ABSTRACT
Synaptic connections in the brain are continuously weakened or strengthened in response to changes in neuronal activity. This process, known as synaptic plasticity, is the cellular basis for learning and memory, and is thought to be altered in several neuronal disorders. An important aspect of synaptic plasticity is the tightly controlled trafficking and synaptic targeting of the AMPA-type glutamate receptors, which are the major mediators of fast excitatory transmission in the brain. This review addresses the role of Rab GTPases in AMPA receptor trafficking in neurons under basal conditions and during activity-induced synaptic plasticity, especially during long-term potentiation (LTP) and long-term depression (LTD). We highlight the importance of the tight spatio-temporal control of Rab activity and suggest that this is critical for proper neuronal functions. We also discuss how abnormal AMPA receptor trafficking and malfunctioning of Rabs can lead to neurologic disorders or memory problems.
Introduction
Overview on Rab proteins
Rab proteins are small monomeric GTPases forming the largest subgroup of the Ras superfamily. Originally, they were named as ra s genes from ra t b rainCitation1 but they are ubiquitously expressed in mammalian cells. Up to date almost 70 different Rab proteins have been identified in humans, playing a central role in the regulation of intracellular membrane traffic. A particular characteristic of Rab proteins is that they specifically mark different membranes and play critical role in ensuring the correct delivery of membrane-bound cargo from the donor to the acceptor compartment (for recent reviews, see refs. Citation2-Citation4).
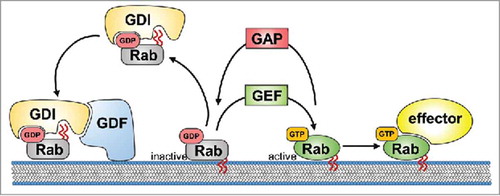
In general, Rab GTPases are molecular switches cycling between an inactive form bound to GDP and an active form bound to GTP (see for an overview of the regulation of Rab activity). Rab proteins can associate with membranes due to the posttranslational covalent attachment of prenyl groups to their C-terminus. Once the geranylgeranylation of a Rab protein takes place, a GDP dissociation inhibitor (GDI) factor chaperones it within the cytosol. GDIs take part in the delivery of inactive, GDP-bound Rab proteins to specific membrane compartments through interactions with membrane-bound GDI displacement factors (GDFs). GDFs recognize GDI-Rab complexes specifically and promote GDI release, thereby facilitating the association of a particular Rab protein with its target membrane. The activation of the GDP-bound Rabs is mediated by guanine nucleotide exchange factors (GEFs), which catalyze the conversion from the GDP-bound to the GTP-bound form. In certain cases, membrane-bound GEFs can be sufficient to lead to the accumulation of Rab GTPases without the involvement of GDFs. Active, GTP-bound Rab proteins exert their function through their effector proteins, providing a diverse array of pathways (see below). As a negative regulator of Rab signaling, the intrinsic GTP hydrolysis of Rab proteins is enhanced by GTPase-activating proteins (GAPs) leading to Rab inactivation. Inactive, GDP-bound Rabs are then removed from their target membrane and kept soluble in the cytoplasm by GDIs (reviewed in refs. Citation5-Citation7).
Figure 1. Life-cycle of Rab proteins. Inactive, GDP-bound Rab is chaperoned within the cytoplasma by GDP dissociation inhibitor (GDI). Membrane-associated GDI displacement factor (GDF) recognizes the Rab–GDI complex and mediates the insertion of the Rab into the target membrane through its prenyl tails (red wavy lines) resulting in release of the GDI into the cytosol. The activation of GDP-bound Rab is mediated by the guanine nucleotide exchange factor (GEF), which mediates the exchange of GDP with GTP. Active, GTP-bound Rab exerts its function through its effector protein(s). Intrinsic GTP hydrolysis of Rab is enhanced by the GTPase-activating protein (GAP) leading to Rab inactivation. Subsequently, inactive, GDP-bound Rab is removed from the membrane and kept in the cytoplasm by GDI.
A diverse set of proteins serves as Rab effectors including motor proteins, kinases and phosphatases, tethering factors or sorting adaptors. There is increasing evidence that each Rab signals through a set of different effectors, allowing the compartment-specific coordination of vesicle transport, budding or fusion as well as receptor signaling. Generally, Rab5 allows entry into the early endosome, whereas Rab4 and Rab11 activate the machinery that is necessary for sorting and recycling membranes and receptors back to the plasma membrane. Rab7 specifically marks late endosomes containing cargo directed toward lysosomal degradation. Regarding secretory transport, Rab1 and Rab2 regulate vesicular traffic between the endoplasmic reticulum (ER) and the Golgi complex, and Rab8 is involved in transport processes from the trans-Golgi network (TGN) toward the plasma membrane (for detailed overview on general Rab activity see refs. Citation2, Citation3, Citation7-Citation9).
Rab proteins in neurons
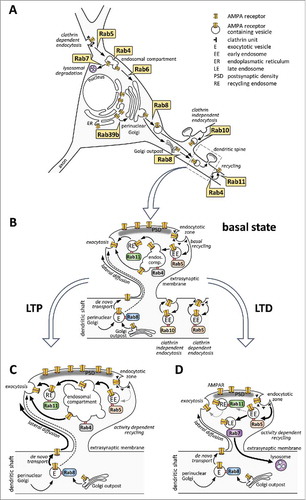
In neurons, elaborated axonal and dendritic branching generates an extreme surface to volume ratio and an enormous flux of membrane traffic, with a need of tightly controlled and activity-dependent delivery of vesicles toward specific membrane compartments. Up to date, approximately 20 Rab proteins have been identified to play important regulatory roles during normal neuronal functions, including axonal outgrowth, synaptic vesicle recycling, vesicular transport to and from the Golgi complex and postsynaptic functions (extensively reviewed in refs. Citation9-Citation12; summarizes data on Rab interactors identified in neuronal cells). So far, the role of only a few Rab proteins and their corresponding GEF, GAP or effectors have been identified during AMPAR vesicular trafficking (interactor proteins are highlighted together with their proven importance in AMPA receptor trafficking in ). In this review we provide an overview on different trafficking routes of AMPAR and how these are coordinated by small GTPases of the Rab family. We also discuss how malfunctioning of Rabs and abnormal AMPAR trafficking can contribute to neurologic disorders or memory problems.
Table 1. Rab GTPase interactors in neuronal cells. Rab GTPase interactor proteins with a reported role in neurons are listed according to their GEF, GAP or effector functions and to their Rab GTPase partners. Major neuronal functions of these proteins are summarized according to literature data. Proteins with a proven role in AMPA receptor trafficking are highlighted in bold lettering.
AMPA receptors and neuronal plasticity
In the central nervous system, most excitatory transmission is mediated by the AMPA-type ionotropic glutamate receptors (AMPARs). Upon binding glutamate that is released from the presynaptic-terminals, AMPARs open and become permeable to Na+, K+ and – depending on the subunit composition – to Ca2+ ions, leading to membrane depolarization. The number and the properties of the available AMPARs at the postsynaptic membrane determine the extent of excitatory postsynaptic currents (EPSCs) formed within the dendritic spines. It is now widely accepted that the amount and composition of AMPA receptors within the postsynaptic density (PSD) of dendritic spines determine synaptic efficacy and affect the excitability of the neuron (reviewed in ref. Citation13).
AMPA receptors are tetrameric structures composed of 4 types of subunits, namely GluA1, GluA2, GluA3 and GluA4. The GluA1, GluA2, and GluA3 subunits are expressed during embryonic development, whereas the GluA4 subunit is mainly present in the late postnatal development and in adults.Citation14 Most AMPARs in the brain contain the GluA2 subunit co-assembled with GluA1 or GluA3, while receptors with GluA4 subunit show a more restricted and developmentally regulated expression. The logistics of the delivery, retention and removal of individual AMPARs with defined subunit compositions at specific synapses is highly complex and fundamentally influences both Hebbian and homeostatic plasticity (reviewed in ref. Citation15). The most studied forms of Hebbian plasticity in the brain are long-term potentiation (LTP) and long-term depression (LTD), which lead to the long-lasting increase and decrease of synaptic strength, respectively. Homeostatic plasticity, however, regulates synaptic activity in a way to keep neuronal activity within a limited range to preserve the stability of neuronal circuits. These mechanisms depend on the number of AMPARs at synapses, which is determined according to the relative rates of exocytosis and endocytosis at the postsynaptic membrane. Although it is highly likely that similar molecular machinery is involved in regulating AMPAR trafficking during Hebbian and homeostatic plasticity,Citation16 some aspects of AMPAR endocytosis clearly differ between events leading to homeostatic scaling or during LTD.Citation17 We will discuss the role of Rab proteins during activity-dependent regulation of AMPAR trafficking only in relation to LTP and LTD as most of the available data deal with these events.
Rab proteins regulating AMPAR trafficking under basal conditions
Already in the ER, AMPA receptors assemble as dimers, which then form heterotetrameric structures leaving the ER. The assembly of the tetramer and the exit from the ER is controlled by a RNA editing step in the GluA2 subunit: the Q/R editing in the pore loop blocks tetrameric assembly and retains the GluA2 protein at the ER whereas the unedited subunits immediately assemble and traffic to their postsynaptic target via the Golgi complex (, enlarged neuronal soma). On the other hand, GluA1, which lacks the ER retention motif found in GluA2, is rapidly exported from the ER. The RNA editing also contributes to the functionality of the receptors because Q/R edited GluA2 subunits are impermeable to Ca2+ ions (reviewed in ref. Citation18).
Figure 2. Rab proteins regulating intracellular trafficking of AMPA receptors (AMPARs) in neurons during basal conditions and synaptic plasticity. (A) Enlarged neuronal soma summarizes neuron-specific, Rab-mediated actions in relation to different trafficking steps (see text for details). (B-D) enlarged spines depict Rab-dependent events in the basal state (B), during long-term potentiation (LTP; C) or long-term depression (LTD; D). (B) In the basal state, de novo transport from the Golgi or from the Golgi outpost takes place through Rab8-associated exocytotic vesicles (designated as E). Exocytosis occurs at the extrasynaptic membrane, mostly at the dendritic shaft, although some data suggest direct delivery of AMPARs to the perisynaptic membrane (dashed arrow). Extrasynaptic AMPARs diffuse laterally toward the synaptic membrane where they get immobilized within the PSD. Clathrin-mediated endocytosis of AMPARs into Rab5-positive early endosomes (indicated by EE) occurs at the endocytotic zone, located perisynaptically or within the dendritic shaft. Clathrin-independent and Rab10-regulated endocytosis of AMPAR-type subunits from lipid rafts was reported in C. elegans neurons, as well. Besides de novo trafficking, continuous recycling directly from Rab4-positive endosomes or through Rab11-associated recycling endosomes (designated as RE) provides the supply for synaptic AMPARs inside the spines as well as within the shafts (depicted in details only inside the spine heads). (C) upon LTP, the amount of synaptic AMPARs is increased by upregulating de novo trafficking toward the plasma membrane and lateral diffusion of newly inserted AMPARs (indicated by thicker arrows). During activity-dependent recycling, the endosomal compartment is increased in size and recycling through Rab11 positive recycling endosomes is elevated. The role of Rab4-dependent delivery to the membrane has yet to be proven during LTP. It is yet unclear how Rab5-dependent endocytosis is changed during LTP. (D) in case of LTD, the loss of synaptic AMPARs is due to increased Rab5-dependent endocytosis. Rab11-positive recycling is reduced, and a large portion of the endocytosed AMPARs is directed to the Rab7-associated late endosome system and toward lysosomal degradation. During this time, Rab11-dependent recycling is still ongoing.
AMPA receptor trafficking from the neuronal cell body to the synapse is also controlled by other mechanisms such as interaction with auxiliary proteins including the stargazing/transmembrane AMPAR regulatory proteins (TARPs)Citation19 or the cornichon family.Citation20 The length and phosphorylation of the intracellular C-terminal domains of AMPA receptors have been considered as further critical determinants of trafficking. Long-tailed AMPAR subunits (GluA1 and GluA4) were reported to rapidly proceed from the ER to the synapse whereas the short-tailed subunits (GluA2 and GluA3) are trafficked more slowly. Recent data, however, questioned the importance of the C-terminal tail, especially during the regulation of the GluA1 transport (reviewed in ref. Citation13; see also refs. Citation21, Citation22). Among Rab proteins, so far only Rab39B has been identified to regulate AMPAR exit from the ER toward the Golgi complexCitation23 (see ). Rab39B localizes to the Golgi and interacts with protein interacting with C-kinase 1 (PICK1), which is necessary for GluA2 transport from the ER to Golgi compartment by selectively binding GluA2/GluA3 heterodimers. Silencing of Rab39B in hippocampal neurons leads to a decrease in surface GluA2 density and an increase in Ca2+ ion permeable GluA1 AMPAR subunits, elevating miniature EPSC amplitudes. Importantly, Rab39B is not involved in activity-dependent AMPAR recycling and endocytosis as Rab39B knock down did not influence LTD.
Surface AMPA receptors can be delivered to the plasma membrane along different trafficking pathways, such as the de novo exocytotic pathway originating from the Golgi apparatus and from recycling pathways involving early and recycling endosomes. Under basal conditions, AMPARs are known to shuttle between internal and surface compartments, with a steady-state of continuous exocytosis and endocytosisCitation24-Citation26 (see the enlarged spines in ). Golgi outposts located in dendritic branch points as well as local protein synthesis from mRNA can also participate in the de novo exocytotic pathway (reviewed by ref.Citation27). Interestingly, local protein synthesis is primarily involved during long-term changes in activity, leading to homeostatic scaling.Citation13
AMPAR delivery to the plasma membrane from the trans-Golgi network (TGN) or from the Golgi outposts located within the dendritic shaft is regulated principally by Rab8.Citation28,Citation29 Although mainly trans-Golgi network (TGN)-localized, Rab8 is also found in close proximity to the postsynaptic plasma membrane and the postsynaptic densityCitation29 and has been indicated to direct the Golgi to plasma membrane delivery of AMPARs during de novo exocytotic pathway under basal conditionsCitation25 as well as during activity-dependent stimuli.Citation28 Although real-time observations promote the findings that AMPARs are inserted extrasynaptically to the plasma membrane, followed by lateral diffusion to the postsynaptic regions, some work has shown a direct insertion of AMPARs through Rab8-mediated transport within the spines.Citation28,Citation30 Rabin8 has been described as a Rab8 GEFCitation31 and optineurin as a Rab8 effectorCitation32,Citation33 in neuronal cells, but so far neither of them has been investigated in relation to AMPAR trafficking.
The recycling pathway of GluA subunit turnover is regulated by a tight, spatially and temporally controlled balance between the rapid internalization of AMPARs associating with the early endosomes and the delivery of AMPARs from the recycling endosomes toward the plasma membrane (depicted within the dendritic shaft of the enlarged spine regions in ). Endocytotic removal of AMPARs occurs at the extrasynaptic membrane within the shaftCitation34 or at the perisynaptic endocytotic zone within the spines.Citation35 During clathrin-mediated internalization, Rab5 regulates uncoating and directs internalized AMPARs toward early endosomes.Citation25,Citation35-Citation37 In C. elegans neurons, there is an additional, clathrin-independent endocytosis of the GLR-1 AMPAR-type subunit. This Rab5-independent endocytosis is mediated by lipid rafts, and AMPARs endocytosed through this mechanism are recycled by Rab10.Citation38
While a role for Rab10 in AMPAR recycling in mammalian neurons remains unclear, the closely related Rab4, as well as Rab11, mediate continuous recycling of endocytosed AMPARs from sorting and recycling endosomes, respectively, during normal conditions.Citation25 Rab4-positive compartments play critical role in spine maintenance during normal conditions.Citation28 GRASP-1, a Rab4 effector has been implicated to mediate the fusion between Rab4 and Rab11 positive endosomes via syntaxin 13 bridging.Citation39,Citation40 Knockdown or overexpression of GRASP-1 perturbs normal spine morphology and leads to abnormal endosomal functions. Rab11 selectively labels recycling endosomes and has been shown to regulate the continuous recycling of previously endocytosed GluA1 subunits to the postsynaptic membrane via frequent entry to and exit from the spines depending on myosin Vb-directed transport.Citation41-Citation44 Rab11 is not solely responsible for targeting recycled AMPARs back to the plasma membrane, as internalized GluA2 subunits can return to the surface directly from the Rab4 positive compartments, as well.Citation25 Increase in the surface amount of AMPARs during corticosterone-induced acute stress also depends on the Rab4-mediated delivery of the AMPARs, regulated by the phosphorylation of its GDI1.Citation45,Citation46 Somewhat contradictory to these data, earlier studies analyzing GluA1 delivery showed that neither dominant-negative Rab4S22N nor Rab11S25N altered AMPAR-mediated basal synaptic transmission,Citation28,Citation29,Citation47 although Rab4-dependent membrane trafficking was critical for spine size maintenance during normal conditions.Citation28
During normal conditions, most of the endocytosed AMPARs return back to the synaptic membrane and therefore transport to the Rab7-labeled late endosomes and toward lysosomal degradation is not a common phenomenon.Citation48 In accordance with this, dominant-negative Rab7N125I did not cause significant differences in the amount of surface AMPARs in hippocampal slices.Citation47
A special way of recycling has been described in C. elegans neurons: the retromer complex sequesters GLR-1 AMPARs into endosomal tubules, where RAB-6.1 and RAB-6.2 regulate the trafficking of the cargo vesicles back to the Golgi or to dendritic Golgi outposts (depicted as Rab6 on ). Subsequently, cargo is transported back to the plasma membrane.Citation49,Citation50 In case of rat hippocampal neurons, Golgi-associated dominant-negative Rab6T27N did not grossly alter glycine-evoked AMPAR insertion to the plasma membrane suggesting that the retrograde transport is negligible under these conditions.Citation44 Very recently, a function for the retromer complex in AMPAR trafficking was also confirmed in mammalian neurons. Here, Temkin and colleagues showed that retromer function is required for exocytosis of AMPAR during LTP but not for basal synaptic transmission in mature hippocampal neurons.Citation51 Whether Rab6 is required for AMPAR trafficking under these conditions, however, still needs to be addressed.
Rab proteins regulating AMPAR trafficking under LTP
Long-term potentiation is accompanied by a rapid increase in the amount of surface AMPARs within the postsynaptic membrane, which confers increased synaptic strength. Increased mobility and lateral diffusion of extrasynaptic AMPARs provide an important supply for newly inserted synaptic AMPARs. Additionally, exocytosis from recycling endosomes and/or from the Golgi complex increased AMPAR insertion into the synaptic membrane within minutes.Citation52 These events are regulated at different levels, including interaction with scaffold proteins and molecular motors, formation of the exocyst complex and regulated release from endosomal compartments (reviewed in more detail by refs. Citation53-Citation55).
Elevated de novo transport of AMPAR subunit (see the thickened arrows on the enlarged spine in ) requires proper Rab8 functioning, as dominant-negative Rab8T22N selectively impairs AMPA receptor currents and abolishes LTP in hippocampal slices.Citation28,Citation29 Interestingly, Rab8T22N highly elevates the relative amount of GluA1 in spines compared with the dendritic shaft following the expression of constitutively active CaMKII, implicating phosphorylation-dependent regulation of Rab8 functions.Citation28
LTP is accompanied by structural changes, including expansion of the spine head and enlargement of the endocytotic compartment.Citation43 It seems that Rab4-associated sorting endosomes are not primarily involved in activity-induced recycling of AMPAR subunits during LTP, as dominant-negative Rab4S22N only slightly reduced EPSC amplitudes in organotypic hippocampal slice cultures.Citation28 Despite the lack of a proven role in LTP, dominant negative Rab4 regulates synaptic efficacy under stress conditions. It is known that acute stress increases AMPAR-mediated synaptic transmission and surface positioning in pyramidal neurons. Corticosterone activates serum- and glucocorticoid-inducible kinase (SGK), which phosphorylates GDI1, regulating the cycling of Rab proteins between membranes and cytosol.Citation45 Thus, in the prefrontal cortex, corticosterone stimulates the formation of the GDI1/Rab4 complex via SGK1-mediated phosphorylation of GDI1, which facilitates Rab4-dependent AMPAR delivery to the surface and potentiates synaptic transmission.Citation45,Citation46
In the case of Rab11, it is widely accepted that its association with recycling endosomes facilitates the delivery of previously endocytosed AMPARs back to the synaptic membrane,Citation44 interacting with myosin Vb and the endosomal adaptor Rab11-FIP2 upon glycine-induced LTP.Citation43 Accordingly, dominant-negative Rab11 inhibits the elevation in synaptic AMPARs induced by cholesterol depletion or during chemically induced LTP,Citation44,Citation56 depletes mobile AMPARs at synapsesCitation57 and blocks LTP formation.Citation28,Citation44 Interestingly, overexpression of wild-type Rab11 leads to robust glycine-induced AMPAR insertionCitation44 while short-term removal or addition of Rab11 recycling endosomes from spines does not impair spine expansion during chemically induced LTP in hippocampal neurons.Citation41
Although dominant-negative Rab5S34N does not influence LTP formation in organotypic cultures,Citation28 it is highly likely that Rab5-mediated endocytosis takes place during LTP. Chemical LTP (cLTP) was shown to increase the synaptic delivery of Ca2+-permeable AMPARs selectively and within minutes, which is followed by a subsequent exchange to Ca2+-impermeable AMPARs.Citation58 Although Rab5-mediated endocytosis was not investigated directly, selective removal of GluA2-free AMPARs during the consolidation phase of cLTP is most probably mediated by Rab5-dependent endocytosis. Importantly, selective retention of GluA2-containing AMPARs during the early phase of LTP is mediated by their association to PICK1 in the endosomal compartments. Upon elevated intracellular Ca2+ ion levels PICK1 is phosphorylated and released from the membrane allowing GluA2 containing AMPARs to insert into the synaptic membrane.Citation58,Citation59 Another study, however, showed that PICK1 is not necessary for maintenance of the basal synaptic complement of AMPARs or NMDAR-dependent LTP. Instead, PICK1 function in AMPAR trafficking seems to be specific to NMDAR-dependent LTD.Citation60 Whether the PICK1-dependent GluA2 retention is of physiological relevance for LTP thus requires further investigation.
So far, we are not aware of any data showing a contribution of Rab7-mediated lysosomal degradation of AMPARs in LTP. However, we assume that basal protein turnover of AMPARs takes place also during LTP, albeit it might not be important for the strengthening of the synapse.
Rab proteins regulating AMPAR trafficking under LTD
During LTD formation (see the enlarged spine in ), synaptic efficacy is reduced due to the loss of synaptic AMPARs, which is often accompanied by structural changes inside the spines, leading to shrinkage (reviewed in more detail in ref. Citation13). During this process, synaptic AMPARs are taken back by Rab5-dependent endocytosis, leading to the formation of Rab5-positive early endosomes containing ex-synaptic AMPARs.Citation35,Citation61 Rab5 activity is a key mediator of AMPAR endocytosis and LTD formation, as blocking Rab5 function by infusing anti-Rab5 antibody or expressing dominant-negative Rab5S53N inhibited serotonin-facilitated LTD formation in the prefrontal cortex. In line with these findings, constitutively active Rab5Q79L caused a gradual depression of mEPSC amplitude, indicating increased endocytotic activity.Citation62 The surface level of AMPARs and its downregulation during chemically evoked LTD in cultured hippocampal neurons was shown to be dependent on the intact Rab5 effector functions of Ras and Rab interactor protein 1 (RIN1), as well.Citation36
Internalized AMPARs are sorted along the recycling or retention pathways, according to neuronal activity.Citation48,Citation61 Blockade of Rab7-dependent trafficking by the dominant-negative Rab7N125I produced a significant reduction in the extent of LTD,Citation47 indicating that Rab7-driven trafficking of AMPARs to lysosomes is important during LTD. Blocking the transport of AMPARs from the recycling endosomes to the postsynaptic membrane by the dominant negative Rab11S25N produced a significant increase in LTD, indicating that a certain amount of internalized AMPARs upon LTD induction recycles back toward the synaptic membrane.Citation47 This is in accordance with previously published data, where vesicles containing AMPARs from the cell surface colocalize with TfR (transferrin receptor) or Rab4,Citation61 highlighting the importance of the recycling pathways even during LTD formation. Rab8, finally, has not been mentioned so far in relation to the development and formation of LTD.
Improper regulation of Rab activity and AMPA receptor trafficking in neuronal disorders
AMPA receptor dysfunction has been reported in a couple of neuronal disorders, with Alzheimer disease (AD) representing the best studied disease so far. Here, amyloid β (Aβ) treatment of neurons induces a reduction of AMPA receptor surface expression through increased endocytosis.Citation63 Accordingly, Aβ treatment facilitates hippocampal LTD and impairs synaptic plasticity and memory.Citation64,Citation65 Some of the earliest neuronal responses in AD are endosomal abnormalities which are associated with an upregulation of Rab5 expression,Citation66 which most likely results in enhanced endocytosis. Furthermore, enhanced Rab7 levelsCitation67 might promote lysosomal degradation of AMPA receptors. So far the molecular mechanisms leading to pathological Rab5 activity have not been well understood. A recent study suggested that elevated levels of βCTF induce APPL1-mediated Rab5 activation on endosomes in AD, a process that is independent from Aβ.Citation68 How Aβ disturbs endocytic signaling thus still awaits investigation.
In the past years, malfunctioning of Rab GTPases and their regulators and effectors has also been implicated in several neurodegenerative and neurodevelopmental disorders. For example, mutations in the Rab5 GEF ALS2 are associated with amyotrophic lateral sclerosis,Citation69 missense mutations in Rab7 cause the Charcot-Marie-Tooth type 2B disease,Citation70,Citation71 whereas Rab8 has been linked to Huntington's disease through its effector optineurin. Here, mutant huntingtin disrupts the Rab8-optineurin complex resulting in an overall deficit in post-Golgi trafficking.Citation33 Furthermore, nonsense or missense mutations of the X-chromosome localized RAB39B resulted in X-linked intellectual disabilityCitation72 and early onset Parkinson disease in affected males.Citation73-Citation76 Although it is likely that alteration of Rab GTPase activity cause the deregulated AMPA receptor trafficking that contributes to these diseases, substantial evidence for this is still missing. In a recent paper, we have shown that the Rab5 GEF RIN1 enhances GluA1 endocytosis due to its Rab5 GEF activity and plays a critical role in AMPAR internalization upon LTD.Citation36 RIN1 is highly expressed within the dendrites of hippocampal neuronsCitation77 and regulates EphA4 receptor internalization.Citation78 Importantly, RIN1−/− mice have deficits in fear learning and extinction and were proposed as a potential model for posttraumatic stress disorder, characterized by enhanced retention of fear-related memories.Citation77,Citation79 Thus, the lack of RIN1 leads to increased amount of AMPARs at the plasma membrane, which cannot be downregulated upon NMDA-dependent cLTD. Because AMPAR downregulation is required during fear extinction in the amygdala,Citation80,Citation81 one interesting possibility is that prolonged fear memory of RIN1−/− mice as well as their inability to forget aversive memories are due to the increased surface level of AMPARs and the inability to downregulate their levels during LTD and fear extinction through Rab5-dependent endocytosis.
Summary and conclusion
Taken together, in the past years several members of the RabGTPase family have been shown to control the trafficking of AMPA receptors under basal and activity-dependent conditions. Because of partially redundant functions within the Rab subfamilies, it can be assumed that even more RabGTPases contribute to exo- and endocytosis of AMPA receptors. While it is intuitively clear that Rab GTPases must be tightly regulated in a spatial and temporal manner to ensure proper trafficking of AMPA receptors and hence synaptic plasticity, the identification of the responsible GEFs and GAPs is still in its infancy. Similarly, only a few Rab effector proteins involved in AMPA receptor trafficking have been characterized so far. Rab effectors are very heterogeneous, and each Rab isoform has many effectors through which it carries out multiple functions, making their identification still challenging. Similarly, several GAPs and GEFs control the activity of a single Rab isoform. Large interactions screens are required to provide more detailed information on the interaction network of RabGTPases in the orchestration of AMPA receptor trafficking under normal and pathological conditions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are indebted to Attila Ignacz for his help with preparing the figures. We apologize to those authors whose original work was not cited due to space limitations.
Funding
Research was supported by the KTIA_NAP_13–2014–0018 and VEKOP-2.3.3–15–2016–00007 grants from the National Research, Development and Innovation Office to KS, by the German Research Foundation to AH (HA 3557/11–2), and by the German-Hungarian Academic Exchange Service No 73539 and PPP Ungarn 57215775 programs to KS and AH, respectively.
References
- Touchot N, Chardin P, Tavitian A. Four additional members of the ras gene superfamily isolated by an oligonucleotide strategy: Molecular cloning of YPT-related cDNAs from a rat brain library. Proc Natl Acad Sci U S A 1987; 84:8210-4; PMID:3317403
- Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci 2015; 128:3171-6; PMID:26272922; https://doi.org/10.1242/jcs.166074
- Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 2014; 6:a022616; PMID:25341920; https://doi.org/10.1101/cshperspect.a022616
- Li G, Marlin MC. Rab family of GTPases. Methods Mol Biol 2015; 1298:1-15; PMID:25800828; https://doi.org/10.1007/978-1-4939-2569-8_1
- Barr F, Lambright DG. Rab GEFs and GAPs. Curr Opin Cell Biol 2010; 22:461-70; PMID:20466531; https://doi.org/10.1016/j.ceb.2010.04.007
- Muller MP, Goody RS. Molecular control of Rab activity by GEFs, GAPs and GDI. Small GTPases 2017:1-17; PMID:28055292; https://doi.org/10.1080/21541248.2016.1276999
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 2009; 10:513-25; PMID:19603039; https://doi.org/10.1038/nrm2728
- Bacaj T, Ahmad M, Jurado S, Malenka RC, Sudhof TC. Synaptic function of Rab11Fip5: Selective requirement for hippocampal long-term depression. J Neurosci 2015; 35:7460-74; PMID:25972173; https://doi.org/10.1523/JNEUROSCI.1581-14.2015
- D'Adamo P, Masetti M, Bianchi V, More L, Mignogna ML, Giannandrea M, Gatti S. RAB GTPases and RAB-interacting proteins and their role in the control of cognitive functions. Neurosci Biobehav Rev 2014; 46(Pt 2):302-14; PMID:24412241; https://doi.org/10.1016/j.neubiorev.2013.12.009
- Binotti B, Jahn R, Chua JJ. Functions of Rab proteins at presynaptic sites. Cells 2016; 5(1). pii: E7; PMID:26861397; https://doi.org/10.3390/cells5010007
- Mignogna ML, D'Adamo P. Critical importance of RAB proteins for synaptic function. Small GTPases 2017:1-13; PMID:28146371; https://doi.org/10.1080/21541248.2016.1277001
- Ng EL, Tang BL. Rab GTPases and their roles in brain neurons and glia. Brain Res Rev 2008; 58:236-46; PMID:18485483; https://doi.org/10.1016/j.brainresrev.2008.04.006
- Henley JM, Wilkinson KA. Synaptic AMPA receptor composition in development, plasticity and disease. Nat Rev Neurosci 2016; 17:337-50; PMID:27080385; https://doi.org/10.1038/nrn.2016.37
- Hall RA, Bahr BA. AMPA receptor development in rat telencephalon: [3H]AMPA binding and western blot studies. J Neurochem 1994; 63:1658-65; PMID:7931321; https://doi.org/10.1046/j.1471-4159.1994.63051658.x
- Chater TE, Goda Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 2014; 8:401; PMID:25505875; https://doi.org/10.3389/fncel.2014.00401
- Diering GH, Gustina AS, Huganir RL. PKA-GluA1 coupling via AKAP5 controls AMPA receptor phosphorylation and cell-surface targeting during bidirectional homeostatic plasticity. Neuron 2014; 84:790-805; PMID:25451194; https://doi.org/10.1016/j.neuron.2014.09.024
- Glebov OO, Tigaret CM, Mellor JR, Henley JM. Clathrin-independent trafficking of AMPA receptors. J Neurosci 2015; 35:4830-6; PMID:25810514; https://doi.org/10.1523/JNEUROSCI.3571-14.2015
- Wright A, Vissel B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front Mol Neurosci 2012; 5:34; PMID:22514516; https://doi.org/10.3389/fnmol.2012.00034
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000; 408:936-43; PMID:11140673; https://doi.org/10.1038/35046031
- Herring BE, Shi Y, Suh YH, Zheng CY, Blankenship SM, Roche KW, Nicoll RA. Cornichon proteins determine the subunit composition of synaptic AMPA receptors. Neuron 2013; 77:1083-96; PMID:23522044; https://doi.org/10.1016/j.neuron.2013.01.017
- Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature 2013; 493:495-500; PMID:23235828; https://doi.org/10.1038/nature11775
- Hosokawa T, Mitsushima D, Kaneko R, Hayashi Y. Stoichiometry and phosphoisotypes of hippocampal AMPA-type glutamate receptor phosphorylation. Neuron 2015; 85:60-7; PMID:25533481; https://doi.org/10.1016/j.neuron.2014.11.026
- Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, Bassani S, Fang H, Van Anken E, Alessio M, et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat Commun 2015; 6:6504; PMID:25784538; https://doi.org/10.1038/ncomms7504
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron 2005; 48:977-85; PMID:16364901; https://doi.org/10.1016/j.neuron.2005.11.030
- Gu Y, Chiu SL, Liu B, Wu PH, Delannoy M, Lin DT, Wirtz D, Huganir RL. Differential vesicular sorting of AMPA and GABAA receptors. Proc Natl Acad Sci U S A 2016; 113:E922-31; PMID:26839408; https://doi.org/10.1073/pnas.1525726113
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci 2007; 27:11112-21; PMID:17928453; https://doi.org/10.1523/JNEUROSCI.2465-07.2007
- Czondor K, Thoumine O. Biophysical mechanisms regulating AMPA receptor accumulation at synapses. Brain Res Bull 2013; 93:57-68; PMID:23174308; https://doi.org/10.1016/j.brainresbull.2012.11.001
- Brown TC, Correia SS, Petrok CN, Esteban JA. Functional compartmentalization of endosomal trafficking for the synaptic delivery of AMPA receptors during long-term potentiation. J Neurosci 2007; 27:13311-5; PMID:18045925; https://doi.org/10.1523/JNEUROSCI.4258-07.2007
- Gerges NZ, Backos DS, Esteban JA. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem 2004; 279:43870-8; PMID:15297461; https://doi.org/10.1074/jbc.M404982200
- Gerges NZ, Backos DS, Rupasinghe CN, Spaller MR, Esteban JA. Dual role of the exocyst in AMPA receptor targeting and insertion into the postsynaptic membrane. EMBO J 2006; 25:1623-34; PMID:16601687; https://doi.org/10.1038/sj.emboj.7601065
- Hattula K, Furuhjelm J, Arffman A, Peranen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 2002; 13:3268-80; PMID:12221131; https://doi.org/10.1091/mbc.E02-03-0143
- Hattula K, Peranen J. FIP-2, a coiled-coil protein, links huntingtin to Rab8 and modulates cellular morphogenesis. Curr Biol 2000; 10:1603-6; PMID:11137014; https://doi.org/10.1016/S0960-9822(00)00864-2
- del Toro D, Alberch J, Lazaro-Dieguez F, Martin-Ibanez R, Xifro X, Egea G, Canals JM. Mutant huntingtin impairs post-Golgi trafficking to lysosomes by delocalizing optineurin/Rab8 complex from the Golgi apparatus. Mol Biol Cell 2009; 20:1478-92; PMID:19144827; https://doi.org/10.1091/mbc.E08-07-0726
- Cooney JR, Hurlburt JL, Selig DK, Harris KM, Fiala JC. Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. J Neurosci 2002; 22:2215-24; PMID:11896161
- Brown TC, Tran IC, Backos DS, Esteban JA. NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron 2005; 45:81-94; PMID:15629704; https://doi.org/10.1016/j.neuron.2004.12.023
- Sziber Z, Liliom H, Morales CO, Ignacz A, Ratkai AE, Ellwanger K, Link G, Szűcs A, Hausser A, Schlett K. Ras and Rab interactor 1 controls neuronal plasticity by coordinating dendritic filopodial motility and AMPA receptor turnover. Mol Biol Cell 2017; 28:285-95; PMID:27852895; https://doi.org/10.1091/mbc.E16-07-0526
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, et al. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci 2009; 29:3206-19; PMID:19279258; https://doi.org/10.1523/JNEUROSCI.4514-08.2009
- Glodowski DR, Chen CC, Schaefer H, Grant BD, Rongo C. RAB-10 regulates glutamate receptor recycling in a cholesterol-dependent endocytosis pathway. Mol Biol Cell 2007; 18:4387-96; PMID:17761527; https://doi.org/10.1091/mbc.E07-05-0486
- Hoogenraad CC, Popa I, Futai K, Martinez-Sanchez E, Wulf PS, van Vlijmen T, Dortland BR, Oorschot V, Govers R, Monti M, et al. Neuron specific Rab4 effector GRASP-1 coordinates membrane specialization and maturation of recycling endosomes. PLoS Biol 2010; 8:e1000283; PMID:20098723; https://doi.org/10.1371/journal.pbio.1000283
- Hoogenraad CC, van der Sluijs P. GRASP-1 regulates endocytic receptor recycling and synaptic plasticity. Commun Integr Biol 2010; 3:433-5; PMID:21057633; https://doi.org/10.4161/cib.3.5.12209
- Esteves da, Silva M, Adrian M, Schatzle P, Lipka J, Watanabe T, Cho S, Futai K, Wierenga CJ, Kapitein LC, Hoogenraad CC. Positioning of AMPA receptor-containing endosomes regulates synapse architecture. Cell Rep 2015; 13:933-43; PMID:26565907; https://doi.org/10.1016/j.celrep.2015.09.062
- Lise MF, Wong TP, Trinh A, Hines RM, Liu L, Kang R, Hines DJ, Lu J, Goldenring JR, Wang YT, et al. Involvement of myosin Vb in glutamate receptor trafficking. J Biol Chem 2006; 281:3669-78; PMID:16338934; https://doi.org/10.1074/jbc.M511725200
- Wang Z, Edwards JG, Riley N, Provance DW, Jr., Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, et al. Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 2008; 135:535-48; PMID:18984164; https://doi.org/10.1016/j.cell.2008.09.057
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science 2004; 305:1972-5; PMID:15448273; https://doi.org/10.1126/science.1102026
- Liu W, Yuen EY, Yan Z. The stress hormone corticosterone increases synaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors via serum- and glucocorticoid-inducible kinase (SGK) regulation of the GDI-Rab4 complex. J Biol Chem 2010; 285:6101-8; PMID:20051515; https://doi.org/10.1074/jbc.M109.050229
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 2011; 16:156-70; PMID:20458323; https://doi.org/10.1038/mp.2010.50
- Fernandez-Monreal M, Brown TC, Royo M, Esteban JA. The balance between receptor recycling and trafficking toward lysosomes determines synaptic strength during long-term depression. J Neurosci 2012; 32:13200-5; PMID:22993436; https://doi.org/10.1523/JNEUROSCI.0061-12.2012
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci 2000; 3:1282-90; PMID:11100149; https://doi.org/10.1038/81814
- Zhang D, Dubey J, Koushika SP, Rongo C. RAB-6.1 and RAB-6.2 promote retrograde transport in C. elegans. PloS One 2016; 11:e0149314; PMID:26891225; https://doi.org/10.1371/journal.pone.0149314
- Zhang D, Isack NR, Glodowski DR, Liu J, Chen CC, Xu XZ, Grant BD, Rongo C. RAB-6.2 and the retromer regulate glutamate receptor recycling through a retrograde pathway. J Cell Biol 2012; 196:85-101; PMID:22213799; https://doi.org/10.1083/jcb.201104141
- Temkin P, Morishita W, Goswami D, Arendt K, Chen L, Malenka R. The retromer supports AMPA receptor trafficking during LTP. Neuron 2017; 94:74-82 e5; PMID:28384478; https://doi.org/10.1016/j.neuron.2017.03.020
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron 2009; 64:381-90; PMID:19914186; https://doi.org/10.1016/j.neuron.2009.08.035
- Esteban JA. Intracellular machinery for the transport of AMPA receptors. Br J Pharmacol 2008; 153(Suppl 1):S35-43; PMID:18026130; https://doi.org/10.1038/sj.bjp.0707525
- Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: Recent advances in AMPA receptor trafficking. Trends Neurosci 2011; 34:258-68; PMID:21420743; https://doi.org/10.1016/j.tins.2011.02.004
- Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: The last 25 years. Neuron 2013; 80:704-17; PMID:24183021; https://doi.org/10.1016/j.neuron.2013.10.025
- Brachet A, Norwood S, Brouwers JF, Palomer E, Helms JB, Dotti CG, Esteban JA. LTP-triggered cholesterol redistribution activates Cdc42 and drives AMPA receptor synaptic delivery. J Cell Biol 2015; 208:791-806; PMID:25753037; https://doi.org/10.1083/jcb.201407122
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron 2009; 63:92-105; PMID:19607795; https://doi.org/10.1016/j.neuron.2009.05.025
- Jaafari N, Henley JM, Hanley JG. PICK1 mediates transient synaptic expression of GluA2-lacking AMPA receptors during glycine-induced AMPA receptor trafficking. J Neurosci 2012; 32:11618-30; PMID:22915106; https://doi.org/10.1523/JNEUROSCI.5068-11.2012
- Yagishita S, Murayama M, Ebihara T, Maruyama K, Takashima A. Glycogen Synthase Kinase 3beta-mediated phosphorylation in the most C-terminal region of protein interacting with C Kinase 1 (PICK1) regulates the binding of PICK1 to glutamate receptor subunit GluA2. J Biol Chem 2015; 290:29438-48; PMID:26472923; https://doi.org/10.1074/jbc.M114.619668
- Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J Neurosci 2010; 30:16437-52; PMID:21147983; https://doi.org/10.1523/JNEUROSCI.4478-10.2010
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 2000; 28:511-25; PMID:11144360; https://doi.org/10.1016/S0896-6273(00)00129-X
- Zhong P, Liu W, Gu Z, Yan Z. Serotonin facilitates long-term depression induction in prefrontal cortex via p38 MAPK/Rab5-mediated enhancement of AMPA receptor internalization. J Physiol 2008; 586:4465-79; PMID:18653660; https://doi.org/10.1113/jphysiol.2008.155143
- Zhao WQ, Santini F, Breese R, Ross D, Zhang XD, Stone DJ, Ferrer M, Townsend M, Wolfe AL, Seager MA, et al. Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem 2010; 285:7619-32; PMID:20032460; https://doi.org/10.1074/jbc.M109.057182
- Bell JD, Park E, Ai J, Baker AJ. PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ 2009; 16:1665-80; PMID:19644508; https://doi.org/10.1038/cdd.2009.106
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med 2008; 14:837-42; PMID:18568035; https://doi.org/10.1038/nm1782
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: Differential effects of APOE genotype and presenilin mutations. Am J Pathol 2000; 157:277-86; PMID:10880397; https://doi.org/10.1016/S0002-9440(10)64538-5
- Ginsberg SD, Alldred MJ, Counts SE, Cataldo AM, Neve RL, Jiang Y, Wuu J, Chao MV, Mufson EJ, Nixon RA, et al. Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer's disease progression. Biol Psychiatry 2010; 68:885-93; PMID:20655510; https://doi.org/10.1016/j.biopsych.2010.05.030
- Kim S, Sato Y, Mohan PS, Peterhoff C, Pensalfini A, Rigoglioso A, Jiang Y, Nixon RA. Evidence that the rab5 effector APPL1 mediates APP-betaCTF-induced dysfunction of endosomes in down syndrome and Alzheimer's disease. Mol Psychiatry 2016; 21:707-16; PMID:26194181; https://doi.org/10.1038/mp.2015.97
- Sheerin UM, Schneider SA, Carr L, Deuschl G, Hopfner F, Stamelou M, Wood NW, Bhatia KP. ALS2 mutations: Juvenile amyotrophic lateral sclerosis and generalized dystonia. Neurology 2014; 82:1065-7; PMID:24562058; https://doi.org/10.1212/WNL.0000000000000254
- Meggouh F, Bienfait HM, Weterman MA, de Visser M, Baas F. Charcot-Marie-Tooth disease due to a de novo mutation of the RAB7 gene. Neurology 2006; 67:1476-8; PMID:17060578; https://doi.org/10.1212/01.wnl.0000240068.21499.f5
- Verhoeven K, De Jonghe P, Coen K, Verpoorten N, Auer-Grumbach M, Kwon JM, FitzPatrick D, Schmedding E, De Vriendt E, Jacobs A, et al. Mutations in the small GTP-ase late endosomal protein RAB7 cause Charcot-Marie-Tooth type 2B neuropathy. Am J Hum Genet 2003; 72:722-7; PMID:12545426; https://doi.org/10.1086/367847
- Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D'Elia E, Vecellio M, Russo S, Cogliati F, Larizza L, et al. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet 2010; 86:185-95; PMID:20159109; https://doi.org/10.1016/j.ajhg.2010.01.011
- Guldner M, Schulte C, Hauser AK, Gasser T, Brockmann K. Broad clinical phenotype in Parkinsonism associated with a base pair deletion in RAB39B and additional POLG variant. Parkinsonism Relat Disord 2016; 31:148-50; PMID:27448726; https://doi.org/10.1016/j.parkreldis.2016.07.005
- Lesage S, Bras J, Cormier-Dequaire F, Condroyer C, Nicolas A, Darwent L, Guerreiro R, Majounie E, Federoff M, Heutink P, et al. Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet 2015; 1:e9; PMID:27066548; https://doi.org/10.1212/NXG.0000000000000009
- Mata IF, Jang Y, Kim CH, Hanna DS, Dorschner MO, Samii A, Agarwal P, Roberts JW, Klepitskaya O, Shprecher DR, et al. The RAB39B p.G192R mutation causes X-linked dominant Parkinson's disease. Mol Neurodegener 2015; 10:50; PMID:26399558; https://doi.org/10.1186/s13024-015-0045-4
- Wilson GR, Sim JC, McLean C, Giannandrea M, Galea CA, Riseley JR, Stephenson SE, Fitzpatrick E, Haas SA, Pope K, et al. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with alpha-synuclein pathology. Am J Hum Genet 2014; 95:729-35; PMID:25434005; https://doi.org/10.1016/j.ajhg.2014.10.015
- Dhaka A, Costa RM, Hu H, Irvin DK, Patel A, Kornblum HI, Silva AJ, O'Dell TJ, Colicelli J. The RAS effector RIN1 modulates the formation of aversive memories. J Neurosci 2003; 23:748-57; PMID:12574403
- Deininger K, Eder M, Kramer ER, Zieglgansberger W, Dodt HU, Dornmair K, Colicelli J, Klein R. The Rab5 guanylate exchange factor Rin1 regulates endocytosis of the EphA4 receptor in mature excitatory neurons. Proc Natl Acad Sci U S A 2008; 105:12539-44; PMID:18723684; https://doi.org/10.1073/pnas.0801174105
- Bliss JM, Gray EE, Dhaka A, O'Dell TJ, Colicelli J. Fear learning and extinction are linked to neuronal plasticity through Rin1 signaling. J Neurosci Res 2010; 88:917-26; PMID:19830836; https://doi.org/10.1002/jnr.22252
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, et al. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A 2007; 104:20955-60; PMID:18165656; https://doi.org/10.1073/pnas.0710548105
- Migues PV, Liu L, Archbold GE, Einarsson EO, Wong J, Bonasia K, Ko SH, Wang YT, Hardt O. Blocking synaptic removal of GluA2-containing AMPA receptors prevents the natural forgetting of long-term memories. J Neurosci 2016; 36:3481-94; PMID:27013677; https://doi.org/10.1523/JNEUROSCI.3333-15.2016
- Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, Helm JR, Bissada N, Cruz-Aguado R, Davidson TL, et al. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proc Natl Acad Sci U S A 2006; 103:9595-600; PMID:16769894; https://doi.org/10.1073/pnas.0510197103
- Mori Y, Matsui T, Fukuda M. Rabex-5 protein regulates dendritic localization of small GTPase Rab17 and neurite morphogenesis in hippocampal neurons. J Biol Chem 2013; 288:9835-47; PMID:23430262; https://doi.org/10.1074/jbc.M112.427591
- Wu KY, He M, Hou QQ, Sheng AL, Yuan L, Liu F, Liu WW, Li G, Jiang XY, Luo ZG. Semaphorin 3A activates the guanosine triphosphatase Rab5 to promote growth cone collapse and organize callosal axon projections. Sci Signal 2014; 7:ra81; PMID:25161316; https://doi.org/10.1126/scisignal.2005334
- Homma Y, Fukuda M. Rabin8 regulates neurite outgrowth in both GEF activity-dependent and -independent manners. Mol Biol Cell 2016; 27:2107-18; PMID:27170183; https://doi.org/10.1091/mbc.E16-02-0091
- Ultanir SK, Hertz NT, Li G, Ge WP, Burlingame AL, Pleasure SJ, Shokat KM, Jan LY, Jan YN. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 Uncovers their roles in dendrite arborization and spine development. Neuron 2012; 73:1127-42; PMID:22445341; https://doi.org/10.1016/j.neuron.2012.01.019
- Sidjanin DJ, Park AK, Ronchetti A, Martins J, Jackson WT. TBC1D20 mediates autophagy as a key regulator of autophagosome maturation. Autophagy 2016; 12:1759-75; PMID:27487390; https://doi.org/10.1080/15548627.2016.1199300
- Hannemann M, Sasidharan N, Hegermann J, Kutscher LM, Koenig S, Eimer S. TBC-8, a putative RAB-2 GAP, regulates dense core vesicle maturation in Caenorhabditis elegans. PLoS Genet 2012; 8:e1002722; PMID:22654674; https://doi.org/10.1371/journal.pgen.1002722
- Sasidharan N, Sumakovic M, Hannemann M, Hegermann J, Liewald JF, Olendrowitz C, Koenig S, Grant BD, Rizzoli SO, Gottschalk A, et al. RAB-5 and RAB-10 cooperate to regulate neuropeptide release in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2012; 109:18944-9; PMID:23100538; https://doi.org/10.1073/pnas.1203306109
- Lee MJ, Jang S, Nahm M, Yoon JH, Lee S. Tbc1d15-17 regulates synaptic development at the Drosophila neuromuscular junction. Mol Cells 2013; 36:163-8; PMID:23812537; https://doi.org/10.1007/s10059-013-0147-1
- Uytterhoeven V, Kuenen S, Kasprowicz J, Miskiewicz K, Verstreken P. Loss of skywalker reveals synaptic endosomes as sorting stations for synaptic vesicle proteins. Cell 2011; 145:117-32; PMID:21458671; https://doi.org/10.1016/j.cell.2011.02.039
- Sumakovic M, Hegermann J, Luo L, Husson SJ, Schwarze K, Olendrowitz C, Schoofs L, Richmond J, Eimer S. UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans. J Cell Biol 2009; 186:897-914; PMID:19797081; https://doi.org/10.1083/jcb.200902096
- Wang L, Liang Z, Li G. Rab22 controls NGF signaling and neurite outgrowth in PC12 cells. Mol Biol Cell 2011; 22:3853-60; PMID:21849477; https://doi.org/10.1091/mbc.E11-03-0277
- Li JY. Rabphilin-3A is transported with fast anterograde axonal transport and associated with synaptic vesicles. Synapse 1996; 23:79-88; PMID:8723712; https://doi.org/10.1002/(SICI)1098-2396(199606)23:2<79::AID-SYN3>3.0.CO;2-D
- Pal A, Severin F, Lommer B, Shevchenko A, Zerial M. Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease. J Cell Biol 2006; 172:605-18; PMID:16476778; https://doi.org/10.1083/jcb.200509091
- Guo X, Farias GG, Mattera R, Bonifacino JS. Rab5 and its effector FHF contribute to neuronal polarity through dynein-dependent retrieval of somatodendritic proteins from the axon. Proc Natl Acad Sci U S A 2016; 113:E5318-27; PMID:27559088; https://doi.org/10.1073/pnas.1601844113
- Schlager MA, Kapitein LC, Grigoriev I, Burzynski GM, Wulf PS, Keijzer N, de Graaff E, Fukuda M, Shepherd IT, Akhmanova A, et al. Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis. EMBO J 2010; 29:1637-51; PMID:20360680; https://doi.org/10.1038/emboj.2010.51
- Seifert W, Kuhnisch J, Maritzen T, Lommatzsch S, Hennies HC, Bachmann S, Horn D, Haucke V. Cohen syndrome-associated protein COH1 physically and functionally interacts with the small GTPase RAB6 at the Golgi complex and directs neurite outgrowth. J Biol Chem 2015; 290:3349-58; PMID:25492866; https://doi.org/10.1074/jbc.M114.608174
- Deng CY, Lei WL, Xu XH, Ju XC, Liu Y, Luo ZG. JIP1 mediates anterograde transport of Rab10 cargos during neuronal polarization. J Neurosci 2014; 34:1710-23; PMID:24478353; https://doi.org/10.1523/JNEUROSCI.4496-13.2014
- Xu XH, Deng CY, Liu Y, He M, Peng J, Wang T, Yuan L, Zheng ZS, Blackshear PJ, Luo ZG. MARCKS regulates membrane targeting of Rab10 vesicles to promote axon development. Cell Res 2014; 24:576-94; PMID:24662485; https://doi.org/10.1038/cr.2014.33
- Lazo OM, Gonzalez A, Ascano M, Kuruvilla R, Couve A, Bronfman FC. BDNF regulates Rab11-mediated recycling endosome dynamics to induce dendritic branching. J Neurosci 2013; 33:6112-22; PMID:23554492; https://doi.org/10.1523/JNEUROSCI.4630-12.2013
- Song M, Giza J, Proenca CC, Jing D, Elliott M, Dincheva I, Shmelkov SV, Kim J, Schreiner R, Huang SH, et al. Slitrk5 mediates BDNF-dependent TrkB receptor trafficking and signaling. Dev Cell 2015; 33:690-702; PMID:26004511; https://doi.org/10.1016/j.devcel.2015.04.009
- Yazaki Y, Hara Y, Tamaki H, Fukaya M, Sakagami H. Endosomal localization of FIP3/Arfophilin-1 and its involvement in dendritic formation of mouse hippocampal neurons. Brain Res 2014; 1557:55-65; PMID:24576489; https://doi.org/10.1016/j.brainres.2014.02.018
- Eva R, Dassie E, Caswell PT, Dick G, ffrench-Constant C, Norman JC, Fawcett JW. Rab11 and its effector Rab coupling protein contribute to the trafficking of beta 1 integrins during axon growth in adult dorsal root ganglion neurons and PC12 cells. J Neurosci 2010; 30:11654-69; PMID:20810886; https://doi.org/10.1523/JNEUROSCI.2425-10.2010
- Stendel C, Roos A, Kleine H, Arnaud E, Ozcelik M, Sidiropoulos PN, Zenker J, Schüpfer F, Lehmann U, Sobota RM, et al. SH3TC2, a protein mutant in Charcot-Marie-Tooth neuropathy, links peripheral nerve myelination to endosomal recycling. Brain 2010; 133:2462-74; PMID:20826437; https://doi.org/10.1093/brain/awq168
- Fukuda M, Kanno E, Yamamoto A. Rabphilin and Noc2 are recruited to dense-core vesicles through specific interaction with Rab27A in PC12 cells. J Biol Chem 2004; 279:13065-75; PMID:14722103; https://doi.org/10.1074/jbc.M306812200
- Kobayashi H, Fukuda M. Rab35 establishes the EHD1-association site by coordinating two distinct effectors during neurite outgrowth. J Cell Sci 2013; 126:2424-35; PMID:23572513; https://doi.org/10.1242/jcs.117846
