ABSTRACT
Rab GTPases, the highly conserved members of Ras GTPase superfamily are central players in the vesicular trafficking. They are critically involved in intracellular trafficking pathway, beginning from formation of vesicles on donor membranes, defining trafficking specificity to facilitating vesicle docking on target membranes. Given the dynamic roles of Rabs during different stages of vesicular trafficking, mechanisms for their spatial and temporal regulation are crucial for normal cellular function. Regulation of Rab GTPase activity, localization and function has always been focused in and around the association of GDP dissociation inhibitor (GDI), Guanine nucleotide Exchange Factor (GEFs) and GTPase accelerating protein (GAP) to Rabs. However, several recent studies have highlighted the importance of different post-translational modifications in regulation of Rab activation and function. This review provides a summary of various post translational modifications (PTMs) and their significance to regulate localization and function of different Rabs.
Introduction
Intracellular vesicular transport mediates rapid and bidirectional membrane traffic between the organelles of endocytic and exocytic pathways. Co-ordinated vesicle budding, vesicle delivery, vesicle tethering, and fusion of the vesicle membrane with that of the target compartment is critical for normal functioning of a cell.Citation1 GTPases from several branches of the Ras superfamily play important role in co-ordinating these processes. Central to this is the family of Rab GTPases, which ensure the delivery of cargo to their correct destinations.
Rabs exhibit high affinity for both GTP and GDP. Bound nucleotide marks the differential localization of Rabs to either membrane or cytosol. In the active GTP-bound form, Rabs are associated with membranes, where they interact with effector proteins that promote diverse steps in vesicular trafficking. When bound to GDP, Rabs are defined as inactive and are predominantly distributed in the cytosol bound to GDI. Binding of either of the nucleotide leads to conformational change in the switch regions of Rabs.Citation2-4 In general, GDP bound inactive Rab present in the cytosol would be targeted to specific membrane in a GDI dependent manner. At the membrane, GEF converts it to a GTP-bound active state. Further, Rab effector proteins bind GTP bound active Rabs, and function in regulating the trafficking. Subsequently, GAP assists hydrolysis of the bound GTP to GDP and thereby converts the Rab back to its inactive state. Further, GDI extracts GDP bound inactive Rab from the membrane and chaperones it in the cytosol till the next Rab cycle begins.Citation5-8
Rabs function in the co-ordination of vesicular trafficking and hence their tight spatio-temporal regulation is critical. Apart from regulation of Rabs by specific association of GDI, GAPs and GEFs during different stages of trafficking process, other mechanisms for their functional regulation are very limited. In this review, we discuss specific examples of Rab GTPases and their functional regulation by various post translational modifications. Here, although limited to specific Rabs, these examples offer some paradigms that may help us to better understand the functional regulation of GTPases by PTMs.
Prenylation
Protein prenylation is well characterized lipid modification, which involves the covalent addition of either farnesyl (15 carbon) or geranylgeranyl (20 carbon) pyrophosphate to proteins via thioether linkages catalyzed by protein prenyl transferases. There are 3 different protein prenyl transferases: protein farnesyltransferase (FTase), protein geranylgeranyl transferase-I (GGTase-I), and Rab geranyl geranyl transferase (RabGGTase or GGTase-II).Citation9 FTase and GGTase-I transfer prenyl groups to proteins containing a C-terminal CAAX motif (where, C- cysteine, A- an aliphatic amino acid, and X- any amino acids). In contrast to the other 2 protein prenyltransferases, RabGGTase does not recognize a 4-amino acid C-terminal sequence, known as a CAAX box, but needs an adaptor protein termed the Rab escort protein (REP) to exert its function. REP recruits newly synthesized Rab GTPases and then presents them to the RabGGTase. These proteins form a tight catalytic ternary complex in which 2 geranylgeranyl groups are transferred onto the C terminus of Rab GTPase.Citation9,10 After geranylgeranylation, REP remains bound to Rab and escorts it to the respective target donor membrane (). Pylypenko et al and Rak et al have determined structures of the RabGGTase–REP-1 and Rab7GG–REP-1 complexes which provide in-sights into the mechanism of REP-mediated Rab prenylation.Citation11,12 These structures have identified 2 binding interfaces of REP and Rab protein: the first one between the Rab-binding platform (RBP) of REP and effector loops of the GTPase and the second one is between the C-terminal-binding region (CBR) of REP and the CBR interacting motif (CIM), which consists of 2 hydrophobic residues near the C terminus of Rab GTPase. Further, elegant studies by Guo et al. and Wu et al. have unravelled the details of specific role of REP in controlling RabGGTase function.Citation13,14 The assembly of catalytic ternary Rab-REP-RabGGTase complex is triggered by the recognition of GTPase domain of Rab by RBP of REP. This low to intermediate affinity complex is further tightened by interaction of CIM with CBR. Further, this complex forms a high affinity ternary complex with RabGGTase via the interaction between α-subunit of RabGGTase and domain II of REP. The weak and largely nonspecific interactions of the C terminus with the active site of RabGGTase further enhance the affinity of the complex. The substrate specificity of RabGGTase is achieved via engagement of the REP molecule.Citation14 REP, on one hand, selectively binds the GTPase core of the Rab proteins and, on the other hand, concentrates its C terminus in the vicinity of the active site of RabGGTase through the CIM-CBR anchor.
Figure 1. Prenylation of Rab. Newly synthesized Rab associates with REP which forms a binary complex. Further, RGGTase binds to REP and forms the Rab-REP-RabGGTase ternary complex. RGGTase catalyze the transfer of prenyl groups to C-terminal cysteine residues. REP remain associated with prenylated Rab and targets it to the membrane.
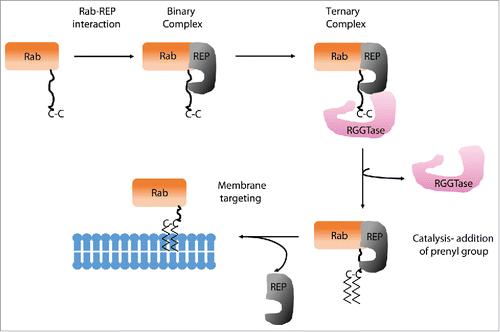
Most of the proteins of Rab superfamily contain 2 cysteine residues, such as CC or CXC at the C-terminus, and undergo 2 geranylgeranylation reactions. The double geranylgeranylation of Rabs makes them rather more hydrophobic than other prenylated proteins and hence, they need to be chaperoned by REP. The presence of 2 GG moieties is essential for faithful targeting of dicysteine Rabs to their appropriate location. Recently, it has been shown that the replacement of dicysteine motif containing Rab proteins such as Rab5a and Rab27a, with a monocysteine motif, such as CSLG or CVLL, led to mistargeting of the mutants to the endoplasmic reticulum/Golgi region rather than their originally designated cellular compartment. Furthermore, Rab27a-CVLL failed to rescue the function of wild-type Rab27a in Rab27a −/− cells. Together, these findings hint toward the importance of prenylation status for the correct targeting and function of Rab proteins.
Postprenylation processing is another factor that may assist the membrane recruitment of Rabs. Carboxyl methylation aids in the enhanced hydrophobicity of farnesylated and geranylgeranylated proteins, where the effect is more prominent in proteins harbouring farnesylation. CAAX-containing Ras and Rho GTPases are processed at endoplasmic reticulum (ER) where CAAX proteases cleave the AAX tripeptide. The newly exposed prenylated cysteine is then modified by carboxyl methylation on the α-carboxyl group by isoprenylcysteine carboxyl methyltransferase. Several studies have supported the role played by methylation in the membrane association of many CAAX proteins, in particular Ras proteins. Though most of the Rabs are modified with geranylgeranyl moiety a subset of Rabs such as Rab8 and Rab13 possess a CAAX motif, and are modified by a single geranylgeranyl moiety. These mono-prenylated Rabs with a CAAX motif have the potential to be processed by CAAX proteolysis and carboxyl methylation. The effect of methylation on singly prenylated Rab8 has been studied, where it has been shown that the loss of isoprenylcysteine carboxyl methyltransferase significantly affects cycle of membrane/cytosol Rab8 partitioning.Citation15 Taken together, it can be concluded that prenylation and post prenylation methylation of Rabs is critical for specific delivery of Rabs to their target intracellular membranes.
Pathogen induced AMPylation and phosphocholination
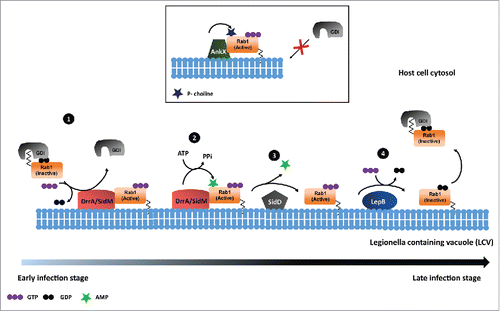
AMPylation (also known as adenylylation) is the covalent attachment of adenosine monophosphate (AMP) to tyrosine or threonine side chains within target proteins. Bacterial pathogen Legionella pneumophila use AMPylation to manipulate the host cell signaling processes upon infection. The intravacuolar pathogen Legionella pneumophila interferes with normal intracellular vesicular transport by several mechanisms, one of which is modulating vesicle transport from endoplasmic reticulum to Golgi. Legionella pneumophila utilize the type IV secretion system called Dot/Icm to translocate proteins called effectors into the host-cell cytosol. Legionella effectors reorient the host cell vesicle trafficking pathway by recruiting GTPase Rab1 to maintain the vacuolar compartment called Legionella containing vacuole (LCV).Citation16,17 One such effector protein is DrrA (also known as SidM), which acts as a GEF for Rab1.Citation18 Earlier DrrA has been shown to possess GDF activity to Rab1 which displace Rab1 from GDI. The GEF activity of DrrA for Rab1 plays an important role during the pathogenesis of Legionella pneumophila within the host cell to maintain Legionella containing vacuole (LCV).Citation19 Müller et al in mid-2010, assigned a novel function to DrrA, which regulates the Rab1 function, in addition to its GEF activity. In a series of experiments the authors showed that DrrA could AMPylate the Tyr 77 residue in the class II switch region of Rab1B. Further, it was shown that AMPylated Rab1 stays in GTP bound state as the access of GAPs (LepB in this case) was restricted. However, the failure of LepB to stimulate GTP hydrolysis on AMPylated Rab1b, hints toward an unknown de-AMPylation mediated mechanism which might regulate the Rab1 GTP hydrolysis and activation-inactivation cycle. Nevertheless, as Rab1 localization to LCVs is transient it can be speculated that the de-AMPylation of Rab1 could lead to its removal from LCVs.Citation20 Further, Neunuebel et al has shown SidD mediated de-AMPylation of Rab1.Citation21 They confirmed that in presence of SidD, the GAP for Rab1 could efficiently associate with Rab1 and deactivate it. Also, Tan et al have provided the experimental evidence for SidD mediated de-AMPylation of Rab1.Citation22 Their studies have shown that de-AMPylation activity of SidD is required for release of Rab1 from Legionella phagosomes. Together, it can be concluded that series of post translational modifications performed by L. pneumophila effectors regulate the activation and deactivation cycle of Rab1. While studying the biologic significance of DrrA mediated AMPylation of Rab1 during Legionella infection, Mukherjee et al. reported a novel modification of Rab1. AnkX, a Fic domain containing protein which is known to possess AMPylation activity earlier, was found to function as phosphocholine transferase that carries out phosphocholination of Rab1 on Ser 79 residue.Citation23 Further, extending their studies to other members of Rab superfamily, they observed DrrA mediated AMPylation as well as AnkX mediated phosphocholination of Rab35. They reported a pronounced defect in the association of GEF (Connecdenn) with phosphocholinated Rab35, which indeed mimics the phenotype observed with AnkX overexpression. Interestingly, Goody et al and Tan et al independently identified, Lpg0696 (Lem3), a Legionella effector, as dephosphorylcholinase.Citation24,25 Though these studies emphasize on the reversible phospho-dephosphocholination of Rab1 during Legionella infection the biologic significance of such event is still elusive. These studies possibly suggest that AnkX/Lem3 system could function at specific cellular compartment providing spatial regulation of Rab1 activity during Legionella infection.
These reports have unravelled a novel modification dependent mechanism which regulates the activation deactivation cycle of Rabs. Further, taking cues from these studies Oesterlin et al. investigated the influence of posttranslational modifications of Rab proteins on the ability to displace GDI from prenylated Rabs.Citation26 They reported that Legionella effectors DrrA and AnkX mediated adenylylation and phosphocholination of Rab1b and Rab35 at Tyr77 and Ser76, abolish GDI binding. Further mechanistic analysis has provided several evidence which show that these modifications indeed inhibit the reformation of Rab: GDI complex.Citation26,27 In conclusion, AnkX and DrrA/SidM mediated phosphocholination and AMPylation of Rabs provide insights for better understanding the membrane extraction/delivery and cytosolic distribution of Rabs ().
Figure 2. Rab1 regulation by P. Legionella. 1) DrrA/SidM acts as GDF for Rab1 to displace GDI as well as acts as GEF to exchange GDP to GTP. 2) DrrA/SidM mediated AMPylation of GTP bound Rab1. 3) SidD mediated de-AMPylation of Rab1. 4) LepB functions as GAP, which leads to GTP hydrolysis and inactivation of Rab1. 5) AnkX mediated phosphocholination of Rab1 that renders it in an activated state.
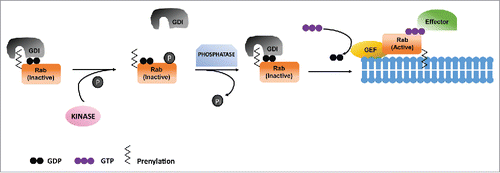
Phosphorylation
In 1991, for the first time Baily et al. provided biochemical evidence confirming phosphorylation mediated functional regulation of Rab GTPases.Citation28 In a series of experiments they showed that M phase inducing protein kinase p34cdc2 phosphorylates Rab1Ap and Rab4p in vitro as well as in vivo. Further, functional experiments revealed that phosphorylated Rab4p was found to be predominantly cytosolic, on the contrary Rab1Ap was membranous.Citation28 Next, Van der Sluijs et al. investigated the molecular mechanism controlling membrane association of Rab4 and its role in mitotic regulation.Citation29 They mapped Ser196, which occurs within a consensus site for p34cdc2 kinase phosphorylation, to be the site of Rab4 phosphorylation. Further, they showed that Ser196 phosphorylation neither affected C-terminal isoprenylation nor carboxymethylation of Rab4 but constitutively phosphorylated Rab4 abolishes its cytosolic abundance. Finally, upon mitotic exit Rab4 was dephosphorylated and reassociated with membranes.Citation29
At the same time, Fitzgerald et al. reported protein kinase C (PKC) mediated phosphoregulation of human Rab6 isoform (Rab6C).Citation30 Here, they demonstrated that in platelets, Rab6 containing 2 PKC consensus phosphorylation sites, gets phosphorylated upon stimulation. Further, they showed that physiologic stimulation of platelets caused a PKC-dependent translocation of Rab6 from platelet particulate fractions to cytosolic fraction. Though they didn't find significant change in Rab6 GTP/GDP binding as well as GTPase activity the translocation to cytosol was evident.Citation30
Lately, Lai et al, have reported Rab8A, 8B and Rab13 phosphorylation at the highly conserved residue of serine 111 in response to PINK1 activation.Citation31 Further, they have mechanistically shown that Rab8 phosphorylation limits its Rabin8 (GEF) mediated activation.Citation31 Recently, Steger et al., used a phosphoproteomics approach to identify LRRK2 substrates and they found a subset of Rab GTPases among many phosphosubstrates.Citation32 The in vivo and in vitro experiments confirmed Rab3a (T86), Rab8a (T72) and Rab10 (T73) as bonafide substrates of LRRK2. They demonstrated that LRRK2 directly phosphorylates these evolutionary conserved residue in the switch II domain of respective Rabs. Further, the phosphorylated Rabs show reduced affinity to their regulatory proteins including Rab GDIs, which results in altered membrane to cytoplasmic pool of Rabs in cell. Further, they observed that pathogenic LRRK2 variants increase phosphorylation of Rabs, which certainly have profound effect on membrane to cytoplasmic ratio of Rabs. As LRRK2 has been associated with Parkinson disease (PD), and the pathogenic LRRK2 variants increase phosphorylation of Rabs, one could speculate a novel regulatory mechanism of Rabs that connects them to PD.Citation32
Other recent study by Levin et al., unravelled TGF-β activated kinase 1 (TAK1) mediated phosphorylation of Rab1.Citation33 They have demonstrated that TAK1 phosphorylates Rab1 at T75 in the dynamic switch II region. Phosphorylated Rab1 fails to interact with GDI, whereas it could associate with GEF or GAP enzymes. Further, they observed that phosphorylated Rab1 to be exclusively localized to membrane suggesting phosphorylation may stimulate Rab1 membrane association. Considering the Rab1 modifications by Legionella effectors in switch II region, this provides an alternate mechanism for Rab1 regulation during infection.
Additionally, in our recent study we identified PTEN mediated dephosphorylation of Rab7 on 2 of the conserved residues, Ser72 and Tyr183.Citation34 In a series of experiments we have demonstrated that phosphorylated Rab7 fails to localize to endosomal membranes. Further, mechanistically we have shown that phosphorylation of Rab7 lead to disruption of its association with GDI as well as GEF (Mon1-Ccz1). Phosphorylation renders Rab7 to have lesser affinity to GTP which results in decreased association of Rab7 with its effector RILP (Rab Interacting Lysosomal Protein). Hence, phosphorylation leads to inactivation of Rab7 which significantly delays EGFR degradation in lysosomes.Citation34 Further, supporting our observations Satpathy et al. also demonstrated that B-cell receptor (BCR) induced phosphorylation of Rab7a at S72 prevents its association with effector proteins and with endo-lysosomal compartments.Citation35
In agreement with the above arguments it can be concluded that phosphoregulation is an important mechanism for functional regulation of Rabs. The Ser/Thr modification of conserved switch II region regulates Rab association with GDI and further dictates their activation through interaction with GEFs. So far, number of kinases have been shown to phosphorylate several Rabs which indeed function to maintain their cytoplasm to membrane ratio. It would be interesting and important to decipher the counteracting phosphatases which dephosphorylate Rabs and maintain their cytoplasm to membrane ratios, in turn controlling the endocytic pathway and cellular homeostasis ().
Other modifications
Apart from well-studied modifications such as prenylation and phosphorylation, isolated examples of other Rab modifications are also reported. For instance, ubiquitination of Rab11a by β2AR/HACE1 complex in regulating Rab11a activity and β2AR recycling was reported. β2AR (a prototypical G-protein coupled receptor) associates with HACE1, a HECT domain containing ubiquitin ligase, and promotes ubiquitination of Rab11a on Lys145. It is known that β2AR associated Rab11a is inactive, but HACE1 mediated ubiquitination of Rab11a leads to dissociation of Rab11a from β2AR. The ubiquitinated Rab11a was active as it promoted the recycling of β2AR, albeit the mechanistic details of its activation are unaddressed.Citation36 Together, these studies unravel a novel mechanism of ubiquitination mediated activation of a Rab GTPase. In addition Qiu et al recently have demonstrated SidE (an effector secreted by L. pneumophila) mediated ubiquitination of multiple Rab small GTPases associated with the endoplasmic reticulum.Citation37
While understanding the role of serotonin (5-hydroxytryptamine; 5-HT) in haemostasis, Walther et al reported that elevated cytoplasmic 5-HT concentrations leads to small GTPase activation in rapid exocytotic processes from platelets. They demonstrated transglutaminase (TGase) mediated covalent attachment of 5-HT to Rab4 (i.e. serotonylation) renders Rab4 to be activated, which results in α-granule exocytosis from platelets.Citation38 In addition to these findings Paulmann et al. recently identified hormone serotonin as a new regulator of insulin secretion. They have shown that glucose stimulation increases intracellular Ca2+, which activates Ca2+ dependent transglutaminases. Further, the activated TGase then utilizes intracellular 5-HT to serotonylate Rab3a and Rab27a, which renders them constitutively active and promotes insulin secretion.Citation39
Conclusions and perspectives
Rab GTPases principally function as regulatory switches that spatiotemporally coordinate membrane trafficking. Appropriate membrane targeting of Rabs to their destination is very critical as far as the trafficking of several cargos is concerned. The mechanistic details regarding the functional regulation of these Rabs are less explored where the only known regulation comes from their association with GEFs, GAPs or GDI. Lately, several reports have demonstrated role of post translational modifications in regulating membrane targeting of Rabs. The emergence of this particular field have enabled us to better understand the functional regulation of Rabs beyond the traditional ones.
Considering the evidence provided by several studies, posttranslational modifications of Rab GTPases have emerged as tentative mechanisms to explain how Rab GTPases can be appropriately targeted to membranes. Several studies have highlighted the importance of prenylation for the membrane targeting of Rabs. Critically, the mono or di-geranylation of Rabs marks the specificity for their localization to the membrane of specific cellular organelle. Each Rab functions at specific cellular organelle thus any mislocalization of Rabs would certainly affect the endocytic as well as exocytic pathways. Modifications such as phosphorylation have constantly been shown to affect the membrane/cytosol distribution of a few Rabs like Rab7a, Rab8 etc. Further, mechanistic details from these studies have unravelled that the phosphorylation regulated association of several Rab regulators like GEFs and/or GAPs. Several experimental evidence have shown that phospho-dephosphorylation of Rab has major effect on Rab activity cycle. For instance the phosphorylation might trap the GDP bound Rab either in the cytosol or at the membrane. The accumulation of excessive inactive GDP bound Rab would affect the endocytic trafficking of several growth factor receptors such as, EGFR, which in turn may result in altered downstream proliferative or survival signaling pathways. Together, this has complicated the view that membrane targeting of Rabs is orchestrated not only by GEFs and GAPs but also by several kinases and phosphatases. Further, this has provided us new avenues to explore the enzymatic machineries such as kinases and phosphatases involved in this process. Additionally, the regulation of Rab1 by effectors of L. pneumophila makes it more curious to explore the human counterparts of the bacterial effectors. Importantly, most of the modifications such as phosphorylation, AMPylation or phosphocholination are targeted to the switch II region of Rabs, a structurally important region for Rab function. Exploring modifications in other structural regions including C-terminal hypervariable region can further give better insights to understand the differential localization of different Rabs. Also, it would be interesting to understand the interdependencies and cross talks between various post translational modifications in regulating Rab activity and functions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was fully supported by Wellcome Trust/DBT India Alliance grant (Intermediate fellowship to S.M; 500230/Z/11/Z). S.M is a recipient of Department of Biotechnology's IYBA award (BT/01/IYBA/2009). SRS acknowledges the support of Senior Research Fellowship from University Grants Commission (UGC), India.
References
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; http://dx.doi.org/10.1038/nrm2728
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 2011; 91:119-49; PMID:21248164; http://dx.doi.org/10.1152/physrev.00059.2009
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A. Rab GTPases at a glance. J Cell Sci 2007; 120:3905-10; PMID:17989088; http://dx.doi.org/10.1242/jcs.015909
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107-17; PMID:11252952; http://dx.doi.org/10.1038/35052055
- Delprato A, Merithew E, Lambright DG. Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5. Cell 2004; 118:607-17; PMID:15339665; http://dx.doi.org/10.1016/j.cell.2004.08.009
- Matsui Y, Kikuchi A, Araki S, Hata Y, Kondo J, Teranishi Y, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein. Mol Cell Biol 1990; 10:4116-22; PMID:2115118; http://dx.doi.org/10.1128/MCB.10.8.4116
- Pan X, Eathiraj S, Munson M, Lambright DG. TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 2006; 442:303-6; PMID:16855591; http://dx.doi.org/10.1038/nature04847
- Soldati T, Shapiro AD, Svejstrup AB, Pfeffer SR. Membrane targeting of the small GTPase Rab9 is accompanied by nucleotide exchange. Nature 1994; 369:76-8; PMID:8164745; http://dx.doi.org/10.1038/369076a0
- Seabra MC, Goldstein JL, Sudhof TC, Brown MS. Rab geranylgeranyl transferase. A multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-X-Cys or Cys-Cys. J Biol Chem 1992; 267:14497-503; PMID:1321151
- Andres DA, Seabra MC, Brown MS, Armstrong SA, Smeland TE, Cremers FP, Goldstein JL. cDNA cloning of component A of Rab geranylgeranyl transferase and demonstration of its role as a Rab escort protein. Cell 1993; 73:1091-9; PMID:8513495; http://dx.doi.org/10.1016/0092-8674(93)90639-8
- Pylypenko O, Rak A, Reents R, Niculae A, Sidorovitch V, Cioaca MD, Bessolitsyna E, Thomä NH, Waldmann H, Schlichting I, et al. Structure of Rab escort protein-1 in complex with Rab geranylgeranyltransferase. Mol Cell 2003; 11:483-94; PMID:12620235; http://dx.doi.org/10.1016/S1097-2765(03)00044-3
- Rak A, Pylypenko O, Niculae A, Pyatkov K, Goody RS, Alexandrov K. Structure of the Rab7:REP-1 complex: insights into the mechanism of Rab prenylation and choroideremia disease. Cell 2004; 117:749-60; PMID:15186776; http://dx.doi.org/10.1016/j.cell.2004.05.017
- Guo Z, Wu YW, Das D, Delon C, Cramer J, Yu S, Thuns S, Lupilova N, Waldmann H, Brunsveld L, et al. Structures of RabGGTase-substrate/product complexes provide insights into the evolution of protein prenylation. EMBO J 2008; 27:2444-56; PMID:18756270; http://dx.doi.org/10.1038/emboj.2008.164
- Wu YW, Goody RS, Abagyan R, Alexandrov K. Structure of the disordered C terminus of Rab7 GTPase induced by binding to the Rab geranylgeranyl transferase catalytic complex reveals the mechanism of Rab prenylation. J Biol Chem 2009; 284:13185-92; PMID:19240028; http://dx.doi.org/10.1074/jbc.M900579200
- Leung KF, Baron R, Ali BR, Magee AI, Seabra MC. Rab GTPases containing a CAAX motif are processed post-geranylgeranylation by proteolysis and methylation. J Biol Chem 2007; 282:1487-97; PMID:17114793; http://dx.doi.org/10.1074/jbc.M605557200
- Derre I, Isberg RR. Legionella pneumophila replication vacuole formation involves rapid recruitment of proteins of the early secretory system. Infect Immun 2004; 72:3048-53; PMID:15102819; http://dx.doi.org/10.1128/IAI.72.5.3048-3053.2004
- Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of Rab1 and Sec 22b to create a replicative organelle. J Exp Med 2004; 199:1201-11; PMID:15117975; http://dx.doi.org/10.1084/jem.20031706
- Murata T, Delprato A, Ingmundson A, Toomre DK, Lambright DG, Roy CR. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nat Cell Biol 2006; 8:971-7; PMID:16906144; http://dx.doi.org/10.1038/ncb1463
- Machner MP, Isberg RR. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev Cell 2006; 11:47-56; PMID:16824952; http://dx.doi.org/10.1016/j.devcel.2006.05.013
- Muller MP, Peters H, Blumer J, Blankenfeldt W, Goody RS, Itzen A. The Legionella effector protein DrrA AMPylates the membrane traffic regulator Rab1b. Science 2010; 329:946-9; PMID:20651120; http://dx.doi.org/10.1126/science.1192276
- Neunuebel MR, Chen Y, Gaspar AH, Backlund PS, Jr, Yergey A, Machner MP. De-AMPylation of the small GTPase Rab1 by the pathogen Legionella pneumophila. Science 2011; 333:453-6; PMID:21680813; http://dx.doi.org/10.1126/science.1207193
- Tan Y, Luo ZQ. Legionella pneumophila SidD is a deAMPylase that modifies Rab1. Nature 2011; 475:506-9; PMID:21734656; http://dx.doi.org/10.1038/nature10307
- Mukherjee S, Liu X, Arasaki K, McDonough J, Galan JE, Roy CR. Modulation of Rab GTPase function by a protein phosphocholine transferase. Nature 2011; 477:103-6; PMID:21822290; http://dx.doi.org/10.1038/nature10335
- Goody PR, Heller K, Oesterlin LK, Muller MP, Itzen A, Goody RS. Reversible phosphocholination of Rab proteins by Legionella pneumophila effector proteins. EMBO J 2012; 31:1774-84; PMID:22307087; http://dx.doi.org/10.1038/emboj.2012.16
- Tan Y, Arnold RJ, Luo ZQ. Legionella pneumophila regulates the small GTPase Rab1 activity by reversible phosphorylcholination. Proc Natl Acad Sci U S A 2011; 108:21212-7; PMID:22158903; http://dx.doi.org/10.1073/pnas.1114023109
- Oesterlin LK, Goody RS, Itzen A. Posttranslational modifications of Rab proteins cause effective displacement of GDP dissociation inhibitor. Proc Natl Acad Sci U S A 2012; 109:5621-6; PMID:22411835; http://dx.doi.org/10.1073/pnas.1121161109
- Pylypenko O, Goud B. Posttranslational modifications of Rab GTPases help their insertion into membranes. Proc Natl Acad Sci U S A 2012; 109:5555-6; PMID:22451945; http://dx.doi.org/10.1073/pnas.1202494109
- Bailly E, McCaffrey M, Touchot N, Zahraoui A, Goud B, Bornens M. Phosphorylation of two small GTP-binding proteins of the Rab family by p34cdc2. Nature 1991; 350:715-8; PMID:1902553; http://dx.doi.org/10.1038/350715a0
- van der Sluijs P, Hull M, Huber LA, Male P, Goud B, Mellman I. Reversible phosphorylation–dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J 1992; 11:4379-89; PMID:1425574
- Fitzgerald ML, Reed GL. Rab6 is phosphorylated in thrombin-activated platelets by a protein kinase C-dependent mechanism: effects on GTP/GDP binding and cellular distribution. Biochem J 1999; 342(Pt 2):353-60; PMID:10455022; http://dx.doi.org/10.1042/bj3420353
- Lai YC, Kondapalli C, Lehneck R, Procter JB, Dill BD, Woodroof HI, Gourlay R, Peggie M, Macartney TJ, Corti O, et al. Phosphoproteomic screening identifies Rab GTPases as novel downstream targets of PINK1. EMBO J 2015; 34:2840-61; PMID:26471730; http://dx.doi.org/10.15252/embj.201591593
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, et al. Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 2016; 5; PMID:26824392; http://dx.doi.org/10.7554/eLife.12813
- Levin RS, Hertz NT, Burlingame AL, Shokat KM, Mukherjee S. Innate immunity kinase TAK1 phosphorylates Rab1 on a hotspot for posttranslational modifications by host and pathogen. Proc Natl Acad Sci U S A 2016; 113:E4776-83; PMID:27482120; http://dx.doi.org/10.1073/pnas.1608355113
- Shinde SR, Maddika S. PTEN modulates EGFR late endocytic trafficking and degradation by dephosphorylating Rab7. Nat Commun 2016; 7:10689; PMID:26869029; http://dx.doi.org/10.1038/ncomms10689
- Satpathy S, Wagner SA, Beli P, Gupta R, Kristiansen TA, Malinova D, Francavilla C, Tolar P, Bishop GA, Hostager BS, et al. Systems-wide analysis of BCR signalosomes and downstream phosphorylation and ubiquitylation. Mol Syst Biol 2015; 11:810; PMID:26038114; http://dx.doi.org/10.15252/msb.20145880
- Lachance V, Degrandmaison J, Marois S, Robitaille M, Genier S, Nadeau S, Angers S, Parent JL. Ubiquitylation and activation of a Rab GTPase is promoted by a beta(2)AR-HACE1 complex. J Cell Sci 2014; 127:111-23; PMID:24190883; http://dx.doi.org/10.1242/jcs.132944
- Qiu J, Sheedlo MJ, Yu K, Tan Y, Nakayasu ES, Das C, Liu X, Luo ZQ. Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 2016; 533:120-4; PMID:27049943; http://dx.doi.org/10.1038/nature17657
- Walther DJ, Peter JU, Winter S, Holtje M, Paulmann N, Grohmann M, Vowinckel J, Alamo-Bethencourt V, Wilhelm CS, Ahnert-Hilger G, et al. Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release. Cell 2003; 115:851-62; PMID:14697203; http://dx.doi.org/10.1016/S0092-8674(03)01014-6
- Paulmann N, Grohmann M, Voigt JP, Bert B, Vowinckel J, Bader M, Skelin M, Jevsek M, Fink H, Rupnik M, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol 2009; 7:e1000229; PMID:19859528; http://dx.doi.org/10.1371/journal.pbio.1000229