ABSTRACT
There is extensive crosstalk between different Rho GTPases, including Cdc42, Rac1, and Rac2, and they can activate or inhibit the activity of each other. Dendritic cells express both Rac1 and Rac2. Due to posttranslational modification of lipid anchors, Rac1 localizes mainly to the plasma membrane whereas Rac2 localizes to the phagosomal membrane where it assembles the NADPH complex. Our recent study of primary immunodeficiency disease caused by mutations in the Cdc42 effector Wiskott-Aldrich syndrome protein (WASp) has shed light on the compensatory mechanisms between Rho GTPases and their effector proteins. WASp-deficient dendritic cells have increased localization and activity of Rac2 to the phagosomal membrane and this allows antigen to be presented on MHC class I molecules to activate cytotoxic CD8+ T cells. This study reveals an intricate balance between Rac2 and WASp signaling pathways and provides an example of compensatory pathways in cells devoid of the Cdc42 effector WASp.
Dendritic cells efficiently take up antigen and present antigenic peptides on major histocompatibility (MHC) class I and II molecules to activate T cells. Antigen uptake, internalization, processing, and presentation require fine-tuned regulation of actin cytoskeleton dynamics to sort antigen into the correct intracellular pathway for loading on MHC I or MHC II molecules. Spontaneous actin assembly is inefficient, and the cell uses several actin nucleating promoting factors to obtain rapid and dynamic actin polymerization.Citation1 The Wiskott-Aldrich syndrome protein (WASp) family of actin nucleating promoting factors all contain a verprolin-cofilin-acidic (VCA) domain that binds to the actin-related protein (Arp)2/3 complex to induce actin polymerization leading to formation of branched filaments.Citation1 The WASp family of proteins includes WASp, neuronal (N)-WASp, and WASp-family verprolin-homologous protein (WAVE)/suppressor of the cyclic AMP receptor (SCAR) 1–3, WASp and SCAR homolog (WASH), and junction-mediating and regulatory protein (JMY).Citation2 The activity of WASp, N-WASp, and WAVE/SCAR 1–3 are regulated by the Rho family of small GTPases Cdc42, Rac1, and Rac2. The small Rho GTPases operate as molecular switches cycling between an active guanosine triphosphate (GTP)-bound and an inactive guanosine diphosphate (GDP)-bound state.Citation3 This cycling is tightly regulated by guanosine nucleotide exchange factors (GEFs), which stimulate the exchange of GDP to GTP, and GTPase-activating proteins (GAPs), which increase the intrinsic rate of GTP hydrolysis.Citation3 Effector proteins of Cdc42 and Rac1 are numerous, and many of them have a GTPase binding domain (GBD), also called Cdc42/Rac-interactive binding (CRIB) motif, that specifically recognize the GTP-bound form of the Rho GTPase. In 1996, WASp was identified as a Cdc42 effector protein and it was later shown that Cdc42 is required for WASp activity.Citation4 At rest, WASp and N-WASp resides in an auto-inhibited conformation due to an intramolecular interaction between the VCA domain and the GTPase binding domain.Citation5-Citation7 Upon binding of Cdc42, the auto-inhibited conformation is released and exposes the VCA domain that allows for recruitment of the Arp2/3 complex and actin polymerization.Citation5-Citation7 Rac1 and Rac2 regulate activation of the multimeric WAVE/Scar regulatory complex including Rac, WAVE, Abi1, and IRSp53, to stimulate actin polymerization by the VCA domain.Citation8-Citation10
Rac1 and Rac2 in NADPH assembly
Rac1 and Rac2 serve an important function in haematopoietic cells such as neutrophils, macrophages, and dendritic cells by assembly of the NADPH oxidase complex. This involves sequential assembly of GTP-bound Rac1 or Rac2 with the flavocytochrome b558 consisting of gp91phox and p22phox with the p67phox, p40phox, p47phox NADPH components. The NADPH oxidase regulates electron transfer from NADPH to molecular oxygen leading to production of superoxide that is rapidly converted into H2O2 and other reactive oxygen species (ROS) essential for phagocyte microbicidal function.Citation11,Citation12 Rho GTPases localize to cellular membranes by posttranslational modification of lipid anchors, including geranylgeranyl or farnesyl isoprenoid modifications.Citation3,Citation13 In dendritic cells, Rac1 localize to the plasma membrane and Rac2 specifically to phagosomes.Citation14 It is of interest to note that Rac2-GTP does not need intermediates when it comes to the NADPH oxidase assembly, while Rac1 and Rac2 activation of WAVE1–3 requires intermediate proteins, such as IRSp53.Citation15 In neutrophils, Rac2 has subcellular localization distinct from that of Rac1 and the absence of Rac2 is associated with mislocalization of Rac1.Citation16 Specific sequences in Rac1 and Rac2 that specify subcellular localization determine the specificity of Rac1 and Rac2 in neutrophil chemotaxis and superoxide generation.Citation16 It remains to be determined if Rac1- and Rac2-mediated assembly of the NADPH complex is linked to association with other Rac1and Rac2 effectors to regulate the actin cytoskeleton in proximity to the phagosomal membrane.
Activation of Rac2 in the absence of WASp in dendritic cells
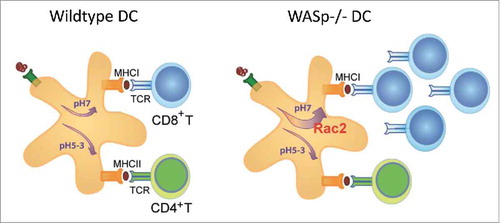
Primary immunodeficiency diseases are caused by loss-of-function mutations in a single gene and provide a unique opportunity to investigate the immune system. The common strategy is to identify loss of a cellular response and interpret this data as the natural function of the gene product of interest. Loss-of-function mutations in the gene encoding WASp in mice and men have been investigated for more than 20 y and have provided critical insights into the role of cell trafficking and cell-to-cell communication during an immune response.Citation17,Citation18 A decreased migratory or cell-to-cell interaction response in WASp-deficient cells has been interpreted by us and many others to mean that WASp directly regulates this response in WASp-sufficient cells. In a recently published paper, we questioned this reasoning and asked if deletion of WASp may trigger activation of other intracellular pathways to compensate for WASp deficiency and provide survival and/or functional capacity of cells.Citation19 We provide evidence for that WASp deficiency skews intracellular signaling to Rac2 activation that assembles the NADPH complex and locally maintains a near neutral pH of endosomes and phagosomes.Citation19 In dendritic cells, exogenous antigen can be degraded by 2 different intracellular pathways. The default pathway fuses antigen-containing phagosomes with lysosomes allowing for antigen degradation at acidic pH and loading of antigenic peptides on MHC class II molecules to activate CD4+ T helper cells ().Citation20 The alternative pathway, referred to as antigen cross-presentation, uses Rac2-mediated assembly of the NADPH complex in the phagosomal membrane. This leads to protons being pumped out from the phagosome, production of reactive oxygen species (ROS), and maintenance of a near neutral pH in the phagosome. Antigen will be transported to the cytosol, degraded by the proteasome, and loaded on MHC class I molecules to activate CD8+ cytotoxic T cells (). Our data suggests that in the absence of WASp, dendritic cells favor the cross-presentation pathway due to increased Rac2 expression, activity, and localization to phagosomes ().Citation19
Figure 1. Schematic presentation of cross-presentation of exogenous antigen in dendritic cells. Rac2 assembles the NADPH oxidase at the phagosomal membrane leading to production of reactive oxygen species. The ATPase pumps out protons (H+) from the phagosome leading to a near neutral pH and antigen escape to the cytosol. Abbreviations: DC; dendritic cell, ROS; reactive oxygen species, TCR; T cell receptor.
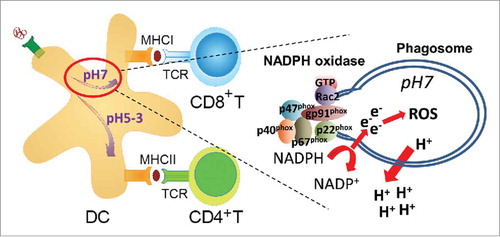
Figure 2. Schematic presentation of antigen presentation in wildtype and WASp−/− dendritic cells. Due to increased Rac2 assembly of the NADPH complex in WASp−/− dendritic cells, antigen is shuttled into the cross-presentation pathway for activation of CD8+ cytotoxic T cells. Abbreviations: DC; dendritic cell, WASp; TCR; T cell receptor, WASp; Wiskott-Aldrich syndrome protein.
Redundant and unique activity of WASp family members
The WASp family of proteins have redundant and unique activities within the cell. N-WASp activity can compensate for critical functions of WASp in lymphocytes since deletion of both WASp and N-WASp in B or T cells leads to severely compromised development and function.Citation21,Citation22 However, N-WASp expression alone in WASp-deficient B and T cells is linked to autoreactivity, possibly due to altered signaling threshold for activation of the B cell and T cell receptor.Citation22-Citation25 Another example of such compensatory mechanism comes from studies of WASp−/− NK cells. Treatment with IL-2 restores normal cytotoxicity of WASp−/− NK cells in vitro and in vivo by increased activation of the WASp family protein WAVE2.Citation26,Citation27 It remains largely unknown how altered expression of WASp family members may affect the activation and cellular localization of Rho GTPases.
Cross-talk between Rho GTPases affecting effector function
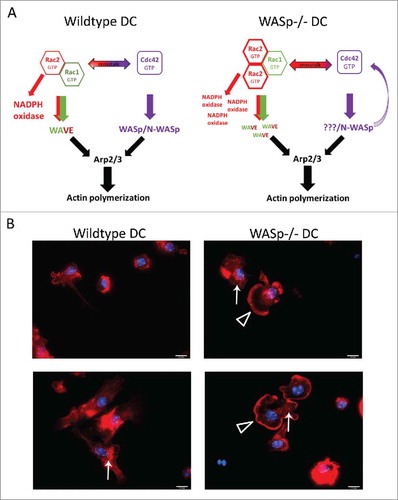
Our finding of increased activation of Rac2 in the absence of WASp provides a new example of the extensive crosstalk between different Rho GTPases. Cdc42, Rac1, and Rac2 can activate or inhibit the activity of each other.Citation13 Cdc42 can inhibit Rac1 and Rac2 activation by acting as a competitor for specific binding sites. Cdc42 can bind to the NADPH oxidase component gp91phox in vitro but is unable to stimulate ROS formation by the NADPH oxidase. Constitutively active Cdc42-Q61L, locked in the GTP-bound state, inhibits ROS production induced by Rac1-Q61L in an NADPH oxidase-expressing Cos7 cell line.Citation28 This suggests that Cdc42-GTP activity may proceed Rac1-GTP activity in assembly of the NADPH complex and that Cdc42 acts as a competitive inhibitor of Rac1- and Rac2-mediated ROS production.Citation28 Inhibition of Cdc42 activity by transduction of the Cdc42-binding domain of WASp into human neutrophils results in enhanced ROS production, consistent with inhibitory cross-talk between Rac2 and Cdc42 in regulating NADPH assembly and activity.Citation29 However, Cdc42 can also stimulate Rac1 and Rac2 activity proximal to cellular membranes. Membrane localization is a key trigger in Rac signaling. Activation of the Rac effector PAK1 (serine/threonine kinase p21 associated kinase1) requires localization of Rac1 to lipid membranes.Citation30,Citation31 Effector proteins such as WASp, N-WASp, and PAK1 sequester active GTP-bound Cdc42 and Rac1/2 at the membrane while dissociation of these Rho GTPases from the membrane usually results in sequestration by Rho guanine nucleotide dissociation inhibitors (RhoGDIs) or in degradation.Citation13 WASp activation, at least initially, requires binding of active GTP-Cdc42.Citation5,Citation6 Therefore, WASp−/− dendritic cells may have a significant pool of active non-sequestered Cdc42 available for various signaling events. When located in proximity at lipid membranes, Cdc42 can directly induce Rac activity. First, in fibroblasts Cdc42 can induce Rac1-dependent cytoskeletal changes, lamellipodia, at the leading edge of the cell.Citation32 Second, a more recent live cell imaging approach in fibroblasts shows that the activity of Cdc42 and Rac1 is spatio-temporally synchronized to stabilize newly expanded membrane and that Rac1 activity remains longer than Cdc42 activity in lamellipodia structures.Citation33 Notably, we detected large lamellipodia-like structures in bone marrow-derived WASp−/− dendritic cells while such structures were rarely detected in wildtype dendritic cells (). This suggests that WASp−/− dendritic cells activate Rac-dependent cytoskeletal changes. Third, Cdc42 can induce local activation of Rac1 at membranes in HeLa and Cos7 cells. Cdc42 binds directly to PAK1 and promotes association with the PAK-interacting GEF α-(PIX) and β-PIX that activate Rac1 at the membrane.Citation34,Citation35 A recent study provides evidence for that PAK2 via interaction with β-Pix and Cdc42 regulates homing and migration of haematopoietic stem cells.Citation36 It is likely that the complex network of activating and inhibitory cross-talk between Cdc42, Rac1, and Rac2 is dependent on cell identity and cellular context at different lipid membranes.
Figure 3. WASp deficiency and the crosstalk between Rac1, Rac2, and Cdc42 in dendritic cells. Wildtype dendritic cells can induce actin polymerization through activation of different pathways that include Rac1 and Rac2 signaling to WAVE and Cdc42 signaling to WASp/N-WASp. WAVE and WASp/N-WASp can activate the Arp2/3 complex and induce actin polymerization. In WASp−/− dendritic cells, the absence of WASp may induce a negative feedback loop through Cdc42,Citation29 which directs the response toward the activation of Rac1 and Rac2. Activation of Rac1 and Rac2 leads to increased WAVE activity and NADPH oxidase activity.Citation14,Citation16,Citation19,Citation56,Citation57 The hypothetical increase of WAVE activation would lead to a compensatory increase in actin polymerization. (B) In support of increased Rac1, Rac2, and WAVE activity in WASp-deficient dendritic cells, we noticed that WASp−/− bone marrow derived dendritic cells formed large lamellipodia, a structure associated with Rac1 and Rac2 activity. Bone marrow-derived dendritic cells were added to glass coverslips, fixed, and labeled with Phalloidin and DAPI to visualize polymerized actin and the nucleus. Open triangle indicates lamellipodia and arrow indicates podosomes. Red; phalloidin, blue; DAPI. Abbreviation: DC; dendritic cell.
Implications for understanding human disease
Our recent study suggests that deletion of a specific protein (WASp) can reprogram dendritic cells and have profound effects on the cellular response (activation of CD8+ T cells). This finding leads the way for increased awareness of strong compensatory mechanisms when studying single-gene defects in patients and mice. Because of the extensive cross-talk between Rho GTPases and the WASp family members, this raises the possibility that activation of alternative intracellular pathways may rescue a detrimental cellular defect. Three patients with mutations in Rac2 have been identified that suffer from a neutrophil immunodeficiency syndrome. All 3 patients harbour a D57N mutation within the Rac2 DX2G motif, conserved in all GTPases, that results in a dominant negative protein. Rac2-D57N neutrophils show complete loss of chemotaxis, ROS production, and polarization in response to a variety of receptor stimuli.Citation37-Citation39 Murine Rac2−/− neutrophils have a similar phenotype and show decreased migration and reduced NADPH activity.Citation40 A striking conclusion of these findings together with the Rac2-D57N patients is that Rac1 insufficiently compensates for Rac2 deficiency in assembly of the NADPH oxidase in neutrophils. This is perhaps due to the specific localization of Rac2 to the phagosomal membrane in neutrophils and dendritic cells. Deletion of both Rac1 and Rac2 in B cells severely compromises B cell development and B cell entry into the white pulp cords of the spleen when compared with deletion of only Rac1 or Rac2 in B cells.Citation41,Citation42 Specific deletion of Rac2 in B cells leads to hyper-phosphorylation of Rac1, suggesting that Rac1 can partly compensate for Rac2 at least in B cell receptor signaling.Citation41 Deletion of both Rac1 and Rac2 in haematopoietic cells leads to rapid egress of haematopoietic stem cells from the bone marrow to the blood and failure of Rac1- and Rac2-deficient haematopoietic stem cells to engraft irradiated recipient mice.Citation43,Citation44 However, Rac1 and Rac2 play distinct roles in actin organization, cell survival, and proliferation and Rac2 is essential for superoxide production and directed migration in neutrophils.Citation43 It would be interesting to determine if some of the effects of Rac2 deficiency are caused by skewed activation toward Cdc42-mediated activation of WASp and N-WASp.
Conclusions and future perspectives
An emerging view from investigation of WAS patient cells and WASp-deficient mice is that WASp deficiency affects specific haematopoietic cells quite differently. Some cells become hypo-responsive in the absence of WASp such as CD4+ T helper cells.Citation45-Citation48 Recent studies show that some haematopoietic cells become hyper-responsive in the absence of WASp such as plasmacytoid DCs,Citation49 B cells,Citation23,Citation25,Citation50,Citation51 and dendritic cell-induced activation of CD8+ T cells.Citation19 Our data from examining dendritic cells and cross-presentation in the absence of WASp suggests that skewed intracellular signaling may significantly contribute to functional outcome. It is possible that some of the cell-specific phenotypes can be attributed to alternative sequestering, expression, and activation of the Rho GTPases Cdc42, Rac1, and Rac2. An interesting aspect for future research will be to compare how different mutations in WASp elicit vastly different immunodeficiency syndromes characterized as WAS or X-linked neutropenia (XLN), the latter caused by constitutive activation of WASp.Citation18,Citation52-Citation54 It is plausibly that some of the clinical findings may be explained by decreased or increased sequestering of GTP-bound Cdc42 when WASp is absent compared with when WASp is constitutively active. Although gene knockout approaches have been valuable in dissecting cellular behavior in the absence of a protein, the next important step will be to examine the role of endogenous proteins in their natural environment. A hurdle to overcome is to correctly visualize WASp and Cdc42 inside the cell. Much of what is known about their localization comes from overexpression studies using mutants of Cdc42 that is locked in the GTP-bound from (Cdc42-L61) or GDP-bound form (Cdc42-N17) or using GFP-fusion proteins, both with natural limitations in localizing endogenously expressed WASp and Cdc42 inside the cell. Modifying the germline-encoded genes by insertion of small specific tags should help in visualization of proteins, for example using the Avitag – BirA ligase system to biotinylate proteins in live cells.Citation55 New antibodies that can distinguish between closely related proteins such as WASp and N-WASp will help resolve the issue with cross-reactivity between homologues proteins in haematopoietic cells.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The work discussed here was supported by a PhD fellowship from Fundação para a Ciência e a Tecnologia #SFRH/BD/47926/2008 and the Queen Silvia foundation to M.A.P.B, and research grants from the Swedish Research Council, the Cancer Society, the Childhood Cancer Society, the European Commission 7th framework program Marie Curie reintegration grant (#249177), Karolinska Institutet, Åke Olsson foundation, Jeansson foundation, Groschinsky Foundation, Åke Wiberg Foundation, Bergvall Foundation, King Gustaf V's 80-year foundation, and the Swedish Medical Society to L.S.W. L.S.W. is a Ragnar Söderberg fellow in Medicine.
References
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 2010; 11:237-51; PMID:20237478; http://dx.doi.org/10.1038/nrm2867
- Moulding DA, Record J, Malinova D, Thrasher AJ. Actin cytoskeletal defects in immunodeficiency. Immunol Rev 2013; 256:282-99; PMID:24117828; http://dx.doi.org/10.1111/imr.12114
- Olson MF. Rho GTPases, their post-translational modifications, disease-associated mutations and pharmacological inhibitors. Small GTPases 2016:1-13; PMID:27548350; http://dx.doi.org/10.1080/21541248.2016.1218407
- Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol 1996; 6:70-5; PMID:8805223; http://dx.doi.org/10.1016/S0960-9822(02)00423-2
- Kim AS, Kakalis LT, Abdul-Manan N, Liu GA, Rosen MK. Autoinhibition and activation mechanisms of the Wiskott-Aldrich syndrome protein. Nature 2000; 404:151-8; PMID:10724160; http://dx.doi.org/10.1038/35004513
- Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol Cell 2003; 11:1215-27; PMID:12769846; http://dx.doi.org/10.1016/S1097-2765(03)00139-4
- Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner MW. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 1999; 97:221-31; PMID:10219243; http://dx.doi.org/10.1016/S0092-8674(00)80732-1
- Machesky LM, Insall RH. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr Biol 1998; 8:1347-56; PMID:9889097; http://dx.doi.org/10.1016/S0960-9822(98)00015-3
- Miki H, Suetsugu S, Takenawa T. WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J 1998; 17:6932-41; PMID:9843499; http://dx.doi.org/10.1093/emboj/17.23.6932
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature 2002; 418:790-3; PMID:12181570; http://dx.doi.org/10.1038/nature00859
- Diekmann D, Abo A, Johnston C, Segal AW, Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 1994; 265:531-3; PMID:8036496; http://dx.doi.org/10.1126/science.8036496
- Nunes P, Demaurex N, Dinauer MC. Regulation of the NADPH oxidase and associated ion fluxes during phagocytosis. Traffic 2013; 14:1118-31; PMID:23980663
- Boulter E, Estrach S, Garcia-Mata R, Feral CC. Off the beaten paths: alternative and crosstalk regulation of Rho GTPases. FASEB J 2012; 26:469-79; PMID:22038046; http://dx.doi.org/10.1096/fj.11-192252
- Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, Moita LF, Amigorena S. The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 2009; 30:544-55; PMID:19328020; http://dx.doi.org/10.1016/j.immuni.2009.01.013
- Lam BD, Hordijk PL. The Rac1 hypervariable region in targeting and signaling: a tail of many stories. Small GTPases 2013; 4:78-89; PMID:23354415; http://dx.doi.org/10.4161/sgtp.23310
- Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol 2004; 5:744-51; PMID:15170212; http://dx.doi.org/10.1038/ni1081
- Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol 2013; 14:7-12; PMID:23212475; http://dx.doi.org/10.1038/nrm3492
- Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol 2010; 10:182-92; PMID:20182458; http://dx.doi.org/10.1038/nri2724
- Baptista MA, Keszei M, Oliveira M, Sunahara KK, Andersson J, Dahlberg CI, Worth AJ, Liedén A, Kuo IC, Wallin RP, et al. Deletion of Wiskott-Aldrich syndrome protein triggers Rac2 activity and increased cross-presentation by dendritic cells. Nat Commun 2016; 7:12175; PMID:27425374; http://dx.doi.org/10.1038/ncomms12175
- Amigorena S, Savina A. Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol 2010; 22:109-17; PMID:20171863; http://dx.doi.org/10.1016/j.coi.2010.01.022
- Westerberg LS, Dahlberg C, Baptista M, Moran CJ, Detre C, Keszei M, Eston MA, Alt FW, Terhorst C, Notarangelo LD, et al. Wiskott-Aldrich syndrome protein (WASP) and N-WASP are critical for peripheral B-cell development and function. Blood 2012; 119:3966-74; PMID:22411869; http://dx.doi.org/10.1182/blood-2010-09-308197
- Cotta-de-Almeida V, Westerberg L, Maillard MH, Onaldi D, Wachtel H, Meelu P, Chung UI, Xavier R, Alt FW, Snapper SB. Wiskott Aldrich syndrome protein (WASP) and N-WASP are critical for T cell development. Proc Natl Acad Sci U S A 2007; 104:15424-9; PMID:17878299; http://dx.doi.org/10.1073/pnas.0706881104
- liuDahlberg CI, Torres ML, Petersen SH, Baptista MA, Keszei M, Volpi S, Grasset EK, Karlsson MC, Walter JE, Snapper SB, et al. Deletion of WASp and N-WASp in B cells cripples the germinal center response and results in production of IgM autoantibodies. J Autoimmun 2015; 62:81-92; PMID:26143192; http://dx.doi.org/10.1016/j.jaut.2015.06.003
- Liu C, Bai X, Wu J, Sharma S, Upadhyaya A, Dahlberg CI, Westerberg LS, Snapper SB, Zhao X, Song W. N-wasp is essential for the negative regulation of B cell receptor signaling. PLoS Biol 2013; 11:e1001704; PMID:24223520; http://dx.doi.org/10.1371/journal.pbio.1001704
- Volpi S, Santori E, Abernethy K, Mizui M, Dahlberg CI, Recher M, Capuder K, Csizmadia E, Ryan D, Mathew D, et al. N-WASP is required for B-cell-mediated autoimmunity in Wiskott-Aldrich syndrome. Blood 2016; 127:216-20; PMID:26468226; http://dx.doi.org/10.1182/blood-2015-05-643817
- Orange JS, Roy-Ghanta S, Mace EM, Maru S, Rak GD, Sanborn KB, Fasth A, Saltzman R, Paisley A, Monaco-Shawver L, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest 2011; 121:1535-48; PMID:21383498; http://dx.doi.org/10.1172/JCI44862
- Kritikou JS, Dahlberg CI, Baptista MA, Wagner AK, Banerjee PP, Gwalani LA, Poli C, Panda SK, Kärre K, Kaech SM, et al. IL-2 in the tumor microenvironment is necessary for Wiskott-Aldrich syndrome protein deficient NK cells to respond to tumors in vivo. Sci Rep 2016; 6:30636; PMID:27477778; http://dx.doi.org/10.1038/srep30636
- Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol 2001; 2:211-5; PMID:11224519; http://dx.doi.org/10.1038/85259
- Diebold BA, Fowler B, Lu J, Dinauer MC, Bokoch GM. Antagonistic cross-talk between Rac and Cdc42 GTPases regulates generation of reactive oxygen species. J Biol Chem 2004; 279:28136-42; PMID:15123662; http://dx.doi.org/10.1074/jbc.M313891200
- del Pozo MA, Alderson NB, Kiosses WB, Chiang HH, Anderson RG, Schwartz MA. Integrins regulate Rac targeting by internalization of membrane domains. Science 2004; 303:839-42; PMID:14764880; http://dx.doi.org/10.1126/science.1092571
- Del Pozo MA, Kiosses WB, Alderson NB, Meller N, Hahn KM, Schwartz MA. Integrins regulate GTP-Rac localized effector interactions through dissociation of Rho-GDI. Nat Cell Biol 2002; 4:232-9; PMID:11862216; http://dx.doi.org/10.1038/ncb759
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995; 81:53-62; PMID:7536630; http://dx.doi.org/10.1016/0092-8674(95)90370-4
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461:99-103; PMID:19693013; http://dx.doi.org/10.1038/nature08242
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell 1998; 1:183-92; PMID:9659915; http://dx.doi.org/10.1016/S1097-2765(00)80019-2
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 1994; 367:40-6; PMID:8107774; http://dx.doi.org/10.1038/367040a0
- Reddy PN, Radu M, Xu K, Wood J, Harris CE, Chernoff J, Williams DA. p21-activated kinase 2 regulates HSPC cytoskeleton, migration, and homing via CDC42 activation and interaction with beta-Pix. Blood 2016; 127:1967-75; PMID:26932803; http://dx.doi.org/10.1182/blood-2016-01-693572
- Williams DA, Tao W, Yang F, Kim C, Gu Y, Mansfield P, Levine JE, Petryniak B, Derrow CW, Harris C, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood 2000; 96:1646-54; PMID:10961859
- Accetta D, Syverson G, Bonacci B, Reddy S, Bengtson C, Surfus J, Harbeck R, Huttenlocher A, Grossman W, Routes J, et al. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol 2011; 127:535-8 e1-2.
- Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, Gonzalez-Aller C, Hiester A, deBoer M, Harbeck RJ, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A 2000; 97:4654-9; PMID:10758162; http://dx.doi.org/10.1073/pnas.080074897
- Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 1999; 10:183-96; PMID:10072071; http://dx.doi.org/10.1016/S1074-7613(00)80019-9
- Walmsley MJ, Ooi SKT, Reynolds LF, Smith SH, Ruf S, Mathiot A, Vanes L, Williams DA, Cancro MP, Tybulewicz VL. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science 2003; 302:459-62; PMID:14564011; http://dx.doi.org/10.1126/science.1089709
- Henderson RB, Grys K, Vehlow A, de Bettignies C, Zachacz A, Henley T, Turner M, Batista F, Tybulewicz VL. A novel Rac-dependent checkpoint in B cell development controls entry into the splenic white pulp and cell survival. J Exp Med 2010; 207:837-53; PMID:20308364; http://dx.doi.org/10.1084/jem.20091489
- Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 2003; 302:445-9; PMID:14564009; http://dx.doi.org/10.1126/science.1088485
- Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med 2005; 11:886-91; PMID:16025125; http://dx.doi.org/10.1038/nm1274
- Morales-Tirado V, Johannson S, Hanson E, Howell A, Zhang J, Siminovitch KA, Fowell DJ. Cutting edge: selective requirement for the Wiskott-Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J Immunol 2004; 173:726-30; PMID:15240657; http://dx.doi.org/10.4049/jimmunol.173.2.726
- Trifari S, Sitia G, Aiuti A, Scaramuzza S, Marangoni F, Guidotti LG, Martino S, Saracco P, Notarangelo LD, Roncarolo MG, et al. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. J Immunol 2006; 177:7451-61; PMID:17082665; http://dx.doi.org/10.4049/jimmunol.177.10.7451
- Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu CH, Hagemann TL, Kwan SP, Ferrini R, Davidson L, et al. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity 1998; 9:81-91; PMID:9697838; http://dx.doi.org/10.1016/S1074-7613(00)80590-7
- Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, dos Santos AO, Nagy A, Grinstein S, et al. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med 1999; 190:1329-42; PMID:10544204; http://dx.doi.org/10.1084/jem.190.9.1329
- Prete F, Catucci M, Labrada M, Gobessi S, Castiello MC, Bonomi E, Aiuti A, Vermi W, Cancrini C, Metin A, et al. Wiskott-Aldrich syndrome protein-mediated actin dynamics control type-I interferon production in plasmacytoid dendritic cells. J Exp Med 2013; 210:355-74; PMID:23337808; http://dx.doi.org/10.1084/jem.20120363
- Recher M, Burns SO, de la Fuente MA, Volpi S, Dahlberg C, Walter JE, Moffitt K, Mathew D, Honke N, Lang PA, et al. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood 2012; 119:2819-28; PMID:22302739; http://dx.doi.org/10.1182/blood-2011-09-379412
- Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, Sather BD, Khim S, Liggitt D, Song W, et al. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med 2011; 208:2033-42; PMID:21875954; http://dx.doi.org/10.1084/jem.20110200
- Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, Van Den Oord JJ, Verhoef GE, Boogaerts MA, Fryns JP, You D, et al. Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet 2001; 27:313-7; PMID:11242115; http://dx.doi.org/10.1038/85886
- Beel K, Cotter MM, Blatny J, Bond J, Lucas G, Green F, Vanduppen V, Leung DW, Rooney S, Smith OP, et al. A large kindred with X-linked neutropenia with an I294T mutation of the Wiskott-Aldrich syndrome gene. Br J Haematol 2009; 144:120-6; PMID:19006568; http://dx.doi.org/10.1111/j.1365-2141.2008.07416.x
- Ancliff PJ, Blundell MP, Cory GO, Calle Y, Worth A, Kempski H, Burns S, Jones GE, Sinclair J, Kinnon C, et al. Two novel activating mutations in the Wiskott-Aldrich syndrome protein result in congenital neutropenia. Blood 2006; 108:2182-9; PMID:16804117; http://dx.doi.org/10.1182/blood-2006-01-010249
- McManus S, Ebert A, Salvagiotto G, Medvedovic J, Sun Q, Tamir I, Jaritz M, Tagoh H, Busslinger M. The transcription factor Pax5 regulates its target genes by recruiting chromatin-modifying proteins in committed B cells. EMBO J 2011; 30:2388-404; PMID:21552207; http://dx.doi.org/10.1038/emboj.2011.140
- Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol 2003; 170:5652-7; PMID:12759446; http://dx.doi.org/10.4049/jimmunol.170.11.5652
- Yamauchi A, Marchal CC, Molitoris J, Pech N, Knaus U, Towe J, Atkinson SJ, Dinauer MC. Rac GTPase isoform-specific regulation of NADPH oxidase and chemotaxis in murine neutrophils in vivo. Role of the C-terminal polybasic domain. J Biol Chem 2005; 280:953-64; PMID:15504745
