts in an endothelial barrier, weakening the vascular wall, just as transformed cells activate mechanisms to prevent the extension of lamellipodia that would induce cell migration and cancer dissemination. It is of note that inflammatory signaling participates in cell migration-related events such as angiogenesis.Citation41-43 In particular, TNF, which induces the greatest upregulation of RhoB, plays a contextual role in promoting or inhibiting cell migration and angiogenesis.Citation44-47 It would be thus interesting to investigate whether the effects of TNF and other cytokines on cell migration and transformation are mediated by an alteration in the intracellular trafficking regulated by RhoB.
Figure 2. Rac endosomal trafficking during plasma membrane extension in migrating cells and in confluent endothelial cells. Left panel. In migrating cells, Rac1 internalization and recycling in Rab5-positive endosomes are necessary for its activation by the endosomal GEF Tiam1 and the subsequent actin-mediated membrane extension.Citation11 Central and right panels. In confluent endothelial cells RhoB and Rac1 co localize in Rab5 and Rab7-positive endosomes. RhoB-GTP increases Rac1 intracellular localization and prevents Rac1 activation and localization at the cell border, where it stabilizes cell-cell junctions, suggesting that RhoB activation affects GTP loading and recycling of endosomal Rac1.Citation7 Active, GTP-loaded Rac is represented by a red star.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The expert technical advice of the confocal microscopy facility of CBMSO is gratefully acknowledged.
Additional information
Funding
References
- Niklas KJ, Newman SA. The origins of multicellular organisms. Evol Dev 2013; 15(1):41-52; PMID:23331916; https://doi.org/10.1111/ede.12013
- Reglero-Real N, García-Weber D, Millán J. Cellular barriers after extravasation: leukocyte interactions with polarized epithelia in the inflamed tissue. Mediators of Inflamm 2016; 2016: p. 1-10; https://doi.org/10.1155/2016/7650260
- Reglero-Real N, Alvarez-Varela A, Cernuda-Morollón E, Feito J, Marcos-Ramiro B, Fernández-Martín L, Gómez-Lechón MJ, Muntané J, Sandoval P, Majano PL, et al. Apicobasal polarity controls lymphocyte adhesion to hepatic epithelial cells. Cell Rep 2014; 8(6):p. 1879-93; PMID:25242329; https://doi.org/10.1016/j.celrep.2014.08.007
- Murray PS, Zaidel-Bar R. Pre-metazoan origins and evolution of the cadherin adhesome. Biol open 2014; 3(12):p. 1183-95; PMID:25395670; https://doi.org/10.1242/bio.20149761
- Rokas A. The origins of multicellularity and the early history of the genetic toolkit for animal development. Annu Rev Genet 2008; 42:p. 235-51; PMID:18983257; https://doi.org/10.1146/annurev.genet.42.110807.091513
- Nichols SA, Roberts BW, Richter DJ, Fairclough SR, King N. Origin of metazoan cadherin diversity and the antiquity of the classical cadherin/β-catenin complex. Proc Natl Acad Sci 2012; 109(32):p. 13046-51; PMID:22837400; https://doi.org/10.1073/pnas.1120685109
- Marcos-Ramiro B, García-Weber D, Barroso S, Feito J, Ortega MC, Cernuda-Morollón E, Reglero-Real N, Fernández-Martín L, Durán MC, Alonso MA, et al. RhoB controls endothelial barrier recovery by inhibiting Rac1 trafficking to the cell border. J Cell Biol 2016; 213(3):385-402; PMID:27138256
- Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J Cell Biol 2001; 152(1):p. 111-26; https://doi.org/10.1083/jcb.152.1.111
- Navarro-Lerida I, Pellinen T, Sanchez SA, Guadamillas MC, Wang Y, Mirtti T, Calvo E, Del Pozo MA. Rac1 nucleocytoplasmic shuttling drives nuclear shape changes and tumor invasion. Dev Cell 2015; 32(3):p. 318-34; PMID:25640224; https://doi.org/10.1016/j.devcel.2014.12.019
- Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res 2004; 301(1):p. 43-9; PMID:15501444; https://doi.org/10.1016/j.yexcr.2004.08.012
- Palamidessi A, Frittoli E, Garré M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell 2008; 134(1):p. 135-47; PMID:18614017; https://doi.org/10.1016/j.cell.2008.05.034
- Strehle A, Schleicher M, Faix J. Trix, a novel Rac guanine-nucleotide exchange factor from Dictyostelium discoideum is an actin-binding protein and accumulates at endosomes. Eur J Cell Biol 2006; 85(9–10):p. 1035-45; PMID:16781009; https://doi.org/10.1016/j.ejcb.2006.05.005
- Menard L, Parker PJ, Kermorgant S. Receptor tyrosine kinase c-Met controls the cytoskeleton from different endosomes via different pathways. Nat Commun 2014; 5:p. 3907; PMID:24835487
- Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell 2004; 7(6):p. 855-69; PMID:15572128; https://doi.org/10.1016/j.devcel.2004.09.019
- Wallar BJ, Deward AD, Resau JH, Alberts AS. RhoB and the mammalian Diaphanous-related formin mDia2 in endosome trafficking. Exp Cell Res 2007; 313(3):p. 560-71; PMID:17198702; https://doi.org/10.1016/j.yexcr.2006.10.033
- Fernandez-Borja M, Janssen L, Verwoerd D, Hordijk P, Neefjes J. RhoB regulates endosome transport by promoting actin assembly on endosomal membranes through Dia1. J Cell Sci 2005; 118(Pt 12):p. 2661-70; PMID:15944396; https://doi.org/10.1242/jcs.02384
- Calvayrac O, Pradines A, Raymond-Letron I, Rouquette I, Bousquet E, Lauwers-Cances V, Filleron T, Cadranel J, Beau-Faller M, Casanova A, et al. RhoB determines tumor aggressiveness in a murine EGFRL858R-induced adenocarcinoma model and is a potential prognostic biomarker for Lepidic lung cancer. Clin Cancer Res 2014; 20(24):p. 6541-50; PMID:25320360; https://doi.org/10.1158/1078-0432.CCR-14-0506
- Bousquet E, Mazières J, Privat M, Rizzati V, Casanova A, Ledoux A, Mery E, Couderc B, Favre G, Pradines A. Loss of RhoB expression promotes migration and invasion of human bronchial cells via activation of AKT1. Cancer Res 2009; 69(15):p. 6092-9; PMID:19602596; https://doi.org/10.1158/0008-5472.CAN-08-4147
- Bousquet E, Calvayrac O, Mazières J, Lajoie-Mazenc I, Boubekeur N, Favre G, Pradines A. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A inhibition. Oncogene 2016; 35(14):p. 1760-9; PMID:26148238; https://doi.org/10.1038/onc.2015.240
- Vega FM, Colomba A, Reymond N, Thomas M, Ridley AJ. RhoB regulates cell migration through altered focal adhesion dynamics. Open Biol 2012; 2(5):p. 120076; PMID:22724071; https://doi.org/10.1098/rsob.120076
- Huang M, Satchell L, Duhadaway JB, Prendergast GC, Laury-Kleintop LD. RhoB links PDGF signaling to cell migration by coordinating activation and localization of Cdc42 and Rac. J Cell Biochem 2011; 112(6):p. 1572-84; PMID:21344485; https://doi.org/10.1002/jcb.23069
- Gerald D, Adini I, Shechter S, Perruzzi C, Varnau J, Hopkins B, Kazerounian S, Kurschat P, Blachon S, Khedkar S, et al. RhoB controls coordination of adult angiogenesis and lymphangiogenesis following injury by regulating VEZF1-mediated transcription. Nat Commun 2013; 4:p. 2824; PMID:24280686; https://doi.org/10.1038/ncomms3824
- Fritz G, Kaina B. rhoB encoding a UV-inducible Ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J Biol Chem 1997; 272(49):p. 30637-44; PMID:9388198; https://doi.org/10.1074/jbc.272.49.30637
- Wojciak-Stothard B, Zhao L, Oliver E, Dubois O, Wu Y, Kardassis D, Vasilaki E, Huang M, Mitchell JA, Harrington LS, et al. Role of RhoB in the regulation of pulmonary endothelial and smooth muscle cell responses to hypoxia. Circ Res 2012; 110(11):p. 1423-34; PMID:22539766; https://doi.org/10.1161/CIRCRESAHA.112.264473
- Vasilaki E, Papadimitriou E, Tajadura V, Ridley AJ, Stournaras C, Kardassis D. Transcriptional regulation of the small GTPase RhoB gene by TGF{β}-induced signaling pathways. FASEB J 2010; 24(3):p. 891-905; PMID:19890017; https://doi.org/10.1096/fj.09-134742
- Kroon J, Tol S, van Amstel S, Elias JA, Fernandez-Borja M. The small GTPase RhoB regulates TNFalpha signaling in endothelial cells. PLoS One 2013; 8(9):p. e75031; PMID:24086429; https://doi.org/10.1371/journal.pone.0075031
- Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-α-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol 1998; 176(1):p. 150-65; PMID:9618155; https://doi.org/10.1002/(SICI)1097-4652(199807)176:1%3c150::AID-JCP17%3e3.0.CO;2-B
- Alberelli MA, De Candia E. Functional role of protease activated receptors in vascular biology. Vascul Pharmacol 2014; 62(2):p. 72-81; PMID:24924409; https://doi.org/10.1016/j.vph.2014.06.001
- Borissoff JI, Spronk HM, Heeneman S, ten Cate H. Is thrombin a key player in the ‘coagulation-atherogenesis’ maze? Cardiovasc Res 2009; 82(3):p. 392-403; PMID:19228706; https://doi.org/10.1093/cvr/cvp066
- Fernandez-Martin L, Marcos-Ramiro B, Bigarella CL, Graupera M, Cain RJ, Reglero-Real N, Jiménez A, Cernuda-Morollón E, Correas I, Cox S et al. Crosstalk between reticular adherens junctions and platelet endothelial cell adhesion molecule-1 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol 2012; 32(8):p. e90-e102; PMID:22723439; https://doi.org/10.1161/ATVBAHA.112.252080
- Breslin JW, Zhang XE, Worthylake RA, Souza-Smith FM. Involvement of local lamellipodia in endothelial barrier function. PLoS ONE 2015; 10(2):p. e0117970; PMID:25658915; https://doi.org/10.1371/journal.pone.0117970
- Tian X, Tian Y, Gawlak G, Meng F, Kawasaki Y, Akiyama T, Birukova AA. Asef controls vascular endothelial permeability and barrier recovery in the lung. Mol Biol Cell 2015; 26(4):p. 636-50; PMID:25518936; https://doi.org/10.1091/mbc.E14-02-0725
- Abu Taha A, Schnittler HJ. Dynamics between actin and the VE-cadherin/catenin complex: novel aspects of the ARP2/3 complex in regulation of endothelial junctions. Cell Adhes Migr 2014; 8(2):p. 125-35; https://doi.org/10.4161/cam.28243
- Timmerman I, Heemskerk N, Kroon J, Schaefer A, van Rijssel J, Hoogenboezem M, van Unen J, Goedhart J, Gadella TW Jr, Yin T et al. A local VE-cadherin and Trio-based signaling complex stabilizes endothelial junctions through Rac1. J Cell Sci 2015; 128(16):p. 3041-54; PMID:26116572; https://doi.org/10.1242/jcs.168674
- Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. Faseb J 2007; 21(11):p. 2776-86; PMID:17428964; https://doi.org/10.1096/fj.06-7660com
- Timmerman I, Hoogenboezem M, Bennett AM, Geerts D, Hordijk PL, van Buul JD. The tyrosine phosphatase SHP2 regulates recovery of endothelial adherens junctions through control of β-catenin phosphorylation. Mol Biol Cell 2012; 23(21):p. 4212-25; PMID:22956765; https://doi.org/10.1091/mbc.E12-01-0038
- van Buul JD, Timmerman I. Small Rho GTPase-mediated actin dynamics at endothelial adherens junctions. Small GTPases 2016; 7(1):p. 21-31; https://doi.org/10.1080/21541248.2015.1131802
- Schlegel N, Waschke J. cAMP with other signaling cues converges on Rac1 to stabilize the endothelial barrier- a signaling pathway compromised in inflammation. Cell Tissue Res 2014; 355(3):p. 587-96; PMID:24322391; https://doi.org/10.1007/s00441-013-1755-y
- Abu Taha A, Taha M, Seebach J, Schnittler HJ. ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Mol Biol Cell 2014; 25(2):p. 245-56; PMID:24227887; https://doi.org/10.1091/mbc.E13-07-0404
- Martinelli R, Kamei M, Sage PT, Massol R, Varghese L, Sciuto T, Toporsian M, Dvorak AM, Kirchhausen T, Springer TA, et al. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J Cell Biol 2013; 201(3):p. 449-65; PMID:23629967; https://doi.org/10.1083/jcb.201209077
- Tas SW, Maracle CX, Balogh E, Szekanecz Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat Rev Rheumatol 2016; 12(2):p. 111-22; PMID:26633288; https://doi.org/10.1038/nrrheum.2015.164
- Naldini A, Carraro F. Role of inflammatory mediators in angiogenesis. Curr Drug Targets Inflamm Allergy 2005; 4(1):p. 3-8; PMID:15720228; https://doi.org/10.2174/1568010053622830
- Kishore R, Qin G, Luedemann C, Bord E, Hanley A, Silver M, Gavin M, Yoon YS, Goukassian D, Losordo DW. The cytoskeletal protein ezrin regulates EC proliferation and angiogenesis via TNF-α-induced transcriptional repression of cyclin A. J Clin Invest 2005; 115(7):p. 1785-96; PMID:15965500; https://doi.org/10.1172/JCI22849
- Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Böhlen P. Tumor necrosis factor type α, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci 1987; 84(15):p. 5277-81; PMID:2440047; https://doi.org/10.1073/pnas.84.15.5277
- Mano-Hirano Y, Sato N, Sawasaki Y, Haranaka K, Satomi N, Nariuchi H, Goto T. Inhibition of tumor-induced migration of bovine capillary endothelial cells by mouse and rabbit tumor necrosis factor. J Natl Cancer Inst 1987; 78(1):p. 115-20; PMID:2432305
- Sato N, Goto T, Haranaka K, Satomi N, Nariuchi H, Mano-Hirano Y, Sawasaki Y. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst 1986; 76(6):p. 1113-21
- Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, Min W. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem 2003; 278(51):p. 51267-76; PMID:14532277; https://doi.org/10.1074/jbc.M310678200
