ABSTRACT
G protein-coupled receptors (GPCRs) constitute a superfamily of cell surface receptors that regulate a variety of cell functions. As the cell surface is the functional destination for most GPCRs, the cell surface targeting process represents a crucial checkpoint in controlling the functionality of the receptors. However, the molecular mechanisms underlying the cell surface delivery of newly synthesized GPCRs remain poorly understood. In this review, we will highlight the role of Rab GTPases in GPCR cell surface transport, particularly post-Golgi traffic, and discuss the underlying molecular mechanisms.
Regulation of intracellular trafficking of G protein-coupled receptors by Rab GTPases
G protein-coupled receptors (GPCRs) are the largest and the most structurally diverse family of signaling proteins.Citation1,2 They regulate a variety of cell functions and are the actual targets of about 30% of drugs on the market to treat human diseases. The precise function of GPCRs is tightly controlled by their appropriate intracellular trafficking, such as ER-Golgi-cell surface export, internalization, recycling and lysosome transport, which dictate the receptors to their functional destinations. After being synthesized and properly assembled in the endoplasmic reticulum (ER), GPCRs are transported forward in vesicles to the cell surface through the Golgi cisternae and the trans-Golgi network (TGN). Once at the cell surface, the receptors are able to bind to their ligands or drugs to produce physiologic or pharmacological responses. Ligand binding to the receptors may induce receptor internalization during which the receptors are moved from the cell surface to the endosomal compartment. Internalized receptors in endosomes can be sorted to the recycling, degradation or retrograde pathways which transport the receptors to the cell surface, lysosomes and the TGN, respectively. Over the past decades, most studies on GPCR trafficking have focused on internalization, recycling and degradation pathways.Citation3-5 However, the molecular mechanisms underlying the anterograde transport of nascent GPCRs en route from the ER through the Golgi to the cell surface remain poorly understood.
Rab GTPases are the largest branch of the Ras-related GTPase superfamily, consisting of more than 60 members, and are involved in almost every step of vesicle-mediated endocytosis and exocytosis. Each Rab GTPase has a distinct subcellular localization pattern that correlates with its function in directing cargo transport between specific subcellular compartments. As with all GTPases, Rab GTPases cycle between the GDP- and GTP-bound states, which is controlled by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs).Citation6-10 In general, Rab GTPases in the GDP-bound state are extracted from membranes by GDP dissociation inhibitors (GDIs) via interacting with the hydrophobic prenyl groups of Rab GTPases. This interaction not only keeps Rab GTPases in the cytoplasm but also controls their recruitment onto the correct subcellular location.Citation8,11 When in the GTP-bound state, Rab GTPases can bind to diverse downstream effectors which may regulate cargo selection, vesicle formation, sorting, motility, tethering and fusion with the appropriate membranes.Citation12-16
Several Rab GTPases have been demonstrated to regulate intracellular trafficking of GPCRs. The most studied Rab GTPase in GPCR trafficking is Rab5, a well characterized GTPase involved in protein trafficking between the plasma membrane and endosomes. Rab5 has been shown to regulate the agonist-evoked internalization of multiple GPCRs.Citation17-27 Constitutive transport of some GPCRs from the cell surface to endosomes may also use the Rab5-mediated pathway.Citation28,29 In contrast, Rab4 and Rab11 are mainly involved in the recycling of internalized receptors from endosomes back to the cell surface membrane, either at distinct recycling steps or via different pathways.Citation17-20,22,25,27,29 Rab7 has been shown to participate in the transport of internalized receptors to the lysosome for degradationCitation20 and in the transport from endosomes to the TGN.Citation28
Our laboratory has focused efforts on understanding the role of Rab GTPases in the biosynthesis pathway of GPCRs.Citation30 We and others have demonstrated that 3 Rab GTPases Rab1, Rab2 and Rab6, which have been known to be involved in vesicle-mediated transport along the early secretory pathway, are required for the ER-to-Golgi transport of GPCRs, including α1-adrenergic receptor (AR), α2-AR, β1-AR, β2-AR, angiotensin II type 1 receptor (AT1R), AT2R and human calcium-sensing receptor.Citation31-40 The following sections will discuss the role of Rab8 and Rab26 in GPCR transport from the Golgi to the cell surface and the possible underlying mechanisms.
Role of Rab8 and Rab26 in the post-Golgi transport of GPCRs
There are 2 Rab8 isoforms, Rab8A and Rab8B, which share 83% identity in their amino acid sequences and have undistinguishable functions. Rab8 isoforms are localized to different subcellular compartments (the Golgi apparatus, plasma membranes, and endosomes) and cell structures (filopodia, lamellipodia, protrusions, ruffles and primary cilia). Rab8 function in protein transport has been extensively investigated, particularly from the TGN to the apical/basolateral membrane under polarized conditions.Citation41,42 Rab26 is relatively less well characterized as compared with many other secretory GTPases. Rab26 was found to be highly expressed at the GolgiCitation43 and on the parotid acinar cell secretory granules.Citation44,45 Rab26 was shown to regulate the maturation of secretory granulesCitation46 and the release of amylase.Citation44,45
The first indication of Rab8 function in GPCR maturation was its association with the post-Golgi membranes that transport newly synthesized rhodopsin to the rod outer segments in retinal photoreceptors.Citation47 A subsequent study showed that expression of dominant negative Rab8 mutants disrupted the post-Golgi transport of rhodopsin in Xenopus.Citation48 We and others have determined the role of Rab8 in the cell surface expression of α2B-AR and β2-AR, 2 prototypic GPCRs which mainly couple to Gi and Gs, respectively, in mammalian cells and primary cultures of neurons.Citation34,49 Attenuation of Rab8 function achieved by transient expression of its dominant negative mutants and shRNA-mediated knockdown significantly reduced the cell surface expression of α2B-AR and β2-AR as measured by intact cell ligand binding. Confocal imaging showed that both receptors were extensively co-localized with the TGN marker p230 in cells expressing the Rab8 mutants. These studies demonstrate that Rab8 is required for post-Golgi traffic of some GPCRs.
Similar to the effect of Rab8, expression of dominant negative Rab26 mutants and siRNA-mediated depletion of Rab26 markedly reduced the cell surface expression of α2A-AR and α2B-AR and caused a massive accumulation of the receptors in the Golgi apparatus.Citation43 These data suggest that Rab26 may function as a crucial regulator for the Golgi-to-cell surface movement of α2-ARs. These data also provide the first evidence indicating that Rab26 may play an important role in the post-Golgi transport of GPCRs.
Differential interaction of Rab8 and Rab26 with GPCRs
To elucidate the molecular mechanisms underlying the function of Rab8 and Rab26 in regulating GPCR transport, we determined if they were able to physically associate with the receptors. Several other Rab GTPases, including Rab4, Rab5, Rab7 and Rab11, have been described to directly interact with GPCRs. For example, Rab4 and Rab11 interact with the C-termini of AT1R, β2-AR and thromboxane A2 receptor to regulate their recycling from endosomes back to the plasma membrane.Citation21,50,51 We found that Rab8 bound to α2B-AR and β2-ARCitation49 and that Rab26 interacted with α2B-ARCitation43 as measured in different protein-protein interaction assays, including co-immunoprecipitation, GST fusion protein pulldown and bioluminescence resonance energy transfer assays.
The most interesting point regarding the interaction of Rab8 and Rab26 with GPCRs is the identification of distinct Rab-binding domains or motifs in α2B-AR and β2-AR. α2B-AR has a relatively large third intracellular loop (ICL3) and short ICL1, ICL2 and C-terminus, whereas β2-AR contains a large C-terminus and short intracellular loops. In GST fusion protein pulldown assays, Rab8-binding domains were mapped to the C-termini of α2B-AR and β2-AR. Site-directed mutagenesis further identified multiple residues in the fragments VFNQ and PWTQTGW of the C-terminus which modulated α2B-AR interaction with Rab8.Citation49 In contrast, the dileucine (LL) motif in the C-terminus was required for the interaction of β2-AR, but not α2B-AR, with Rab8. The LL motif is highly conserved in the membrane-proximal C-termini of the family A GPCRsCitation52 and is required for the ER export of several GPCRs, including α2B-AR and β2-AR.Citation52-55 These data suggest that Rab8 function in the post-Golgi trafficking of different GPCRs is mediated through distinct mechanisms. These data also imply that the LL motif may regulate β2-AR transport from both the ER and the Golgi.
In contrast to Rab8 interacting with the C-termini of α2B-AR and β2-AR,Citation49 Rab26 was shown to bind to the ICL3 of α2B-AR.Citation43 These data suggest that one GPCR may utilize different intracellular domains to interact with distinct Rab GTPases to control their export trafficking (). However, if Rab8 and Rab26 could simultaneously bind to α2B-AR and how they cooperatively regulate the post-Golgi export of α2B-AR remain unknown. It is possible that Rab8 and Rab26 mediate α2B-AR transport along different routes (). It is also possible that Rab8 and Rab26 may regulate α2B-AR transport at different steps from different sub-compartments in the same pathway ( and ).
Figure 1. Possible mechanisms by which Rab8 and Rab26 mediate the Golgi-to-cell surface transport of α2B-AR. A, Rab8 and Rab26 mediate 2 separate pathways that deliver the receptor to the cell surface. Rab8, in its inactive form presumably localized in the cytoplasm, interacts with the C-terminus of α2B-AR, whereas Rab26, in its active form tethered to the membrane, binds to the ICL3 of the receptor. B and C, Rab8 and Rab26 regulate α2B-AR transport at different steps in the same route. PM, plasma membrane.
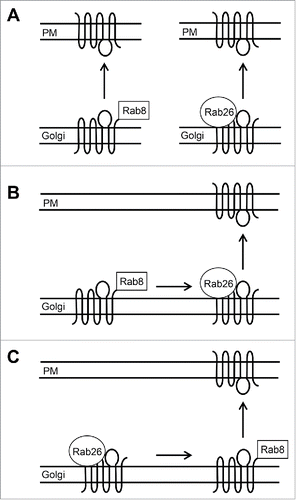
Another striking difference between the interactions of Rab8 and Rab26 with the receptors is their activation dependence. Both α2B-AR and β2-AR were found to associate with Rab8, preferentially in its inactive, GDP-bound form.Citation49 It is possible that both receptors may function as GEFs to promote the activation of Rab8. This possibility is supported by the fact that some cargo proteins can activate transport machinery to modulate their transport. For example, AT1R is able to interact with and activate Rab5 GTPase which regulates the internalization process of the receptor.Citation21 It is also possible that the receptors may function as anchoring proteins for Rab8 localization to the TGN through providing docking sites for inactive, GDP-bound Rab8. In contrast, α2B-AR interaction with active, GTP-bound form of Rab26 was much stronger than with inactive, GDP-bound form of Rab26.Citation43 These data suggest that α2B-AR may be a downstream effector of Rab26 and the Rab26 interaction may enhance the recruitment of the receptors onto Rab26-coordinated transport vesicles.
As discussed above, different GPCRs interact with one Rab via different motifs and one GPCR may bind to different Rab GTPases via different domains. These data, together with other studies demonstrating that GPCRs are able to directly associate with Sec24 isoforms,Citation56 the components of ER-derived COPII vesicles that mediate cargo transport exclusively from the ER to the Golgi, and GGA3,Citation57 the component of clathrin-coated vesicles that transport cargo between endosomes and the TGN, strongly support that the cargo GPCRs may physically bind to components of the transport machinery to control their export trafficking. Differential interaction of distinct GPCRs with Rab GTPases may form a unique transport machinery for each individual receptor to export to the cell surface.
Conclusions and perspectives
It is apparent that Rab GTPases control the proper GPCR expression at the cell surface and different Rab GTPases may regulate different steps in the biosynthesis and processing of GPCRs. It is also intriguing to note that some Rab GTPases may directly associate with the receptors they regulate, suggesting their specific function in GPCR trafficking. However, the exact molecular mechanisms underlying the function of Rab GTPases in GPCR export remain largely unknown. The regulatory role of Rab GTPases in the cell surface export trafficking of the GPCR superfamily has just begun to be revealed and numerous questions remain to be addressed. As only a few Rab GTPases have been investigated in GPCR transport, future studies should search for more Rabs and define the specificity between various Rab GTPases and distinct GPCR members. In addition, the function of RabGEFs and RabGAPs in the trafficking of GPCRs has not been studied. Although Rab GTPases influence the cell surface expression of GPCRs at the steady-state, their function in receptor export kinetics as well as receptor sorting to different transport routes remains unknown.
Importantly, the dysfunction of Rab GTPases are implicated in the development of human diseases but their exact roles under these pathological conditions are elusive.Citation58 One possibility is that Rab dysfunction may actually cause the defective trafficking of some GPCRs, leading to the disruption of GPCR-mediated signaling pathways. It should be pointed out that in addition to Rab GTPases, receptor activity modifying proteins, ER chaperones, escort proteins and gatekeepers may facilitate GPCR maturation, promote receptor delivery to the plasma membrane and stabilize receptor conformation.Citation59-62 To further explore the function of Rab GTPases as well as many other regulatory proteins in the export trafficking of nascent GPCRs will not only greatly enhance our understanding of GPCR targeting to their functional destinations but also reveal novel therapeutic targets for human diseases involving abnormal trafficking and signaling of GPCRs.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Natural Science Foundation of China Grant 81370168 (to G. Wang) and National Institutes of Health Grant R01GM118915 (to G. Wu).
References
- Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 2002; 3:639-50; PMID:12209124; http://dx.doi.org/10.1038/n/rm908
- Kobilka BK. Structural insights into adrenergic receptor function and pharmacology. Trends Pharmacol Sci 2011; 32:213-8; PMID:21414670; http://dx.doi.org/10.1016/j/.tips.2011.02.005
- Puthenveedu MA, von Zastrow M. Cargo regulates clathrin-coated pit dynamics. Cell 2006; 127:113-24; PMID:17018281; http://dx.doi.org/10.1016/j/.cell.2006.08.035
- Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol 2008; 48:601-629; PMID:17995450; http://dx.doi.org/10.1146/a/nnurev.pharmtox.48.113006.094646
- Smith JS, Rajagopal S. The beta-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors. J Biol Chem 2016; 291:8969-77; PMID:26984408; http://dx.doi.org/10.1074/j/bc.R115.713313
- Frasa MA, Koessmeier KT, Ahmadian MR, Braga VM. Illuminating the functional and structural repertoire of human TBC/R/ABGAPs. Nat Rev Mol Cell Biol 2012; 13:67-73; PMID:22251903; http://dx.doi.org/10.1038/n/rm3364
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 2007; 129:865-77; PMID:17540168; http://dx.doi.org/10.1016/j/.cell.2007.05.018
- Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev 2013; 93:269-309; PMID:23303910; http://dx.doi.org/10.1152/p/hysrev.00003.2012
- Fukuda M. TBC proteins: GAPs for mammalian small GTPase Rab? Biosci Rep 2011; 31:159-68; PMID:21250943; http://dx.doi.org/10.1042/B/SR20100112
- Novick P. Regulation of membrane traffic by Rab GEF and GAP cascades. Small GTPases 2016; 7:252-6; PMID:27427966; http://dx.doi.org/10.1080/21541248.2016.1213781
- DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 2005; 15:356-63; PMID:15921909; http://dx.doi.org/10.1016/j/.tcb.2005.05.001
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 2001; 81:153-208; PMID:11152757
- Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 2006; 103:11821-7; PMID:16882731; http://dx.doi.org/10.1073/p/nas.0601617103
- Martinez O, Goud B. Rab proteins. Biochim Biophys Acta 1998; 1404:101-12; PMID:9714762; http://dx.doi.org/10.1016/S/0167-4889(98)00050-0
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/10.1038/n/rm2728
- Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 2013; 25:414-9; PMID:23639309; http://dx.doi.org/10.1016/j.ceb.2013.04.002
- Seachrist JL, Anborgh PH, Ferguson SS. beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J Biol Chem 2000; 275:27221-8; PMID:10854436
- Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. Recycling and resensitization of the neurokinin 1 receptor. Influence of agonist concentration and Rab GTPases. J Biol Chem 2004; 279:30670-9; PMID:15128739; http://dx.doi.org/10.1074/j/bc.M402479200
- Roosterman D, Schmidlin F, Bunnett NW. Rab5a and rab11a mediate agonist-induced trafficking of protease-activated receptor 2. Am J Physiol Cell Physiol 2003; 284:C1319-29; PMID:12540381; http://dx.doi.org/10.1152/a/jpcell.00540.2002
- Fan GH, Lapierre LA, Goldenring JR, Richmond A. Differential regulation of CXCR2 trafficking by Rab GTPases. Blood 2003; 101:2115-24; PMID:12411301; http://dx.doi.org/10.1182/b/lood-2002-07-1965
- Seachrist JL, Laporte SA, Dale LB, Babwah AV, Caron MG, Anborgh PH, Ferguson SS. Rab5 association with the angiotensin II type 1A receptor promotes Rab5 GTP binding and vesicular fusion. J Biol Chem 2002; 277:679-85; PMID:11682489; http://dx.doi.org/10.1074/j/bc.M109022200
- Dale LB, Seachrist JL, Babwah AV, Ferguson SS. Regulation of angiotensin II type 1A receptor intracellular retention, degradation, and recycling by Rab5, Rab7, and Rab11 GTPases. J Biol Chem 2004; 279:13110-8; PMID:14711821; http://dx.doi.org/10.1074/j/bc.M313333200
- Iwata K, Ito K, Fukuzaki A, Inaki K, Haga T. Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. Eur J Biochem 1999; 263:596-602; PMID:10406971; http://dx.doi.org/10.1046/j/.1432-1327.1999.00549.x
- Murph MM, Scaccia LA, Volpicelli LA, Radhakrishna H. Agonist-induced endocytosis of lysophosphatidic acid-coupled LPA1/E/DG-2 receptors via a dynamin2- and Rab5-dependent pathway. J Cell Sci 2003; 116:1969-80; PMID:12668728; http://dx.doi.org/10.1242/j/cs.00397
- Grimsey NL, Goodfellow CE, Dragunow M, Glass M. Cannabinoid receptor 2 undergoes Rab5-mediated internalization and recycles via a Rab11-dependent pathway. Biochim Biophys Acta 2011; 1813:1554-60; PMID:21640764; http://dx.doi.org/10.1016/j/.bbamcr.2011.05.010
- Yang J, Sun H, Zhang J, Hu M, Wang J, Wu G, Wang G. Regulation of beta-adrenergic receptor trafficking and lung microvascular endothelial cell permeability by Rab5 GTPase. Int J Biol Sci 2015; 11:868-78; PMID:26157342; http://dx.doi.org/10.7150/i/jbs.12045
- Castillo-Badillo JA, Sánchez-Reyes OB, Alfonzo-Méndez MA, Romero-Ávila MT, Reyes-Cruz G, García-Sáinz JA. alpha1B-adrenergic receptors differentially associate with Rab proteins during homologous and heterologous desensitization. PLoS One 2015; 10:e0121165; PMID:25799564; http://dx.doi.org/10.1371/j/ournal.pone.0121165
- Snyder JC, Rochelle LK, Lyerly HK, Caron MG, Barak LS. Constitutive internalization of the leucine-rich G protein-coupled receptor-5 (LGR5) to the trans-Golgi network. J Biol Chem 2013; 288:10286-97; PMID:23439653; http://dx.doi.org/10.1074/j/bc.M112.447540
- Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem 2004; 279:36013-21; PMID:15210689; http://dx.doi.org/10.1074/j/bc.M403990200
- Wang G, Wu G. Small GTPase regulation of GPCR anterograde trafficking. Trends Pharmacol Sci 2012; 33:28-34; PMID:22015208; http://dx.doi.org/10.1016/j/.tips.2011.09.002
- Wu G, Zhao G, He Y. Distinct pathways for the trafficking of angiotensin II and adrenergic receptors from the endoplasmic reticulum to the cell surface: Rab1-independent transport of a G protein-coupled receptor. J Biol Chem 2003; 278:47062-9; PMID:12970354; http://dx.doi.org/10.1074/j/bc.M305707200
- Filipeanu CM, Zhou F, Fugetta EK, Wu G. Differential regulation of the cell-surface targeting and function of beta- and alpha1-adrenergic receptors by Rab1 GTPase in cardiac myocytes. Mol Pharmacol 2006; 69:1571-8; PMID:16461589; http://dx.doi.org/10.1124/m/ol.105.019984
- Li Y, Wang G, Lin K, Yin H, Zhou C, Liu T, Wu G, Qian G. Rab1 GTPase promotes expression of beta-adrenergic receptors in rat pulmonary microvascular endothelial cells. Int J Biochem Cell Biol 2010; 42:1201-9; PMID:20417717; http://dx.doi.org/10.1016/j/.biocel.2010.04.009
- Dupre DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, Hébert TE. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem 2006; 281:34561-73; PMID:16959776; http://dx.doi.org/10.1074/j/bc.M605012200
- Filipeanu CM, Zhou F, Claycomb WC, Wu G. Regulation of the cell surface expression and function of angiotensin II type 1 receptor by Rab1-mediated endoplasmic reticulum-to-Golgi transport in cardiac myocytes. J Biol Chem 2004; 279:41077-84; PMID:15252015; http://dx.doi.org/10.1074/j/bc.M405988200
- Yin H, Li Q, Qian G, Wang Y, Li Y, Wu G, Wang G. Rab1 GTPase regulates phenotypic modulation of pulmonary artery smooth muscle cells by mediating the transport of angiotensin II type 1 receptor under hypoxia. Int J Biochem Cell Biol 2011; 43:401-8; PMID:21095238; http://dx.doi.org/10.1016/j/.biocel.2010.11.010
- Zhang X, Wang G, Dupré DJ, Feng Y, Robitaille M, Lazartigues E, Feng YH, Hébert TE, Wu G. Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor. J Pharmacol Exp Ther 2009; 330:109-17; PMID:19357319; http://dx.doi.org/10.1124/j/pet.109.153460
- Zhuang X, Adipietro KA, Datta S, Northup JK, Ray K. Rab1 small GTP-binding protein regulates cell surface trafficking of the human calcium-sensing receptor. Endocrinology 2010; 151:5114-23; PMID:20861236; http://dx.doi.org/10.1210/e/n.2010-0422
- Hammad MM, Kuang YQ, Morse A, Dupre DJ. Rab1 interacts directly with the beta2-adrenergic receptor to regulate receptor anterograde trafficking. Biol Chem 2012; 393:541-6; PMID:22628317; http://dx.doi.org/10.1515/h/sz-2011-0284
- Dong C, Wu G. Regulation of anterograde transport of adrenergic and angiotensin II receptors by Rab2 and Rab6 GTPases. Cell Signal 2007; 19:2388-99; PMID:17716866; http://dx.doi.org/10.1016/j/.cellsig.2007.07.017
- Sato T, Mushiake S, Kato Y, Sato K, Sato M, Takeda N, Ozono K, Miki K, Kubo Y, Tsuji A, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 2007; 448:366-9; PMID:17597763; http://dx.doi.org/10.1038/n/ature05929
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007; 129:1201-13; PMID:17574030; http://dx.doi.org/10.1016/j/.cell.2007.03.053
- Li C, Fan Y, Lan TH, Lambert NA, Wu G. Rab26 modulates the cell surface transport of alpha2-adrenergic receptors from the Golgi. J Biol Chem 2012; 287:42784-94; PMID:23105096; http://dx.doi.org/10.1074/j/bc.M112.410936
- Yoshie S, Imai A, Nashida T, Shimomura H. Expression, characterization, and localization of Rab26, a low molecular weight GTP-binding protein, in the rat parotid gland. Histochem Cell Biol 2000; 113:259-63; PMID:10857477; http://dx.doi.org/10.1007/s/004180000130
- Nashida T, Imai A, Shimomura H. Relation of Rab26 to the amylase release from rat parotid acinar cells. Arch Oral Biol 2006; 51:89-95; PMID:16076461; http://dx.doi.org/10.1016/j/.archoralbio.2005.06.005
- Tian X, Jin RU, Bredemeyer AJ, Oates EJ, Bł azewska KM, McKenna CE, Mills JC. RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation. Mol Cell Biol 2010; 30:1269-84; PMID:20038531; http://dx.doi.org/10.1128/M/CB.01328-09
- Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS. rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci 1995; 108( Pt 1):215-24; PMID:7738098
- Moritz OL, Tam BM, Hurd LL, Peränen J, Deretic D, Papermaster DS. Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell 2001; 12:2341-51; PMID:11514620; http://dx.doi.org/10.1091/m/bc.12.8.2341
- Dong C, Yang L, Zhang X, Gu H, Lam ML, Claycomb WC, Xia H, Wu G. Rab8 interacts with distinct motifs in {alpha}2B- and {beta}2-adrenergic receptors and differentially modulates their transport. J Biol Chem 2010; 285:20369-80; PMID:20424170; http://dx.doi.org/10.1074/j/bc.M109.081521
- Hamelin E, Theriault C, Laroche G, Parent JL. The intracellular trafficking of the G protein-coupled receptor TPbeta depends on a direct interaction with Rab11. J Biol Chem 2005; 280:36195-205; PMID:16126723; http://dx.doi.org/10.1074/j/bc.M503438200
- Parent A, Hamelin E, Germain P, Parent JL. Rab11 regulates the recycling of the beta(2)-adrenergic receptor through a direct interaction. Biochem J 2009; 418:163-72; PMID:18983266; http://dx.doi.org/10.1042/B/J20080867
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J Biol Chem 2004; 279:30741-50; PMID:15123661; http://dx.doi.org/10.1074/j/bc.M313881200
- Schulein R, Hermosilla R, Oksche A, Dehe M, Wiesner B, Krause G, Rosenthal W. A dileucine sequence and an upstream glutamate residue in the intracellular carboxyl terminus of the vasopressin V2 receptor are essential for cell surface transport in COS.M6 cells. Mol Pharmacol 1998; 54:525-35; PMID:9730911
- Carrel D, Hamon M, Darmon M. Role of the C-terminal di-leucine motif of 5-HT1A and 5-HT1B serotonin receptors in plasma membrane targeting. J Cell Sci 2006; 119:4276-84; PMID:17003106; http://dx.doi.org/10.1242/j/cs.03189
- Sawyer GW, Ehlert FJ, Shults CA. A conserved motif in the membrane proximal C-terminal tail of human muscarinic m1 acetylcholine receptors affects plasma membrane expression. J Pharmacol Exp Ther 2010; 332:76-86; PMID:19841475; http://dx.doi.org/10.1124/j/pet.109.160986
- Dong C, Nichols CD, Guo J, Huang W, Lambert NA, Wu G. A triple Arg motif mediates alpha2B-adrenergic receptor interaction with Sec 24C/D\ and export. Traffic 2012; 13:857-68; PMID:22404651; http://dx.doi.org/10.1111/j/.1600-0854.2012.01351.x
- Zhang M, Davis JE, Li C, Gao J, Huang W, Lambert NA, Terry AV Jr, Wu G. GGA3 Interacts with a G Protein-Coupled Receptor and Modulates Its Cell Surface Export. Mol Cell Biol 2016; 36:1152-63; PMID:26811329; http://dx.doi.org/10.1128/M/CB.00009-16
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/10.1152/p/hysrev.00059.2009
- Achour L, Labbe-Jullie C, Scott MG, Marullo S. An escort for GPCRs: implications for regulation of receptor density at the cell surface. Trends Pharmacol Sci 2008; 29:528-35; PMID:18760490; http://dx.doi.org/10.1016/j/.tips.2008.07.009
- Doly S, Shirvani H, Gäta G, Meye FJ, Emerit MB, Enslen H, Achour L, Pardo-Lopez L, Yang SK, Armand V, et al. GABAB receptor cell-surface export is controlled by an endoplasmic reticulum gatekeeper. Mol Psychiatry 2016; 21:480-90; PMID:26033241; http://dx.doi.org/10.1038/m/p.2015.72
- Hay DL, Pioszak AA. Receptor Activity-Modifying Proteins (RAMPs): New Insights and Roles. Annu Rev Pharmacol Toxicol 2016; 56:469-87; PMID:26514202; http://dx.doi.org/10.1146/a/nnurev-pharmtox-010715-103120
- Dong C, Filipeanu CM, Duvernay MT, Wu G. Regulation of G protein-coupled receptor export trafficking. Biochim Biophys Acta 2007; 1768:853-70; PMID:17074298; http://dx.doi.org/10.1016/j/.bbamem.2006.09.008
