ABSTRACT
Uncontrolled metastasis significantly contributes to high lethality of patients suffering from ovarian cancer. To date, the detailed molecular mechanisms which account for ovarian tumor cell spreading and metastasis remain largely unknown. In a recent study, we have demonstrated that aberrantly high expression of the non-receptor tyrosine kinase FER is responsible for ovarian tumor cell metastasis both in vitro and in vivo. Mechanistically, we indentified Hepatocyte Growth Factor Receptor HGFR/MET as a novel substrate of FER, and through which the kinase FER modulates ovarian cancer cell motility and invasiveness in a ligand-independent manner. We also observed aberrantly high expression of PAK1 kinase in cancer cells, and RNAi-mediated knockdown of FER kinase inactivated the RAC1-PAK1 signaling pathway and decreased metastatic potential of CAOV4 ovarian cancer cells. Overall, our study revealed a previously uncharacterized, pro-metastatic role of the kinase FER in ovarian cancer through the MET-RAC1-PAK1 pathway. Further efforts are essential to investigating beneficial outcomes towards targeting the RAC1-PAK1 signaling pathway in reducing metastatic burden of this deadly disease.
KEYWORDS:
Ovarian cancer is the leading cause of death resulting from gynecological malignancies, and ranks the fifth most frequent cause of cancer-related death for women.Citation1 This year in the United States alone, more than 25000 women will be diagnosed with ovarian cancer, and more than 16000 women will die of this disease. Five-year survival rates for women diagnosed with stage I or stage II ovarian cancer are 90% and 70%, respectively. Unfortunately, however, there is no reliable screening test for the early detection of this ‘silent killer’,as less than 35% of women are diagnosed before Stage III, with the five-year survival for Stage III or IV being less than 25%.Citation2 Therefore, improvement in treatment is an urgent need for this devastating disease.
One major impediment to successful treatment is the inability to detect ovarian cancer at an early stage, resulting in disease progression to an advanced stage with extensive metastasis. The unique feature of ovarian cancer metastasis where normal peritoneal fluid can be harnessed to transport exfoliated ovarian carcinoma cells throughout the peritoneal cavity freely to adjacent organs makes prognosis of ovarian cancer even worse.Citation3 It is almost impossible to render patients free of disease with surgery due to this dispersive feature. Any effort(s) to pinpoint the molecular basis for ovarian carcinoma dissemination and metastasis will provide key information to guide development of next-generation therapeutic interventions which can effectively improve progression-free survival after surgery.
For this purpose, we decided to apply both biochemical and biological approaches to elucidate the molecular mechanism that controls ovarian cancer cell metastasis. Interestingly, our preliminary results indicated aberrant increase in Tyr142 phosphorylation and nuclear distribution of β-Catenin in 11 ovarian carcinoma-derived cell lines compared to two human ovarian surface epithelial (HOSE) cell lines.Citation4 Three tyrosine kinases have been reported to be responsible for this phosphorylation-induced nuclear translocation of β-Catenin, including MET,Citation5 FYNCitation6 and FER.Citation7 Compared to the controls, only FER was significantly up-regulated in all 11 ovarian cancer cell lines examined, and this elevation was also confirmed by immunohistochemical staining of ovarian tumor samples.Citation4 Unexpectedly, neither Tyr142 phosphorylation of β-Catenin, nor transactivation of β-Catenin regulated genes including tcf-1 and lef-1 was decreased in FER knockdown cells, potentially due to compensation from other tyrosine kinase(s). However, this up-regulation of FER was very critical to cell motility, since shRNA knockdown robustly decreased migration ability of five different high-grade serous ovarian cancer cell lines.Citation4 This was also true in ovarian cancer cell invasion assays. The impaired migration and invasion ability upon FER loss was not due to a proliferative rate decrease, since no overt change in cell growth was noticed in Ki-67 staining.Citation4
To further investigate the role of FER kinase in ovarian cancer cell migration and invasion in vivo, we employed two mouse models; one sub-cutaneous injection model in which cell movement is blood-dependent, the other, an intraperitoneal injection model in which cell movement is blood-independent.Citation4 Consistent with our previous cell-based assays, loss of FER showed no effect on subcutaneous tumor growth, however, the ability of ovarian cancer cell metastasis to the lung was significantly decreased in the absence of FER. In line with the conclusion from this model, our blood-independent, intraperitoneal injection model also demonstrated that ovarian cancer cells with diminished levels of FER displayed a profound reduction in their ability to metastasize to surrounding organs/tissues, including peritoneal wall, diaphragm, omentum, mesentery, ovary, stomach and liver. Collectively, this evidence clearly suggested an essential role of FER kinase in controlling ovarian cancer cell metastasis.
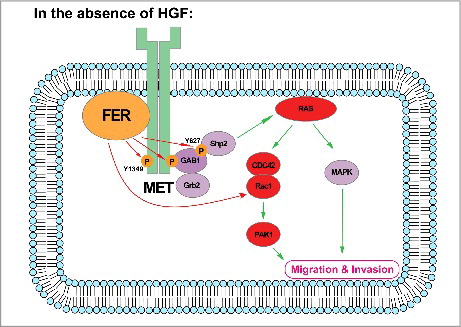
What's the molecular mechanism adopted by FER to modulate ovarian cancer cell motility and invasiveness? To answer this question, we applied global tyrosine phosphorylation comparison between cells with and without FER.Citation4 To our surprise, results from a series of biochemical analyses confirmed hepatocyte growth factor receptor, HGFR/MET, as a novel substrate of FER, and its function is key to FER-mediated cell migration and invasion. Furthermore, we demonstrated that FER phosphorylated a signaling relay site on MET, Tyr1349. This promoted a kinase-independent scaffolding function of MET to recruit GAB1. Upon recruitment, FER can further phosphorylate GAB1 at Tyr627, a key motif for SHP2 binding, and activate the downstream SHP2-ERK signaling pathway.
It has been well-characterized that both MET and GAB1, within the HGF-MET pathway, play an indispensible role in cell migration, invasion and metastasis. Particularly, the bidentate docking site of Tyr1349&1356 in MET is important to the functional role of MET in metastasis. Experimental mutation of these sites profoundly prevents metastasis induced by TPR-MET, the constitutively active form of MET, in vivo in mice.Citation8 Meanwhile, loss-of-function analysis using both GAB1-null fibroblasts and GAB1 RNAi-mediated knockdown in tumor cells also demonstrated its necessity in MET-mediated invadopodia formation and cell invasion.Citation9 Our results demonstrated that a non-receptor tyrosine kinase could harness molecular components from this signaling pathway to modulate cancer cell metastasis in a ligand-independent manner. Importantly, we also illustrated that the output signaling from this alternative regulation was comparable to those from ligand-dependent regulation, further highlighting the physiological significance of this new regulation. Lastly, although potent and effective inhibitors of the receptor protein tyrosine kinase MET are available, many HGF-MET antagonists fail to abolish downstream signal propagation.Citation10–Citation13 We believe this novel ‘ligand- and autophosphorylation-independent activation of MET’ model could shed some light on this conundrum and potentially guide future improvement of related therapy.
In addition, accumulating evidence suggests that RAS-MAPK and RAC1 signaling, downstream of the receptor tyrosine kinase MET and GAB1, are important in the early steps of metastasis.Citation14 There are two GTPase-involved signaling pathways downstream of MET and GAB1; the RAS-RAC1-PAK pathway and the RAP1-FAK pathway. RAC1, along with RAC2, RAC3 and RhoG, form a Rac subfamily within the Rho family of GTPases.Citation15 Rac proteins stimulate lamellipodium and membrane ruffle formation, and induce membrane extension.Citation16 It has been shown that in T cells, dominant-negative RAC1 inhibits chemokine-induced adhesion to integrin ligands.Citation17 Alternatively, RAP1 is a member of the RAS superfamily of small GTPases, whose function has been implicated in a variety of integrin-mediated ‘inside-out’ signaling events.Citation18 Signals through the RAC1-PAK and RAP1-FAK pathways propagate to the cell membrane and modulate cadherin and integin adhesion molecules and thereby impact cell migration.Citation14 Consistently, we observed an active form of RAC1 in ovarian cancer cells, and this activation was compromised in the absence of FER.Citation4 On the contrary, by using FAK as a downstream effector of RAP1, we did not observe any change in FAK phosphorylation and activation upon FER loss in ovarian cancer cells, indicating RAC1 is the major GTPase downstream of the MET-GAB1 pathway that regulates ovarian cancer cell metastasis.
The GTPase RAC1 regulates cell motility directly through the Ser/Thr kinase PAK (p21-activated kinase). Interestingly, PAK was initially identified as a binding partner of RAC1 and CDC42, and this binding is important for kinase activation.Citation19,20 Biochemically, RAC1 interacts with the PBD (p21-binding domain) of PAK, and this association releases the PBD from the kinase domain thereby activating the kinase.Citation20 Active PAK can further phosphorylate LIM kinase (LIMK), which in-turn phosphorylates and inhibits cofilin, thus regulating actin dynamics and cell motility.Citation15 In our study, we observed robustly elevated expression of PAK1, but not PAK2 or PAK4, in most ovarian cancer-derived cells compared to both normal HOSE controls.Citation4 We could not detect any expression of PAK3 in the same cell extracts, probably due to its restricted expression within dendritic cells.Citation21 Furthermore, loss of FER led to inactivation of PAK1, illustrated by the decreased phosphorylation of its activating site Ser1444. These results are consistent with the reduced activation of RAC1 we observed in FER-deficient ovarian cancer cells, and highlight the importance of the RAC1-PAK1 signaling pathway in regulating ovarian cancer cell motility.
Over-expression and hyper-activation of PAK1 has been reported in many malignancies, including breast, colon and ovarian cancer.Citation21,22 The gene loci of PAK1, which resides within region 11q13, is frequently amplified, particularly in ovarian cancer.Citation23 Importantly, this chromosomal amplification is associated with poor prognosis in patients suffering from ovarian and breast cancers.Citation24,25 In addition to migration and invasion, cell survival can be modulated through the activity of PAK1 over the pro-apoptotic protein BAD.Citation26 Activation of PAK1 has also been identified as a component of the DNA damage response, indicating its function in cellular sensitivity to ionizing radiation.Citation27 Recent work from Chernoff's group demonstrates that PAK1-amplified ovarian cancer cells are significantly more sensitive to genetic and pharmacologic inhibition of PAK1, implying PAK1 amplification could serve as a potential patient selection criterion for PAK1-targeted therapy.Citation22 Consistent with our current study, we also found aberrantly high expression of PAK1 in the majority of ovarian cancer cell lines we tested, and furthermore, that the FER-mediated MET-GAB1 signaling axis is important for activation of PAK14. Together, this evidence provides insight for future molecular-targeted therapies in ovarian cancer, and offers the potential for exploring combinatorial therapeutic avenues.
The fact that we suggest FER could impact GTPase activity of RAC1 in an indirect, MET-GAB1-dependent model doesn't necessarily mean this regulation couldn't be direct. A study from the Heisterkamp group illustrated that FER could phosphorylate RhoGDIα (Rho GDP-Dissociation Inhibitor α), and this tyrosine phosphorylation prevents subsequent binding of RAC to RhoGDIα.Citation28 Over-expression of FER also correlated with enhanced tyrosine phosphorylation and activation of Vav2,Citation29 a RAC guanine exchange factor (GEF). Work from the Craig group further demonstrated the residue on Vav2 that undergoes FER regulation is Tyr172.Citation30 We are actively investigating whether or not FER could modulate RAC1 activity in ovarian cancer through a direct manner. With the development of phospho-tyrosine antibodies against RhoGDIα, Vav2 and FER, we could apply immunohistochemical staining to those xenograft tumor samples (in the presence/absence of FER) previously collected. Further investigation into these regulatory modules in ovarian tumor microarray samples will establish subtype(s) of ovarian cancer which are subject to this direct regulation. Efforts from these studies will definitely enhance our understanding of the important role that the tyrosine kinase FER plays in ovarian tumor maintenance, progression and metastasis, and shed light on better treatment regimes for ovarian cancer patients ().
Figure 1. Working model: In the absence of the ligand HGF, the receptor HGFR/MET serves as a scaffold protein on plasma membrane. Non-receptor tyrosine kinase FER binds to phospholipids through its F-BAR domain. Meanwhile, the kinase also directly interacts with and phosphorylates MET on Tyr1349, and this phosphorylation equips the receptor with the ability to recruit GAB1. Upon recruitment, GAB1 could be further phosphorylated by FER on Tyr627, a key motif for SHP2 binding. The signaling relay eventually leads to activation of the RAS-MAPK pathway, as well as the CDC42/RAC1-PAK1 pathway, both of which are important to modulate cell motility and invasiveness. Evidence also suggest FER could regulate RAC1 in a direct manner.
Up-regulation and activation of FER has been reported in many malignancies, including lung,Citation31 hepatic,Citation32 prostate,Citation33 breastCitation34 and ovarian cancer.Citation35 Furthermore, the oncogenic function of FER in controlling cell motility, invasion, suppression of apoptosis, and drug resistanceCitation36,37 have been well-characterized. Our recent work has also made important fundamental discoveries that raise several testable and translational questions for the near future, including simultaneous targeting FER and MET in ovarian cancer, the resolution of which may ultimately justify the development of appropriate FER inhibitors.
Acknowledgements
We thank Dr. Christopher Bonham (CSHL) for proofreading the manuscript. This work was supported by ShanghaiTech University Startup grant (F-0202-17-041) to G.F.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi:10.3322/caac.21254. PMID:25559415.
- Bast RC Jr, Hennessy B, Mills GB. The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer. 2009;9:415–28. doi:10.1038/nrc2644. PMID:19461667.
- Longuespee R, Boyon C, Desmons A, Vinatier D, Leblanc E, Farre I, Wisztorski M, Ly K, D'Anjou F, Day R, et al. Ovarian cancer molecular pathology. Cancer Metastasis Rev. 2012;31:713–32. doi:10.1007/s10555-012-9383-7. PMID:22729278.
- Fan G, Zhang S, Gao Y, Greer PA, Tonks NK. HGF-independent regulation of MET and GAB1 by nonreceptor tyrosine kinase FER potentiates metastasis in ovarian cancer. Genes Dev. 2016;30:1542–57. doi:10.1101/gad.284166.116. PMID:27401557.
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 2004;18:2225–30. doi:10.1101/gad.317604. PMID:15371335.
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Duñach M. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin Interaction. Mol Cell Biol. 2003;23:2287–97. doi:10.1128/MCB.23.7.2287-2297.2003. PMID:12640114.
- Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, Balsamo J. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J Cell Sci. 2004;117:3207–19. doi:10.1242/jcs.01174. PMID:15226396.
- Giordano S, Bardelli A, Zhen Z, Menard S, Ponzetto C, Comoglio PM. A point mutation in the MET oncogene abrogates metastasis without affecting transformation. Proc Natl Acad Sci U S A. 1997;94:13868–72. doi:10.1073/pnas.94.25.13868. PMID:9391119.
- Rajadurai CV, Havrylov S, Zaoui K, Vaillancourt R, Stuible M, Naujokas M, Zuo D, Tremblay ML, Park M. Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. J Cell Sci. 2012;125:2940–53. doi:10.1242/jcs.100834. PMID:22366451.
- Gordon MS, Sweeney CS, Mendelson DS, Eckhardt SG, Anderson A, Beaupre DM, Branstetter D, Burgess TL, Coxon A, Deng H, et al. Safety, pharmacokinetics, and pharmacodynamics of AMG 102, a fully human hepatocyte growth factor-neutralizing monoclonal antibody, in a first-in-human study of patients with advanced solid tumors. Clin Cancer Res. 2010;16:699–710. doi:10.1158/1078-0432.CCR-09-1365. PMID:20068101.
- Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37–45. doi:10.1016/j.molmed.2009.11.005. PMID:20031486.
- Cepero V, Sierra JR, Corso S, Ghiso E, Casorzo L, Perera T, Comoglio PM, Giordano S. MET and KRAS gene amplification mediates acquired resistance to MET tyrosine kinase inhibitors. Cancer Res. 2010;70:7580–90. doi:10.1158/0008-5472.CAN-10-0436. PMID:20841479.
- Petti C, Picco G, Martelli ML, Trisolini E, Bucci E, Perera T, Isella C, Medico E. Truncated RAF kinases drive resistance to MET inhibition in MET-addicted cancer cells. Oncotarget. 2015;6:221–33. doi:10.18632/oncotarget.2771. PMID:25473895.
- Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89–103. doi:10.1038/nrc3205. PMID:22270953.
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi:10.1038/nrm2476. PMID:18719708.
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi:10.1146/annurev.cellbio.21.020604.150721. PMID:16212495.
- Garcia-Bernal D, Wright N, Sotillo-Mallo E, Nombela-Arrieta C, Stein JV, Bustelo XR, Teixidó J. Vav1 and Rac control chemokine-promoted T lymphocyte adhesion mediated by the integrin alpha4beta1. Mol Biol Cell. 2005;16:3223–35. doi:10.1091/mbc.E04-12-1049. PMID:15872091.
- Boettner B, Van Aelst L. Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol. 2009;21:684–93. doi:10.1016/j.ceb.2009.06.004. PMID:19615876.
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–6. doi:10.1038/367040a0. PMID:8107774.
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–97. doi:10.1016/S0092-8674(00)00043-X. PMID:10975528.
- Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene. 2009;28:2545–55. doi:10.1038/onc.2009.119. PMID:19465939.
- Prudnikova TY, Villamar-Cruz O, Rawat SJ, Cai KQ, Chernoff J. Effects of p21-activated kinase 1 inhibition on 11q13-amplified ovarian cancer cells. Oncogene. 2016;35:2178–85. doi:10.1038/onc.2015.278. PMID:26257058.
- Shrestha Y, Schafer EJ, Boehm JS, Thomas SR, He F, Du J, et al. PAK1 is a breast cancer oncogene that coordinately activates MAPK and MET signaling. Oncogene 2012;31:3397–408.
- Brown LA, Kalloger SE, Miller MA, Shih Ie M, McKinney SE, Santos JL, Swenerton K, Spellman PT, Gray J, Gilks CB, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008;47:481–9. doi:10.1002/gcc.20549. PMID:18314909.
- Lundgren K, Holm K, Nordenskjold B, Borg A, Landberg G. Gene products of chromosome 11q and their association with CCND1 gene amplification and tamoxifen resistance in premenopausal breast cancer. Breast Cancer Res. 2008;10:R81. doi:10.1186/bcr2150. PMID:18823530.
- Schurmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, Bokoch GM. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol. 2000;20:453–61. doi:10.1128/MCB.20.2.453-461.2000. PMID:10611223.
- Motwani M, Li DQ, Horvath A, Kumar R. Identification of novel gene targets and functions of p21-activated kinase 1 during DNA damage by gene expression profiling. PloS One. 2013;8:e66585. doi:10.1371/journal.pone.0066585. PMID:23950862.
- Fei F, Kweon SM, Haataja L, De Sepulveda P, Groffen J, Heisterkamp N. The Fer tyrosine kinase regulates interactions of Rho GDP-Dissociation Inhibitor alpha with the small GTPase Rac. BMC Biochem. 2010;11:48. doi:10.1186/1471-2091-11-48. PMID:21122136.
- Itoh T, Hasegawa J, Tsujita K, Kanaho Y, Takenawa T. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci Signal. 2009;2:ra52. doi:10.1126/scisignal.2000393. PMID:19738202.
- Ahn J, Truesdell P, Meens J, Kadish C, Yang X, Boag AH, Craig AW. Fer protein-tyrosine kinase promotes lung adenocarcinoma cell invasion and tumor metastasis. Mol Cancer Res. 2013;11:952–63. doi:10.1158/1541-7786.MCR-13-0003-T. PMID:23699534.
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–203. doi:10.1016/j.cell.2007.11.025. PMID:18083107.
- Li H, Ren Z, Kang X, Zhang L, Li X, Wang Y, Xue T, Shen Y, Liu Y. Identification of tyrosine-phosphorylated proteins associated with metastasis and functional analysis of FER in human hepatocellular carcinoma cells. BMC Cancer. 2009;9:366. doi:10.1186/1471-2407-9-366. PMID:19835603.
- Zoubeidi A, Rocha J, Zouanat FZ, Hamel L, Scarlata E, Aprikian AG, Chevalier S. The Fer tyrosine kinase cooperates with interleukin-6 to activate signal transducer and activator of transcription 3 and promote human prostate cancer cell growth. Mol Cancer Res. 2009;7:142–55. doi:10.1158/1541-7786.MCR-08-0117. PMID:19147545.
- Albeck JG, Brugge JS. Uncovering a tumor suppressor for triple-negative breast cancers. Cell. 2011;144:638–40. doi:10.1016/j.cell.2011.02.030. PMID:21376226.
- Ren H, Tan ZP, Zhu X, Crosby K, Haack H, Ren JM, Beausoleil S, Moritz A, Innocenti G, Rush J, et al. Identification of anaplastic lymphoma kinase as a potential therapeutic target in ovarian cancer. Cancer Res. 2012;72:3312–23. doi:10.1158/0008-5472.CAN-11-3931. PMID:22570254.
- Greer P. Closing in on the biological functions of Fps/Fes and Fer. Nat Rev Mol Cell biol. 2002;3:278–89. doi:10.1038/nrm783. PMID:11994747.
- Craig AW. FES/FER kinase signaling in hematopoietic cells and leukemias. Front Biosci (Landmark Ed). 2012;17:861–75. doi:10.2741/3961. PMID:22201778.
