ABSTRACT
In the last two decades, extracellular vesicle-mediated communication between cells has become a major field in cell biology. However, the function of extracellular vesicles is far from clear, especially due to the disparity of released vesicles by cells. Basically, one must consider vesicles budding from the cell plasma membrane (ectosomes) and vesicles released upon fusion of an endosomal multivesicular compartment (exosomes). Moreover, even for exosomes, we report and discuss here the possibility that different routes regulated by specific Rab GTPases might produce exosomes having various biologic functions.
KEYWORDS:
History and definition of exosomes
Besides designating the multienzyme ribonuclease complex, in recent years, the term “Exosome” has defined the different types of vesicles released by various cells. It is critical to discriminate these vesicles, since their mode of biogenesis is likely directly related to their physiologic function and/or to the state of the productive cell. This is pivotal in the context of their regulation by small GTPases, which is the purpose of this review. We have restricted the data to papers strictly related to exosomes and Rab GTPases, and consequently a large piece of work related to the biogenesis and function of exosomes is not presented here, but can be found in recent reviews on exosomes.
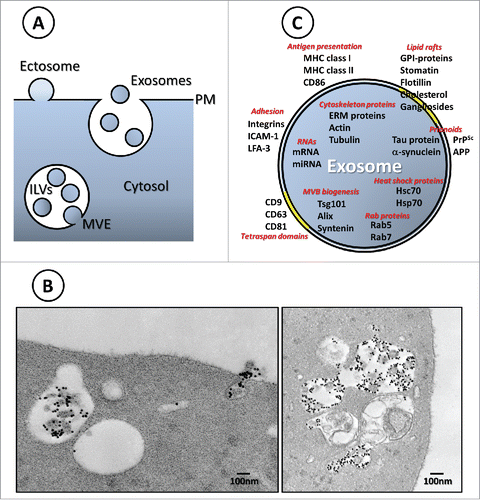
First coined by Trams et al.,Citation1 the term exosome was used to name exfoliated membrane vesicles collected from supernatants of normal or neoplastic cell lines. These surface-shed vesicles have diameters varying from 40 to 1,000 nm and contain plasma membrane 5′-nucleotidase activity, some of which possessed a high concentration of tumor antigens and were shed at a higher rate by highly metastatic cells.Citation2 These vesicles, also called microvesicles, microparticles, oncosomes or shedding particles, are formed from outward budding of the plasma membrane. We will collectively call them “ectosomes,” as originally proposed for isolated vesicles released by activated human polymorphonuclear neutrophils.Citation3,4 In contrast, the term exosomes, as used in 1987 by Rose Johnstone,Citation5 refers to vesicles released in the extracellular medium upon fusion of a multivesicular endosome (MVE) with the cell surface ), as first demonstrated to occur during reticulocyte maturation.Citation6,7 However, since some ectosomes and exosomes are of similar size (approximately 60–100 nm) regardless of the process of formation, it remains difficult to evaluate the compartment of origin of these 2 kinds of vesicles without evidencing the producing event at the ultrastructural level. Moreover, there appears to be molecular overlap between the 2 events. Components, such as the endosomal sorting complexes required for transport (ESCRT) machinery, that are directly involved in multivesicular endosome formationCitation8,9 are also associated with plasma membrane domains, which give rise to vesicle budding from the cell surface.Citation10,11 Consequently, a mixed population of vesicles of different intracellular origin are generally copurified as recently pointed out by carefully analyzing the proteomic composition of vesicle subtypes.Citation12
Figure 1. (A) Ectosome vs. exosome biogenesis. (B) Left panel: Electron microscopy image of a multivesicular endosome (MVE) and a fusion event of an MVE with the reticulocyte plasma membrane. Transferrin-gold is labeling the exosome surface. Right panel: Autophagic structures are observed in continuity or close to MVE containing Tf-gold labeled ILVs.Citation14 (C) Representation of the composition of exosomes emphasizing on potential functions. Note that these components can also be present in other types of extracellular vesicles.
Biogenesis, composition and functions
In the early 1980s, the labs of Rose Johnstone and Philip Stahl demonstrated secretion of the transferrin receptor (TfR) in association with small membrane vesicles from reticulocytes of different species.Citation6,7 Using different approaches and the power of electron microscopy, they followed the fate of the TfR during reticulocyte maturation, from its usual recycling pathway to a secretion route involving inward budding from the endosomal membrane, leading to the formation of a multivesicular endosome. The TfR accumulates in the small intraluminal vesicles (ILVs) of the MVE and is released from the cell by fusion of the MVE with the reticulocyte plasma membrane.Citation6,13 Moreover, we have evidenced by EM amphisomes arising from the fusion of MVE containing nascent exosomes (Tf-gold labeled) with autophagic structures )(14), a process that likely accounts for the secretion of mitochondria occurring during the last stage of erythropoiesis.Citation15 At this time, exosomes were thus considered as a way to expel unwanted cellular components.
Studies on exosomes were restricted to the reticulocyte model until 1996, when Hans Geuze and his group discovered that B-lymphocytes secrete similar vesicles.Citation16 Importantly, these exosomes contain major histocompatibility complex class II molecules, opening the door toward a potential role for these vesicles in immunological processes.Citation17,18 Exosomes were thus envisaged as an acellular mode of communication between cells. Since then, studies on these vesicles have expanded and exosomes have been detected in various biological fluids, including plasma, urine, and pleural effusion. A few years later, mRNA and miRNA were found in mast cell exosomes,Citation19 and it was determined that after entering another cell, exosomal mRNA can be translated and miRNA can regulate gene expression,Citation19,20 demonstrating a fascinating mechanism for nucleic acid exchange between cells.Citation21 However, many studies did not fully characterize the origin of the vesicles and the term extracellular vesicle (EV) was suggested to be more appropriate. On this subject, we refer the reader to a recent review summarizing the similarities and differences between ectosomes and exosomes.Citation22
Sorting of cargo proteins ) within exosomes is still not completely clear and likely depends on the budding process (for review see refCitation23) Various mechanisms involving the ESCRT machinery,Citation24 a ceramide-based mechanism,Citation25 tetraspanin proteins,Citation26,27 AlixCitation28-30 or, more recently, Hsc7031 have been described as potential mechanisms that are driving protein sorting and/or budding events.
In the present review we will focus on exosome secretion and its regulation by small GTPases of the Rab family.
Secretion and regulation
The small Rab GTPases consist of a family of proteins composed of approximately 70 distinct proteins, all belonging to the super family of Ras GTPases. They classically function as molecular switch by cycling between GTP- and GDP-bound states. The GTP-bound form (i.e. the active form) can interact with effectors, promoting various steps and contributing to vectorial membrane traffic. Rab GTPases are considered as organelle markers since each Rab protein regulates a distinct intracellular transport step.Citation32 They are associated with the endocytic and secretion pathways, and are recognized for their roles in both membrane transport and fusion.Citation33 The biogenesis compartment of extracellular vesicles is important with regards to their regulation by GTPases since, in general, Rab proteins regulate vesicular traffic and exosome formation while Rho/Rac proteins are rather involved in ectosome formation through rearrangements of the actin cytoskeleton and ARF6-regulated endosomal recycling.Citation34,35 Surprisingly, Rab22A, which had been described as an endosomal associated protein in different cell lines, was recently shown to be involved in ectosome formation.Citation36 Intratumoral hypoxia triggers changes in gene expression mediated by hypoxia-inducible factors (HIFs). Rab22A is one of the various genes whose expression is increased through HIFs in breast cancer. Rab22A GTPase was shown to colocalize with budding ectosomes at the plasma membrane of MDA-MB-231 cells, while Rab22A knockdown decreased ectosome formation and concomitantly impaired breast cancer cell invasion.Citation36
Even though a majority of the work on exosomes focuses on their extracellular functions, some papers characterize the regulation of exosome biogenesis and secretion by various Rab GTPases. We will present these data and discuss the possibility of Rabs regulation playing a role in exosome production by cells.
Rab GTPases and exosomes: Cargoes vs. actors
As previously mentioned, exosomes were first discovered in reticulocytes. As consequence, the presence of Rab GTPases was originally investigated on reticulocytes exosomes. Vesicular trafficking is reduced in reticulocytes due to the disappearance of the secretion pathway with enucleation at the erythroblast stage. Moreover, post-endocytic trafficking is limited to recycling since lysosomes are dying out. Thus, Rab4 and Rab5 GTPases were tested by GTP-binding and western blot in different reticulocyte and erythrocyte fractions, and it was found that Rab4 was enriched within exosomes while Rab5 was concentrated in plasma membrane ghosts. These results argued against a role for vesicle-trapped Rab4 in exosomes secretion, and conversely suggested that reticulocyte exosomes are used to clear out the “needless” GTPase, which accordingly disappeared in erythrocytes.Citation37
Since then, and especially by means of proteomic analysis, numerous Rab proteins have been detected in exosomes. Referring to Exocarta (www.exocarta.org), a web-based compendium for exosomal cargo, more than 28 Rab isoforms have been characterized in exosomes from various sources. Within those, most of the Rabs characterized are GTPases involved in the endocytic pathway, while Rab proteins having a role in ER-Golgi transport are less frequently found. This highlights the endosomal origin of the multivesicular compartment, resulting from various membrane transport steps regulated by Rabs. One may propose that Rabs are present in exosomes as contaminants, but, alternatively, sequestration might be a way to assist formation of Rab domains,Citation38 or Rab conversionCitation39 if the Rab protein to be removed is not correctly released in the cytosol. In addition, we recently showed that, during reticulocyte maturation, effectors of small GTPases can be downregulated through the exosomal pathway. This is the case for RASA3 (Ras GTPase activating Protein 3), which is lost completely during the last stage of erythroid differentiation.Citation40,41
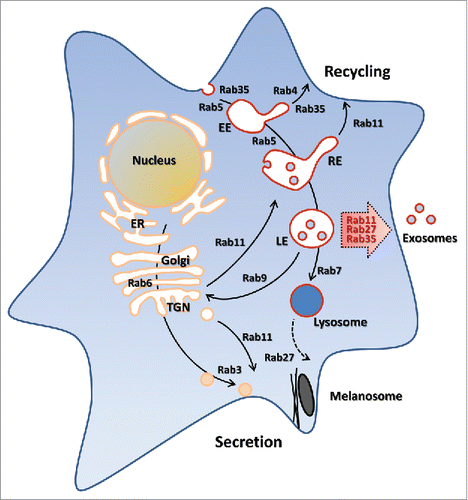
Conversely, a few Rab proteins (i.e., Rab11, Rab35 and Rab27) have been shown to play a direct and significant role in exosome biogenesis and secretion. As anticipated, they are all involved in the transport of endolysosomal vesicles toward the plasma membrane . Rab11 and Rab35 regulate the recycling of membrane components from the endosomal compartment to the plasma membrane. This recycling event is also used in a polarized manner to facilitate various cell processes such as cytokinesis, cell migration and neurite outgrowth.Citation42-44 On the other hand, the secretory Rab27 is specialized in transport of late endosomal/lysosome-like compartments to the plasma membrane.Citation45 Rab27 has also been shown to regulate exocytic events in a sequential manner together with the other secretory GTPase Rab346 and jointly with the recycling Rab11.Citation47
Slow recycling Rab11
The Rab11 subfamily includes 3 members: Rab11a, Rab11b and Rab11c (also named Rab25). Rab11a was the first member characterized and is ubiquitously expressed,Citation48 while Rab11b and Rab25 show a more restricted tissue expression pattern.Citation49,50 The Rab11 subfamily is involved in recycling from an endosomal compartment to the plasma membrane. In epithelial cells, a common recycling endosome (CRE) and an apical recycling endosome (ARE) are used to specifically deliver material to either apical or basolateral plasma membrane with differential dependence on Rab11a and Rab25.Citation51 In non-polarized cells (e.g. haematopoietic cells), Rab11a regulates slow transport from a perinuclear recycling endosome compartment, contrary to Rab4, which is involved in fast recycling after endocytosis. Rab11 was thus proposed to regulate the transport of MVE to the plasma membrane during differentiation of erythroid cells. The erythroleukemia K562 cell line susceptible to differentiate into erythroid precursors and expressing high levels of Rab1152 was used in pioneering experiments.Citation53 Overexpression of the wild type form of Rab11 slightly stimulated exosome secretion in K562 cell, as measured by different markers previously used for reticulocyte exosomes studies.Citation54 Conversely, the inhibition of Rab11 function by overexpression of a dominant-negative mutant (Rab11 S25N) decreased exosome release.Citation53 In addition, intracellular Ca2+ was involved in exosome release from K562 cells, and Rab11 promotes docking and fusion of MVE in a calcium-dependent manner.Citation55,56 Rab11 was also shown to contribute to exosome secretion in neuronal cells,Citation57 in the context of the p75 neurotrophin receptor, a signaling receptor that regulates different aspects of neuronal physiology. After ligand-mediated endocytosis, a portion of endocytic p75 neurotrophin receptor pool is sorted from a Rab5- to a Rab11-positive compartment evading lysosomal route, and accumulates in a compartment positive for the tetraspanin CD63. These CD63-positive organelles have a multivesicular structure as observed by electron microscopy, and release their content into extracellular medium upon KCl depolarization.Citation57 These studies demonstrate the existence of an exosomal pathway dependent on Rab11 recycling route and calcium sensitization. Although the molecular mechanism of Rab11 function in exosome secretion has yet to be deciphered, especially regarding downstream effectors of Rab11, studies in Drosophila could bring some insights into the mechanism. Indeed, a recent study showed that vesicles are secreted at the Drosophila larval neuromuscular junction,Citation58 and 2 concomitant studies performed on Drosophila S2 cells demonstrated by knockdown experiments that Rab11, but not Rab35 or Rab27, participate in the production of vesicles containing the transmembrane protein Evenness Interrupted (Evi) and its binding partner Wingless (Wg).Citation59,60 These released vesicles share characteristics of exosomes, such as the size as assessed by EM and the density range as estimated after migration through sucrose gradient. Moreover, immuno-electron microscopic analysis provided evidence of multivesicular structures with intraluminal vesicles labeled for Evi. These Multivesicular Bodies (MVB) fuse with the presynaptic membrane at specific sites that are likely separate from the active zones where Rab3 synaptic vesicles discharge their content. Finally, the Rab11 effector Myosin5 was shown to be required for exosome release,Citation60 while the participation of syntaxin 1A is under debate,Citation59,60 adding another layer of complexity to the regulatory process. Nevertheless, these studies in Drosophila may help to decipher the mechanism in other organisms.
Fast recycling Rab35
Rab35 was first identified and cloned from a human skeletal muscle cDNA library.Citation61 Rab35 regulates a fast endocytic/recycling step for various proteins (e.g., transferrin) to the plasma membrane. Accordingly, Rab35 is mainly localized at the plasma membrane and in internal structures corresponding to clathrin-coated vesicles (CCV), sorting endosome and endosomal tubules.Citation62 More recent studies have demonstrated that Rab35 recruits the lipid phosphatase OCRL immediately after CCV scission from the plasma membrane, allowing PI(4,5)P2 hydrolysis into PtdIns(4)P and CCV uncoating.Citation63 Another function related to vesicular trafficking emerged during cytokinesis. Indeed, Rab35 silencing or overexpression of a dominant-negative mutant showed that the small GTPase is essential for bridge stability and abscission.Citation62
Oligodendrocytes insulate axons with a myelin sheath, providing a trophic support as well as promoting rapid impulse conduction. The proteolipid protein (PLP) plays a major role in maintaining the multilamellar structure of myelin sheath. PLP was found to be associated with exosomes released by a murine oligodendroglial cell line. PLP reaches multivesicular compartments in a Rab5-dependent manner, and is then sorted into intraluminal vesicles formed by a mechanism sensitive to sphingomyelinase inhibition, involving ceramide production without involvement of the ESCRT machinery.Citation25 A screening strategy evidenced a role for Rab35 in exosome secretion from oligodendroglial cells.Citation64 A Rab GTPase-activating protein (GAP) library was screened and identified the TBC1D10 family as regulating the secretion PLP-EGFP-associated exosomes. The three members of the TBC1D10 family, especially TBC1D10B which has the highest effect on exosome secretion, have a strong and specific GAP-activity on Rab35. Accordingly, inhibition of exosome secretion by the expression of the GAP was abolished when a GTP-blocked mutant of Rab35 was coexpressed with TBC1D10B. Conversely, expression of a dominant-negative Rab35 mutant or knockdown of Rab35 resulted in a decrease of exosome-associated PLP. In addition, total internal reflection fluorescence (TIRF) demonstrated that expression of the GTP-blocked mutant of Rab35 increases docking/tethering of endosomal vesicles at the plasma membrane, and raising the intracellular concentration of Ca2+ promotes vesicle fusion with the plasma membrane.Citation64 Subsequent work confirmed that Rab35-regulated exosome release by oligodendrocytes is indeed increased by the elevation of intracellular Ca2+ levels and by glutamate Ca2+- signaling.Citation65,66
Rab35-regulated exosomes released from oligodendrocytes provide a trophic support for axons and may protect neurons from oxidative stress and starvation.Citation66 However, the physiological certainty of this function is tempered by the fact that microglia was found to be the preferential brain cell type taking up oligodendrocytes exosomes. Indeed, a study revealed that microglia rather that astrocytes, neurons or oligodendrocytes internalize the vesicles by macropinocytosis and degrade exosomes through a “silent” manner preventing an immune response in microglia,Citation67 reminiscent of our work on the clearance of reticulocytes exosomes.Citation68
Similar to Rab11, the detailed mechanism of Rab35 downstream sequence leading to exosomal secretion remains to be clarified. To date, none of the various effectors of Rab35 identified so farCitation69 have been shown to be involved in exosome secretion.
Secretory Rab27
Rab27 is widely conserved in metazoans with 2 isoforms existing in vertebrates, Rab27A and Rab27B. They are both involved in the regulated secretory pathway, often in a redundant manner. However, in melanocytes and cytotoxic lymphocytes (CTL), only Rab27A exists, in contrast to platelets where Rab27A and Rab27B are both present. Of note, Rab27A was the first Rab GTPase found to be involved in human disease. Indeed, mutations in Rab27A lead to the human type 2 Griscelli syndrome, an autosomal recessive disorder that is characterized by (i) hypopigmentation due to deficient melanosome transport, and (ii) loss of cytotoxic killing activity by CTL caused by an impairment in lytic granules secretion.Citation70 Rab27A/B are also expressed on various secretory granules in endocrine, exocrine and immune cells, and are considered as secretory Rab GTPases, like Rab3.
The first study to establish a role for Rab27A and Rab27B in the regulation of exosome release was performed on HeLa cells transfected with the transactivator CIITA, using shRNA screen and immunodetection by flow cytometry of exosome components using vesicles immunoadsorbed on beads.Citation71 Lentivirus–mediated depletion of Rab27A or Rab27B induced a decrease in the amount of secreted exosomes without changing their protein content, ruling out a role in protein sorting in ILVs. Moreover, the size of CD63-positive MVEs was significantly increased in Rab27A-knockdown cells while their distribution was similar to the ones of control cells, as assessed by immunofluorescence. EM studies confirmed these findings, whereas MVEs of Rab27B-knockdown cells appeared smaller and showed a clustered distribution. TIRF was used to study the role of Rab27 in MVE docking at the plasma membrane. Rab27A and Rab27B depletion induced a higher mobility of MVEs just beneath the plasma membrane, while the specific depletion of only Rab27B increased both the proportion and velocity of vesicles likely moving along microtubules. Collectively, these data suggest that the 2 isoforms work separately along the exosomal pathway.
Among the numerous effectors of Rab27,Citation72 2 were shown to be involved in exosomes secretion in HeLa cells. Slp4 (granuphilin-a/b) and Slac2b (exophilin-5) knockdown cells were found to secrete smaller amounts of exosomes as assessed by flow cytometry and beads-based assay, as well as WB detection of exosome components upon large scale vesicle purification. In addition, it was found that the phenotype induced by each effector knockdown is similar to the one of the Rab27-knockdown phenotypes, suggesting that Slp4 and Slac2b function through Rab27A and Rab27B, respectively.Citation71
Since then, several studies were performed attempting to assess Rab27-dependence of exosome secretion. Rab27A regulation of exosome release was confirmed using breast cancer cell lines and nanoparticle tracking analysis.Citation73 Strikingly, the function of released exosomes can be investigated in a more integrated manner. For example, 2 carcinoma cell lines with different metastatic capacity and knocked down for Rab27A were tested for tumor growth when injected subcutaneously in mice.Citation74 Rab27A knockdown in both cell lines decreased the amount of in vitro secreted vesicles containing bona fide exosomal proteins (Tsg101, Alix, CD63), and in vivo tumor growth of the highly metastatic cells was impaired compare with their scramble small hairpins-treated counterparts. Similarly, this tumor-promoting role was found in another study investigating exosomes released by highly metastatic melanoma cells. Rab27-regulated exosomes were found to educate bone marrow progenitor cells that are necessary for the metastatic progression.Citation75
At the cellular level, invadopodia is a key site for Rab27a-regulated exosome secretion.Citation76 Rab27A knockdowns in invasive carcinoma and breast cancer cell lines decrease exosome secretion and invadopodia-associated matrix degradation, an effect phenocopied by synaptotagmin-7 knockdown. Synaptotagmin-7 is a calcium-dependent phospholipid-binding protein that plays a role in exocytosis, strengthening the connection between exosomes and invadopodia. Moreover, using 3D culture assays, Rab27A was shown to regulate invasive migration by delivering MT1-MMP associated with exosomes at invadopodia. In another context, T regulatory (Treg) cells secrete exosomes that are used to transfer specific miRNAs to T helper 1 (Th1) cells and suppress Th1 proliferation and cytokine secretion.Citation77 Abrogation of this non cell-autonomous gene regulation was obtained by using Rab27A and Rab27B-deficient Treg cells failing to release exosomes, as well as Treg cells lacking Dicer function and impaired for the production of mature miRNAs, or Treg cells transfected with hairpin inhibitors of Let-7d, a miRNA specifically involved in the process through its exosome transfer.
Do the involved Rabs sign a functional role for exosomes?
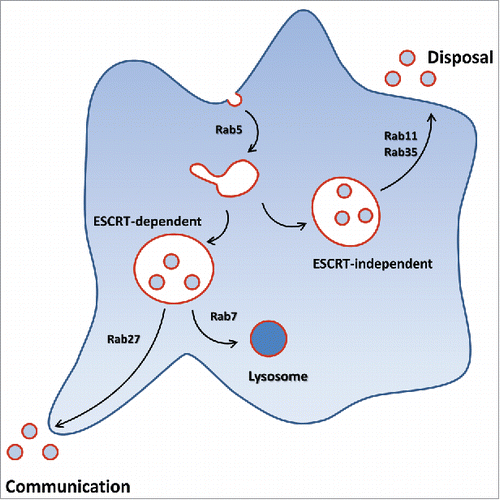
Some years ago, we proposed that exosomes can originate from different endosomal compartments.Citation78 This would explain why various Rab GTPases could regulate the exosomal release. This is likely cell-dependent, reflecting the intracellular traffic present in each cell type. Moreover, cells release specific subpopulations of exosomes with distinct molecular and biological properties.Citation79 It is still unclear if this exosome diversity is already present in MVEs and caused by various budding processes (e.g., ESCRT, ceramide-dependent) leading to specific protein sorting into ILVs, or if MVEs originating on different traffic routes, and thus potentially enriched in different cargoes, lead to exosome release; although both hypotheses might not be mutually exclusive. A related question is: is there a relationship between the Rab GTPases regulating exosome release and the functional role of exosome subpopulations?
A disposal route
A first element of a response is to consider the producing cell and exosomes as a way to regulate its homeostasis or fate. The best example is the red cell differentiating system and the complete loss of TfR, and the adjustment of other cell surface proteins (e.g., AQP-1) from the reticulocyte surface to accommodate environmental conditions through the exosomal pathway.Citation80 The fact that lysosomes are almost inexistent in reticulocytes indicates that exosomes are released through an endocytic/recycling route. Accordingly, K562 erythroleukemia cells release exosomes by a Rab11-regulated pathway.Citation53 As mentioned earlier, Rab11 regulates secretion of exosomes containing the P75 neurotrophin receptor and contributes to its downregulation.Citation57 AQP-2 is a critical water channel in the kidney, whose regulation on the surface of principal cells of the collecting duct is mediated by a Rab11-positive storage compartment.Citation81 Interestingly, secretion of exosomes containing AQP-2 in urine has been reported, again likely indicating a disposal function of the vesicles. Similarly, exosomes containing the epithelial Na channel also regulated through a Rab11-positive compartment in the distal nephron are released in the urine.Citation82 Finally, Rab11-regulated secretion of Wingless associated with exosomes is not involved in Wg gradient-formation in wing discs of drosophila.Citation59 With respect to the other recycling GTPase Rab35, exosomes released by oligodendrocytes were found mostly taken up by microglia weakening the argument toward a physiological communication role.Citation67 Along the same line, oligodendrial cells use exosome secretion as a way to maintain cholesterol homeostasis when the cells are submitted to intracellular accumulation of cholesterol.Citation83
Rab11- and Rab35-regulated exosomal secretion does not seem to be ESCRT-dependent,Citation25,59,84 contrary to the one dependent on Rab27.Citation76 Whether Rab11 and Rab35 may share some regulators or effectors during exosomal secretion remains to be clarified. Note in this respect that RAL-1 is involved in MVB biogenesis and fusion with the plasma membrane in Caenorhabditis elegans, and that RalA and RalB are required for secretion of exosomes in 4T1 mammary tumor cells.Citation85 Nevertheless, it was shown that RAL-1 acts independently of the exocyst complex, a direct target of Ral GTPasesCitation85 and Rab11.Citation86
All together these studies support the hypothesis of Rab11- and Rab35-regulated exosome secretion as a disposal way to regulate cellular levels of specific components . However, these exosomes could be vectors in some human diseases such as in pathogen infection or neurodegenerative diseases. For example, pathogen components can be carried by exosomes, as was shown for the anthrax toxin that translocates into ILVs after endocytosis is released associated with exosomes through a Rab11 and Rab35-regulated pathway and can then be delivered to naïve cells.Citation84 Similarly, the cellular components eliminated by exosomes could be toxic by themselves by propagating diseases.Citation87 For example, when the prion protein scrapie (PrPsc) is associated with exosomes, they become susceptible to spread the infectious PrPsc to recipient cells.Citation88 One could speculate that PrPsc protein is released through Rab35 or Rab11-regulated vesicles. In fact, GPI-anchoring and propensity to aggregate are 2 characteristics favoring the sorting of proteins into exosomes from the recycling pathway.Citation54,89
A communication device
Another appealing hypothesis for the role of exosomes is their potential as intercellular communication carriers. In fact, most of the studies in the exosomal field have focused on these aspects. Rab27 is involved in the secretion of specific components contained in compartments of different origins (e.g., melanosome, secretory lysosome, dense granule) depending on cell types. Release of these components (respectively: melanin, lytic granules or serotonin) is finely tuned by Rab27 assignment and has a specific function once secreted. In all fairness, Rab27 is the only small GTPase for which released exosomes have been demonstrated to clearly induce a change in naïve cells.Citation74 Horizontal transfer of proteins, such as the receptor tyrosine kinase MET, has been demonstrated to occur from melanoma exosomes to bone marrow progenitors.Citation75 Similarly, carcinoma cells were shown to induce differentiation of fibroblasts into tumor-promoting stromal myofibroblasts through TGFβ1 and heparin sulfate associated with Rab27-regulated exosomes.Citation90 Moreover, miRNA transfer and gene regulation into recipient cells has been demonstrated to occur via exosomes secreted through a Rab27-regulated manner.Citation77 Rab27A and Rab27B were both found to be required for exosomes secretion by HeLa cells,Citation71 while Rab27B is not consistently required in 2 other carcinoma cells.Citation74 Interestingly, Rab27 is overexpressed in some cancers.Citation91 Capacity to be secreted at specific sites such as invadopodiaCitation76 might also be a characteristic for Rab27-regulated exosomes , reminding the ability of the other secretory GTPase Rab3 to target neurotransmitter release at active zones. Note, however, that Rab27-regulated exosomes can also be used by cells as a disposal tool. Carcinoma cells were shown to produce exosomes containing tumor-suppressor miRNAs by a mechanism arrested by silencing Rab27A or Rab27B, but insensitive to the sphingomyelinase inhibitor GW4869, and acquire metastatic properties by secretion of the miRNA-associated exosomes.Citation91 It is tempting to speculate that miRNAs are components more specifically transported within Rab27-regulated exosomes, which might be related to the association of GW-bodies with MVBs.Citation92
Tumor cells regulate their morphology and invasion capacity to adapt to the environment by using vesicle secretion. The switch between mesenchymal and amoeboid phenotypes is correlated with formation of invadopodia through exosome secretion while the rounded morphology is correlated with microvesicles blebbing, respectively.Citation93 This clearly indicates membrane remodeling through membrane traffic and cytoskeleton reorganization in which Rab and Rho proteins have differential roles.
A greater part of the studies on exosomes as a communication device examines pathologic effects of the vesicles, mainly in cancer, promoting tumor growth, invasiveness and metastasis. As previously mentioned, microvesicles released by tumor cell plasma membrane blebbing contribute as well to these cancer-associated processes.Citation94 A physiologic communication function of exosomes is more difficult to evidence, contrary to an imbalance of normal state, meeting on this aspect “disposal” exosomes carrying toxic components.
In all cases, exosomes could be used as a prognostic factor. A lot of work is being performed in order to implement released vesicles as biomarkers in cancer. However, the presence of ectosomes generally released in higher amounts by tumor cells renders difficult the possibility to know the contribution of exosomes versus ectosomes.
Despite growing evidence for a role in communication in pathological conditions, exosomal secretion and its regulation are still not fully understood. Additionally one might ask, what is the role of exosomal secretion during steady-state conditions? Do they play a role in cell differentiation? Are they vectors of communication? Are small GTPases constitutively regulating exosomal secretions or are they recruited only under certain conditions? Many questions that remain unanswered as of today; but for which answers may bring one's attention to new functions for small GTPases in the context of vesicular trafficking and exosomal secretion.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from Cancéropôle GSO (to MV) and by an Allied World St. Baldrick's Scholar (to LB), Pediatric Cancer Foundation (to LB), and the National Institute of Health (NIH) National Heart, Lung, and Blood Institute (NHLBI) HL134043–01A1 (to Luanne L Peters and LB).
References
- Trams EG, Lauter CJ, Salem N, Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981; 645(1):63-70; PMID:6266476; https://doi.org/10.1016/0005-2736(81)90512-5
- Taylor DD, Taylor CG, Jiang CG, Black PH. Characterization of plasma membrane shedding from murine melanoma cells. Inter J Cancer 1988; 41(4):629-35; PMID:3356493; https://doi.org/10.1002/ijc.2910410425
- Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol 1999; 163(8):4564-73; PMID:10510400
- Stein JM, Luzio JP. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. T Biochem J 1991; 274(Pt 2):381-6. Epub 1991/03/01
- Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 1987; 262(19):9412-20
- Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 1983; 97:329-39; PMID:6309857; https://doi.org/10.1083/jcb.97.2.329
- Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell 1983; 33:967-77; PMID:6307529; https://doi.org/10.1016/0092-8674(83)90040-5
- Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001; 106(2):145-55; PMID:11511343; https://doi.org/10.1016/S0092-8674(01)00434-2
- Conibear E. An ESCRT into the endosome. Mol Cell 2002; 10(2):215-6; PMID:12191463; https://doi.org/10.1016/S1097-2765(02)00601-9
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol 2006; 172(6):923-35; PMID:16533950; https://doi.org/10.1083/jcb.200508014
- Taylor DD, Gercel-Taylor C. Tumour-derived exosomes and their role in cancer-associated T-cell signalling defects. Br J Cancer 2005; 92(2):305-11; PMID:15655551
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113(8):E968-77. Epub 2016/02/10; PMID:26858453; https://doi.org/10.1073/pnas.1521230113
- Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 1985; 101(3):942-8; PMID:2993317; https://doi.org/10.1083/jcb.101.3.942
- Dardalhon V, Géminard C, Reggio H, Vidal M, Sainte-Marie J. Fractionation analysis of the endosomal compartment during rat reticulocyte maturation. Cell Biol Int 2002; 26(8):669-78; PMID:12175670; https://doi.org/10.1006/cbir.2002.0917
- Ney PA. Normal and disordered reticulocyte maturation. Curr Opin Hematol 2011; 18(3):152-7; PMID:21423015; https://doi.org/10.1097/MOH.0b013e328345213e
- Raposo G, Nijman HW, Stoorvogel W, Leidendekker R, Harding CV, Meleif CJM, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996; 183:1161-72; PMID:8642258; https://doi.org/10.1084/jem.183.3.1161
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 2009; 9(8):581-93; PMID:19498381; https://doi.org/10.1038/nri2567
- Denzer K, Kleijmeer MJ, Heijnen HFG, Storrvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000; 113:3365-74; PMID:10984428
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Na Cell Biol 2007; 9(6):654-9; PMID:17486113; https://doi.org/10.1038/ncb1596
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sánchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2011; 2:282. Epub 2011/04/21; PMID:21505438; https://doi.org/10.1038/ncomms1285
- Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016; 164(6):1226-32. Epub 2016/03/12; PMID:26967288; https://doi.org/10.1016/j.cell.2016.01.043
- Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trend Cell Biol 2015; 25(6):364-72; PMID:25683921; https://doi.org/10.1016/j.tcb.2015.01.004
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Revi Cell Dev Biol 2014; 30:255-89. Epub 2014/10/08; PMID:25288114; https://doi.org/10.1146/annurev-cellbio-101512-122326
- Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Théry C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Scie 2013; 126(Pt 24):5553-65. Epub 2013/10/10; PMID:24105262; https://doi.org/10.1242/jcs.128868
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008; 319(5867):1244-7; PMID:18309083; https://doi.org/10.1126/science.1153124
- Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, Vázquez J, Yáñez-Mó M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem 2013; 288(17):11649-61. Epub 2013/03/07; PMID:23463506; https://doi.org/10.1074/jbc.M112.445304
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 1998; 273:20121-7; PMID:9685355; https://doi.org/10.1074/jbc.273.32.20121
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 2012; 14(7):677-85. Epub 2012/06/05; PMID:22660413; https://doi.org/10.1038/ncb2502
- Géminard C, de Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TfR for sorting into exosomes. Traffic 2004; 5(3):181-95; https://doi.org/10.1111/j.1600-0854.2004.0167.x
- Ghossoub R, Lembo F, Rubio A, Gaillard CB, Bouchet J, Vitale N, Slavík J, Machala M, Zimmermann P. Syntenin-ALIX exosome biogenesis and budding into multivesicular bodies are controlled by ARF6 and PLD2. Nat Communicat 2014; 5:3477. Epub 2014/03/19; PMID:24637612; https://doi.org/10.1038/ncomms4477
- Uytterhoeven V, Lauwers E, Maes I, Miskiewicz K, Melo MN, Swerts J, Kuenen S, Wittocx R, Corthout N, Marrink SJ, et al. Hsc70-4 deforms membranes to promote synaptic protein turnover by endosomal microautophagy. Neuron 2015; 88(4):735-48. Epub 2015/11/22; PMID:26590345; https://doi.org/10.1016/j.neuron.2015.10.012
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2(2):107-17; PMID:11252952; https://doi.org/10.1038/35052055
- Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harbor Perspect Biol 2014; 6(11):a022616. Epub 2014/10/25; PMID:25341920; https://doi.org/10.1101/cshperspect.a022616
- Antonyak MA, Wilson KF, Cerione RA. R(h)oads to microvesicles. Small GTPases 2012; 3(4):219-24; PMID:22906997; https://doi.org/10.4161/sgtp.20755
- D'Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Gene Dev 2012; 26(12):1287-99. Epub 2012/06/21; PMID:22713869; https://doi.org/10.1101/gad.192351.112
- Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, Chaturvedi P, Green JJ, Semenza GL. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A 2014; 111(31):E3234-42; PMID:24938788; https://doi.org/10.1073/pnas.1410041111
- Vidal MJ, Stahl PD. The small GTP-binding proteins Rab4 and ARF are associated with vesicles released during reticulocyte maturation. Eur J Cell Biol 1993; 60(2):261-7; PMID:8330623
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 2000; 149(4):901-14; PMID:10811830; https://doi.org/10.1083/jcb.149.4.901
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005; 122(5):735-49. Epub 2005/09/07; PMID:16143105; https://doi.org/10.1016/j.cell.2005.06.043
- Blanc L, Ciciotte SL, Gwynn B, Hildick-Smith GJ, Pierce EL, Soltis KA, Cooney JD, Paw BH, Peters LL. Critical function for the Ras-GTPase activating protein RASA3 in vertebrate erythropoiesis and megakaryopoiesis. Proc Natl Acad Sci U S A 2012; 109(30):12099-104. Epub 2012/07/10; PMID:22773809; https://doi.org/10.1073/pnas.1204948109
- Peters LL, Paw BH, Blanc L. The scat mouse model highlights RASA3, a GTPase activating protein, as a key regulator of vertebrate erythropoiesis and megakaryopoiesis. Small GTPases 2013; 4(1):47-50. Epub 2012/12/12; PMID:23221813; https://doi.org/10.4161/sgtp.23013
- Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, Fawcett JW. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci 2010; 30(35):11654-69. Epub 2010/09/03; PMID:20810886; https://doi.org/10.1523/JNEUROSCI.2425-10.2010
- Yu X, Prekeris R, Gould GW. Role of endosomal Rab GTPases in cytokinesis. Eur J Cell Biol 2007; 86(1):25-35. Epub 2006/12/13; PMID:17157409; https://doi.org/10.1016/j.ejcb.2006.10.002
- Ramel D, Wang X, Laflamme C, Montell DJ, Emery G. Rab11 regulates cell-cell communication during collective cell movements. Nat Cell Biol 2013; 15(3):317-24. Epub 2013/02/05; PMID:23376974; https://doi.org/10.1038/ncb2681
- Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci 2008; 65(18):2801-13. Epub 2008/08/30; PMID:18726178; https://doi.org/10.1007/s00018-008-8351-4
- Bustos MA, Lucchesi O, Ruete MC, Mayorga LS, Tomes CN. Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc Nat Acad Sci U S A 2012; 109(30):E2057-66. Epub 2012/07/04; PMID:22753498; https://doi.org/10.1073/pnas.1121173109
- Menager MM, Menasche G, Romao M, Knapnougel P, Ho CH, Garfa M, Raposo G, Feldmann J, Fischer A, de Saint Basile G. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol 2007; 8(3):257-67. Epub 2007/01/24; PMID:17237785; https://doi.org/10.1038/ni1431
- Sakurada K, Uchida K, Yamaguchi K, Aisaka K, Ito S, Ohmori T, Takeyama Y, Ueda T, Hori Y, Ohyanagi H, et al. Molecular cloning and characterization of a ras p21-like GTP-binding protein (24KG) from rat liver. Biochem Biophys Res Communicat 1991; 177(3):1224-32. Epub 1991/06/28; PMID:1711847; https://doi.org/10.1016/0006-291X(91)90672-T
- Goldenring JR, Shen KR, Vaughan HD, Modlin IM. Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney, and lung. J Biol Chem 1993; 268(25):18419-22. Epub 1993/09/05; PMID:8360141
- Lai F, Stubbs L, Artzt K. Molecular analysis of mouse Rab11b: a new type of mammalian YPT/Rab protein. Genomics 1994; 22(3):610-6. Epub 1994/08/01; PMID:8001972; https://doi.org/10.1006/geno.1994.1434
- Golachowska MR, Hoekstra D, van ISC. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trend Cell Biol 2010; 20(10):618-26. Epub 2010/09/14; PMID:20833047; https://doi.org/10.1016/j.tcb.2010.08.004
- Green EG, Ramm E, Riley NM, Spiro DJ, Goldenring JR, Wessling-Resnick M. rab11 is associated with transferrin-containing recycling compartments in K562 cells. Biochem Biophys Res Commun 1997; 239:612-6; PMID:9344879; https://doi.org/10.1006/bbrc.1997.7520
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. J Cell Sci 2002; 115:2505-15; PMID:12045221
- Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci 1997; 110(16):1867-77; PMID:9296387
- Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem 2003; 278(22):20083-90; PMID:12639953; https://doi.org/10.1074/jbc.M301642200
- Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic 2005; 6(2):131-43. Epub 2005/01/07; PMID:15634213; https://doi.org/10.1111/j.1600-0854.2004.00257.x
- Escudero CA, Lazo OM, Galleguillos C, Parraguez JI, Lopez-Verrilli MA, Cabeza C, Leon L, Saeed U, Retamal C, Gonzalez A, et al. The p75 neurotrophin receptor evades the endolysosomal route in neuronal cells, favouring multivesicular bodies specialised for exosomal release. J Cell Sci 2014; 127(Pt 9):1966-79. Epub 2014/02/27; PMID:24569882; https://doi.org/10.1242/jcs.141754
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 2009; 139(2):393-404. Epub 2009/10/20; PMID:19837038; https://doi.org/10.1016/j.cell.2009.07.051
- Beckett K, Monier S, Palmer L, Alexandre C, Green H, Bonneil E, Raposo G, Thibault P, Le Borgne R, Vincent JP. Drosophila S2 cells secrete wingless on exosome-like vesicles but the wingless gradient forms independently of exosomes. Traffic 2013; 14(1):82-96. Epub 2012/10/06; PMID:23035643; https://doi.org/10.1111/tra.12016
- Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J Biol Chem 2012; 287(20):16820-34. Epub 2012/03/23; PMID:22437826; https://doi.org/10.1074/jbc.M112.342667
- Chua CE, Lim YS, Tang BL. Rab35–a vesicular traffic-regulating small GTPase with actin modulating roles. FEBS Lett 2010; 584(1):1-6; PMID:19931531; https://doi.org/10.1016/j.febslet.2009.11.051
- Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol 2006; 16(17):1719-25; PMID:16950109; https://doi.org/10.1016/j.cub.2006.07.020
- Cauvin C, Rosendale M, Gupta-Rossi N, Rocancourt M, Larraufie P, Salomon R, Perrais D, Echard A. Rab35 GTPase triggers switch-like recruitment of the lowe syndrome lipid phosphatase OCRL on newborn endosomes. Curr Biol 2016; 26(1):120-8. Epub 2016/01/05; PMID:26725203; https://doi.org/10.1016/j.cub.2015.11.040
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Grønborg M, Möbius W, Rhee J, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol 2010; 189(2):223-32; PMID:20404108; https://doi.org/10.1083/jcb.200911018
- Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, Nave KA, Schild H, Trotter J. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteom Clin Applicat 2007; 1(11):1446-61. Epub 2007/11/01.
- Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave KA, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol 2013; 11(7):e1001604. Epub 2013/07/23; PMID:23874151; https://doi.org/10.1371/journal.pbio.1001604
- Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch UK, Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 2011; 124(Pt 3):447-58. Epub 2011/01/19; PMID:21242314; https://doi.org/10.1242/jcs.074088
- Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood 2007; 110(9):3407-16; PMID:17666570; https://doi.org/10.1182/blood-2007-04-085845
- Chaineau M, Ioannou MS, McPherson PS. Rab35: GEFs, GAPs and effectors. Traffic 2013; 14(11):1109-17. Epub 2013/08/03; PMID:23905989
- Van Gele M, Dynoodt P, Lambert J. Griscelli syndrome: a model system to study vesicular trafficking. Pigment Cell Melanoma Res 2009; 22(3):268-82. Epub 2009/02/27; PMID:19243575; https://doi.org/10.1111/j.1755-148X.2009.00558.x
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010; 12(1):19-30; sup pp 1-13; PMID:19966785; https://doi.org/10.1038/ncb2000
- Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013; 14(9):949-63. Epub 2013/05/18; PMID:23678941; https://doi.org/10.1111/tra.12083
- Zheng Y, Campbell EC, Lucocq J, Riches A, Powis SJ. Monitoring the Rab27 associated exosome pathway using nanoparticle tracking analysis. Exp Cell Res 2013; 319(12):1706-13. Epub 2012/10/25; PMID:23092844; https://doi.org/10.1016/j.yexcr.2012.10.006
- Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Théry C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res 2012; 72(19):4920-30; PMID:22865453; https://doi.org/10.1158/0008-5472.CAN-12-0925
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18(6):883-91. Epub 2012/05/29; PMID:22635005; https://doi.org/10.1038/nm.2753
- Hoshino D, Kirkbride KC, Costello K, Clark ES, Sinha S, Grega-Larson N, Tyska MJ, Weaver AM. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep 2013; 5(5):1159-68. Epub 2013/12/03; PMID:24290760; https://doi.org/10.1016/j.celrep.2013.10.050
- Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014; 41(1):89-103. Epub 2014/07/19; PMID:25035954; https://doi.org/10.1016/j.immuni.2014.05.019
- De Gassart A, Géminard C, Hoekstra D, Vidal M. Exosome secretion: the art of reutilizing nonrecycled proteins? Traffic 2004; 5(11):896-903
- Willms E, Johansson HJ, Mager I, Lee Y, Blomberg KE, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep 2016; 6:22519. Epub 2016/03/05; PMID:26931825; https://doi.org/10.1038/srep22519
- Blanc L, Vidal M. Reticulocyte membrane remodeling: contribution of the exosome pathway. Curr Opin Hematol 2010; 17(3):177-83; PMID:20173636
- Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol 2008; 130(2):197-209; PMID:18566824; https://doi.org/10.1007/s00418-008-0457-0
- Frindt G, Gravotta D, Palmer LG. Regulation of ENaC trafficking in rat kidney. J General Physiol 2016; 147(3):217-27. Epub 2016/02/18; PMID:26880754; https://doi.org/10.1085/jgp.201511533
- Strauss K, Goebel C, Runz H, Mobius W, Weiss S, Feussner I, Simons M, Schneider A. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease. J Biol Chem 2010; 285(34):26279-88; PMID:20554533; https://doi.org/10.1074/jbc.M110.134775
- Abrami L, Brandi L, Moayeri M, Brown MJ, Krantz BA, Leppla SH, van der Goot FG. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep 2013; 5(4):986-96. Epub 2013/11/19; PMID:24239351; https://doi.org/10.1016/j.celrep.2013.10.019
- Hyenne V, Apaydin A, Rodriguez D, Spiegelhalter C, Hoff-Yoessle S, Diem M, Tak S, Lefebvre O, Schwab Y, Goetz JG, et al. RAL-1 controls multivesicular body biogenesis and exosome secretion. J Cell Biol 2015; 211(1):27-37. Epub 2015/10/16; PMID:26459596; https://doi.org/10.1083/jcb.201504136
- Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T. Sec 15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 2004; 279(41):43027-34. Epub 2004/08/05; PMID:15292201; https://doi.org/10.1074/jbc.M402264200
- Aguzzi A, Lakkaraju AK. Cell biology of prions and prionoids: a status report. Trend Cell Biol 2016; 26(1):40-51. Epub 2015/10/13; PMID:26455408; https://doi.org/10.1016/j.tcb.2015.08.007
- Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, Laude H, Raposo G. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A 2004; 101(26):9683-8; PMID:15210972; https://doi.org/10.1073/pnas.0308413101
- Rabesandratana H, Toutant JP, Reggio H, Vidal M. Decay-accelerating factor (CD55) and membrane inhibitor of reactive lysis (CD59) are released within exosomes during in vitro maturation of reticulocyte. Blood 1998; 91:2573-80; PMID:9516159
- Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, Wymant J, Jones AT, Kynaston H, Mason MD, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 2015; 34(3):290-302. Epub 2014/01/21; PMID:24441045; https://doi.org/10.1038/onc.2013.560
- Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res 2014; 74(20):5758-71. Epub 2014/09/28; PMID:25261234; https://doi.org/10.1158/0008-5472.CAN-13-3512
- Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 2009; 11(9):1143-9; PMID:19684575; https://doi.org/10.1038/ncb1929
- Sedgwick AE, Clancy JW, Olivia Balmert M, D'Souza-Schorey C. Extracellular microvesicles and invadopodia mediate non-overlapping modes of tumor cell invasion. Scient Rep 2015; 5:14748. Epub 2015/10/16; PMID:26458510; https://doi.org/10.1038/srep14748
- Martins VR, Dias MS, Hainaut P. Tumor-cell-derived microvesicles as carriers of molecular information in cancer. Curr Opin Oncol 2013; 25(1):66-75. Epub 2012/11/21; PMID:23165142; https://doi.org/10.1097/CCO.0b013e32835b7c81