ABSTRACT
GTP-ases of the Rab family (about 70 in human) are key regulators of intracellular transport and membrane trafficking in eukaryotic cells. Remarkably, almost one third associate with membranes of the Golgi complex and TGN (trans-Golgi network). Through interactions with a variety of effectors that include molecular motors, tethering complexes, scaffolding proteins and lipid kinases, they play an important role in maintaining Golgi architecture.
Introduction
The Golgi complex is at the crossroad between the biosynthetic/secretory pathway and the endocytic pathway. Although it adopts an apparently stable organization (Golgi stacks) at the steady state, it is a highly dynamic organelle. For instance, Golgi resident enzymes have been proposed to recycle continuously to pre-Golgi compartments and the endoplasmic reticulum (ER), although the extent of this recycling is still a matter of debate.Citation75,83 Late Golgi and TGN membranes are, at least in part, consumed to produce post-Golgi transport carriers. The morphology of the Golgi at steady state thus relies on a tightly regulated balance between membranes arriving or leaving at its cis-side and membranes arriving or leaving at its trans-side.
GTPases of the Rab family (about 70 members in human) are master regulators of vesicular transport and membrane trafficking in eukaryotic cells. By interacting with a wide variety of effectors, that include molecular motors, tethering complexes, scaffolding proteins and lipid kinases, they regulate virtually all transport steps of vesicular traffic between cell compartments, from the biogenesis of transport carriers to their movement along the cytoskeleton and their tethering with target membranes (for reviews, seeCitation34,82). Remarkably, about one third of known human Rabs have been found associated with membranes at the ER/Golgi/TGN level (for review, see.Citation42 Since they regulate in and out as well as intra-Golgi trafficking, Rab proteins are expected to have a major role in Golgi architecture and maintenance, and functional alterations of several Rabs and Rab-interacting proteins, in particular Rab effectors, have been shown to disrupt Golgi morphology (see and ).
Figure 1. Illustrative shared and distinguishing traits of Golgi important Rab proteins. Rab proteins share a GTPase cycle between nucleotide-bound, membreane associated and –free states and functional amino acid motifs colored as indicated in (A). However, activated Rab proteins recruit distinct effectors to regulate their respective pathways (B). As indicated in (C). Rab proteins have distinct locations with respect to the Golgi complex and vary in abundance as quantified for purified cell fractions.Citation8,10,13,15,17,19,21,22,28,30,39,40,41,54,56,57,58,68,76,79,86,90,93,94
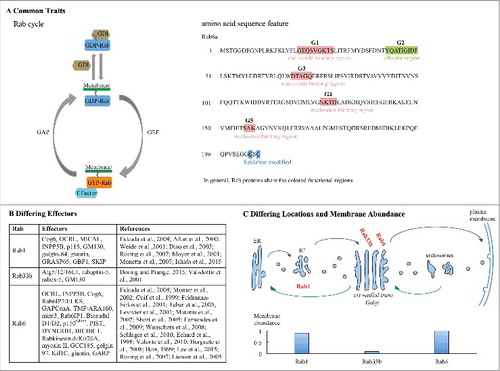
Table 1. Effect of altered rab expression on golgi ribbon organization.
Several excellent reviews have recently addressed the role of Rab GTP-ases in Golgi organization and function.Citation44,64,73 Our goal here is to summarize and discuss the recent literature on this topic. We will distinguish three groups of Rabs, those associated to the cis-side of the Golgi complex/intermediate compartment (IC), those associated towards the late Golgi/TGN/post-Golgi/endosome and secretory vesicles, and those primarily associated with Golgi membranes (“bona fide” Golgi Rabs).
- Pre-Golgi and cis-Golgi Rabs (Rab1, Rab2, Rab18, Rab41/Rab6d, Rab43)
The best known Rab that regulates pre-Golgi membrane trafficking events is Rab1 (Ypt1 in yeast S. cerevisiae). The Rab1 family comprises two isoforms with a high degree of sequence similarity (∼92 and 86%, respectively), Rab1a and Rab1b. Rab1a and Rab1b have long been thought to fulfill redundant functions but a recent study suggests that it may not be the case as Golgi fragmentation induced by Rab1a siRNA cannot be rescued by overexpression of Rab1b.Citation1
Rab1b was originally found associated with ER and Golgi.Citation66 Later studies showed that Rab1a and Rab1b are predominantly associated with membranes of the so-called IC, an ERGIC53/p58 positive, network of pleomorphic membranes located between ER exit sites (ERES) and Golgi.Citation72 Whether IC is a transient and motile compartment that gives rise to cis-Golgi cisternae or whether it is a stable compartment connected by bi-directional vesicular traffic with ER and Golgi is still a matter of debate. Depending on the model, the functions of Rab1 could be slightly different: in the biogenesis of ER-derived COPII vesicles and the tethering of COPII vesicles and/or COPII positive IC membranes with cis-Golgi,Citation2 or primarily in the formation of membrane domains on the IC and of a specialized IC sub-compartment next to the centrosome called pcIC (for review, seeCitation73).
As with Ypt1p in yeast,Citation37 Rab1, at least Rab1b, appears also to be involved in COPI-dependent retrograde transport between Golgi and ER.Citation4 Rab1 function in this pathway could rely on its interaction with GBF1, the exchange factor for Arf1 involved in the biogenesis of COPI vesicles.Citation4 Finally, Rab1 has also been shown to be involved in intra-Golgi transportCitation66 but this function remains poorly characterized, in Golgi bypass pathways,Citation71 in transport of melanosomes,Citation35 and recently in actin-based remodeling of ER and Golgi membranes through its interaction with WHAMM.Citation69
The pivotal role of Rab1 in bi-directional transport between ER and Golgi explain why alteration of Rab1 function(s) has a strong effect on Golgi morphology. The microinjection of GDP-bound form of Rab1a (S25N) or a mutant defective in guanine nucleotide binding (N124I) causes Golgi disassembly.Citation96 Similar effect is observed by overexpressing the dominant-negative forms of Rab1a and Rab1bCitation61,66,88 or following Rab1 depletion by siRNA.Citation5 Golgi collapse is also observed by overexpressing TBC1D20, a Rab1 GAP in vivo.Citation32 However, Golgi collapse following inhibition of Rab1 function is stronger than that observed after the inhibition of the other players of the ER to Golgi transport pathways such as Sar1 or the tethering factor p115, a Rab1 effector.Citation2,32 This is likely because Rab1 is not solely involved in ER to Golgi anterograde pathway (see above). The overexpression of Rab1b was also shown to cause Golgi enlargement.Citation67 Interestingly, this enlargement is likely due to increased expression of genes encoding proteins involved in membrane transport or Golgi structure, such as GM130, GRASP65, and GRASP55.Citation67
The role of Rab1 in the maintenance of Golgi architecture has been recently confirmed by two high-content siRNAs-based screens. In HeLa cells, depletion of Rab1a and Rab1b causes, similar to that of Rab2 (see below), a strong fragmentation of the Golgi complex.Citation25 Similar results were obtained in HeLa-S3 cells by Aizawa and Fukuda.Citation1
Another important player in pre-Golgi trafficking is Rab2. Rab2 family comprises two isoforms, Rab2a and Rab2b. As for Rab1, a recent study showed that Rab2a and Rab2b may have non-redundant role in Golgi morphology, which is supported by the identification of Rab2b-specific effector, GARI-L4, involved in Golgi compaction.Citation1
Although the precise localization of Rab2 has been less investigated than that of Rab1, the two proteins appear to mainly associate with similar compartments at steady state, i.e the IC and cis-Golgi membranes (for review, seeCitation73). Rab2 was originally found to work, like Rab1, in the anterograde transport pathway between ER and Golgi.Citation88 However, later work suggested that Rab2 could primarily function in the opposite direction (Golgi to ER). In support of this hypothesis, Rab2 was shown to promote the formation of retrograde COPI vesicles, in conjunction with two of its known effectors, an atypical kinase C iota/lambda (aPKC) and glyceraldehyde-3 phosphate dehydrogenase (GAPDH).Citation89
Evidence also exists that Rab2 plays additional roles at the Golgi level. Active Rab2 (GTP-bound) was shown to directly interact with golgin-45 and both proteins form a complex with GRASP55.Citation78 Rab2 also interacts with other Golgins, including GCC185 and GMAP-210.Citation33,80 Golgins are long coiled-coil proteins that specifically localize to cis, medial and trans Golgi membranes. An elegant recent study by Wong and MunroCitation97 showed that Golgins can, like tentacles, capture in vivo transport vesicles from different origins (ER or endosomes-derived as well as intra-Golgi vesicles) and thus act as tethers of transport vesicles with Golgi membranes. In addition to Rab2, Golgins bind several other Rabs (see below), mostly through their coiled-coil regions, but the significance of Rab binding remains unclear. Concerning Rab2, it was shown that GMAP-210 recruits transport vesicles via its N-terminus ALPS domain and that Rab2 binding occurs in the central coiled-coil region of GMAP-210 and downstream of vesicle tethering.Citation74 A tentative hypothesis is that Rab2 binding promotes a conformational change of GMAP-210, allowing the Rab2 positive transport vesicles to get closer to the Golgi membranes. Flexibility has been recently documented for another Golgin, GCC185.Citation11
In contrast to Rab1, the role of Rab2 in Golgi architecture has not been extensively investigated. However, the overexpression of the Rab2 GAP, TBC1D20, induces the redistribution of Golgi-resident enzymes to the ER.Citation32 Depletion of golgin-45 disrupts the Golgi apparatusCitation78 and GMAP-210 is required for Golgi assembly.Citation74 Finally, Rab2 depletion by siRNAs was found to strongly disrupt the Golgi in the two high-content siRNAs-based screens mentioned above.
Three other proteins, Rab18, Rab43 and Rab41/Rab6d, have also been implicated in pre-Golgi trafficking events. Rab43 was found associated with several membranes (ER/Golgi in;Citation14 medial Golgi in;Citation12 TGN in.Citation23 Several regulatory functions have thus been attributed to Rab43, including ER to Golgi anterograde trafficking, perhaps through an interaction with the dynein/dynactin complex,Citation14 retrograde transport between endosomes and TGN (using Shiga toxin as a marker of this pathway)Citation23 and anterograde trafficking of a subset of membrane cargo through the medial Golgi.Citation12
Whatever at which steps of transport Rab43 exactly plays a role, the alteration of its function has strong effects on Golgi morphology. The overexpression of the dominant-negative mutant of Rab43 (GFP-Rab43-T32N) results in the redistribution of various Golgi/TGN markers, including GM130, Mannosidase II and TGN46 into scattered punctae colocalizing with ERES, a phenotype reminiscent of that obtained upon disruption of microtubules with nocodazole.Citation14 High overexpression of GFP-Rab43 causes the fragmentation of ManII and giantin-positive compartments. Fragmentation of GM-130 and TGN46 positive compartments was also observed in cells overexpressing RN-tre, proposed to act as a GAP (GTP-ase activating protein) for Rab43 in vivo.Citation32
Less information is available on Rab18 and its exact function(s) remain(s) poorly characterized. Rab18 seems to be predominantly associated with ER membranes,Citation14,26,47 and to lipid droplets.Citation63
Overexpression of GFP-Rab18 or the activated mutant Rab18 Q67L (but not GFP-Rab18S22N) causes a redistribution of Golgi markers near ER-exit sites (ERES) and Rab18 depletion fragments the Golgi.Citation14 These findings support a role for Rab18 in COPI-independent Golgi to ER transport.Citation14 Of note, the other functions attributed to Rab18 are regulation of lipid transport and ER architecture.Citation26,47
Rab41, also termed Rab6d,Citation43 shares 60% similarity with other members of the Rab6 family (80% in its central region). Depletion of Rab41 by siRNA or the overexpression of the GDP-bound forms inhibit transport of VSV-G between ER and Golgi without having an effect on retrograde transport between endosomes and Golgi.Citation43 Both treatments have a profound effect on the organization of the Golgi ribbon, which is fragmented into a cluster of punctate elements. This suggests a direct role of Rab41 in Golgi architecture. Recently, three Rab41 effectors were identified (dynactin 6, syntaxin 8, and Kif18A), and depletion of two of them (dynactin 6 and syntaxin 8) give a phenotype similar to Rab41 knock-down, suggesting that they are involved in Golgi organization.Citation45
- Late Golgi/TGN and post-Golgi Rabs (Rab3, Rab7b, Rab8, Rab9a/b, Rab10, Rab11a, Rab13, Rab14, Rab21, Rab22b/Rab31, Rab29, Rab39a)
Many Rabs have been associated to late Golgi/TGN compartments. They include Rab7b, Rab8, Rab9a/b, Rab10, Rab11a, Rab13, Rab14, Rab29, Rab31/Rab22b, and Rab39a. However, these Rabs localize primarily to endosomal compartments (although their steady state localization may vary from one cell type to another), reflecting the dynamic nature of the interface between late Golgi/TGN membranes and endosomes.
Members of the Rab6 family also localize to late Golgi/TGN membranes and to post-Golgi vesicles. However, Rab6 does not associate with endosomal membranes and regulates intra-Golgi and Golgi to ER trafficking. Rab6 will be thus considered as a “bona fide” Golgi Rab (see next paragraph).
The Rab GTPases mentioned above have been implicated in many transport pathways that have been reviewed elsewhere.Citation9,16,55,60,82 In most cases, the depletion or the alteration of the function (s) of these GTPases have no effect or much less pronounced effect on Golgi morphology than of those located at the ER-Golgi interface. This suggests that the regulation of membrane flux at the late Golgi/TGN-endosome interface is not playing a major role in the regulation of Golgi morphology. Alteration of Golgi morphology (Golgi fragmentation) have been however observed following Rab8a depletionCitation1 but the significance of this result remains unclear.
A role for Rab22b/Rab31 and Rab29 in TGN morphology have also been documented.Citation59,92 However, the functions of these GTP-ases are very poorly known and how they regulate TGN morphology remain to be investigated.
Of note, it was recently shown, unexpectedly, that depletion of members of the Rab3 family (Rab3a-d) and that of Rab21, a protein that associates with early endosomes, affects Golgi morphology.Citation25 The possible reasons for such an effect are discussed in this article.
- “Bona fide” Golgi Rabs (Rab6, Rab19, Rab30, Rab33b, Rab34, Rab36, Rab39b)
Several Rab proteins localize almost exclusively to the Golgi and might well be considered to be « bona fide » Golgi Rabs. They include Rab19, Rab30, Rab33b, Rab34, Rab36, and Rab39b. Rab34 and Rab36 interact with the Rab7a effector RILP and regulate the spatial distribution of late endosomes, lysosomes and melanosomes.Citation51 Rab39b is preferentially expressed in brain and mutations in the RAB39b gene are associated with neuronal diseasesCitation27. Rab39b was recently shown to regulate ER to Golgi trafficking of the GluA2 receptor.Citation52 Very little is known about Rab19 and Rab30 functions. To our knowledge, the contribution of Rab19, Rab30, Rab34, Rab36 and Rab39b to Golgi architecture has not been investigated, except for Rab30 whose depletion impacts Golgi morphology.Citation38 Of note, Rab19 and Rab30 interact with Golgins.Citation80
The Rab33 family comprises two isoforms, the b isoform being ubiquitously expressed at relatively low levelsCitation28 while the a isoform is only expressed in brain and cells of the immune system. Rab33b localizes to the medial Golgi cisternae.Citation101 Rab33b has been implicated in Golgi-to-ER retrograde transportCitation36,90 but also in autophagy as it directly interacts with Atg16L.Citation24
The overexpression of wild-type Rab33b or its GTP-locked form induces the redistribution of Golgi enzymes into the ER.Citation36,91 Evidence exists for a functional connection between Rab6 (see below) and Rab33b, possibly through a Rab6/Rab33b « cascade ». This Rab cascade may contribute to Golgi compartmentalization and membrane domain formation as suggested by Pfeffer.Citation65 These points are discussed in detail in a recent review.Citation44 In humans, mutations in Rab33b that affect GTP-binding results in vastly decreased protein abundance and defects in skeletal formation and early death.Citation3,18
The best studied Golgi associated protein Rab, and the most abundant, is Rab6 (Ypt6p in yeast). We take this example as being illustrative of the extent to which we understand the relative role of local Rab recruited protein machines in Golgi structure/function relationships.
The type member of the Rab6 family, Rab6A, was discovered almost 30 years agoCitation29,100 and the encoding gene has been shown to be essential to mammalian development in utero.Citation77 The Rab6 family comprises 4 proteins, named Rab6A, Rab6A’, Rab6B and Rab6C. Rab6A’ is generated by alternative splicing of the RAB6A gene and differs from Rab6A by only three amino acids.Citation20 Both proteins are ubiquitously expressed and are together the most abundant Golgi-associated Rab protein.Citation28 Rab6B is encoded by a separate gene and is mostly expressed in neurons and neuroendocrine cells.Citation62 The exact function of the neuronal isoform Rab6B is unknown. RAB6C is a primate-specific retrogene transcribed in a limited number of human tissues. It encodes a protein with altered biochemical properties compared to other Rab6 isoforms that localizes to centrosome and is involved in cell cycle progression.Citation99 A slightly more distant protein, Rab41, can be considered to be a 5th member of the Rab6 family.Citation43
Numerous studies have established the essential role played by Rab6A/A’ in the regulation of several transport steps at the level of the Golgi complex, including retrograde transport between endosomes and the endoplasmic reticulum via the Golgi complex, anterograde transport between Golgi and the plasma membrane, as well as in the homeostasis of Golgi membranes.Citation31,46,48,49,50,84,95 As expected, these multiple roles in Rab6 in Golgi derived trafficking are then mediated by the recruitment of a wide array of Rab6 effector proteins to the Golgi complex. At least 15 different Rab6 effectors have been identified.Citation7,30 In a manner not well understood yet, effector recruitment must be localized and context sensitive. In brief, Rab6 can and does promote the formation of localized, multicomponent protein machines that initiate and sustain individual Golgi trafficking pathways. Probably the best studied case is the myosin II, Rab6, Kif20A complex at the trans Golgi/TGN. This complex forms locally and promotes the extension of Golgi derived tubules and the eventual release of vesicles;Citation53,54). In the depth study of this complex holds great promise of answering the question of how local, context sensitive Rab action can occur. This is an important and general problem in both secretory and endocytic pathways as Rab protein in general have many effectors.
Considering the numerous roles of Rab6 in Golgi associated trafficking events, one might expect that Rab6 depletion or alteration of its functional state would produce little net effect on Golgi morphological organization as the various individual effects would cancel one another out. In the case of Rab6 inactivation, GDP-locked Rab6 T27N overexpressionCitation36,48,85,98 or Rab6 depletion through siRNA treatment,Citation84,97 the effects seen by light microscopy appear slight. There is a slight compaction of the juxtanuclear Golgi ribbon and a delay, but no strong inhibition, in anterograde and retrograde transport through the organelle (e.g.Citation84). The length of cisternae however increases by about 2x fold in si-RNA depleted cells and in MEFs derived from Rab6 knock-out mice.Citation6,84 In HeLa cells, Rab6 depletion also leads to an increase in the number of Golgi stacks.Citation84 Considering the wide variety of trafficking pathways supported by Rab6, this outcome might well be considered to be the expected result. Both anterograde and retrograde trafficking pathways should be slowed. In net, the balance between trafficking pathways appears to be roughly maintained. However, in striking contrast, overexpression of wild type Rab6 or GTP-locked Rab6 results in a BFA-like Golgi phenotype; Golgi resident proteins redistribute to the endoplasmic reticulum,Citation36,49 a result that strongly indicates a preferential biasing of Golgi trafficking pathways towards retrograde trafficking to the ER. How might this outcome be explained? We propose that the most plausible explanation is that the binding constants of various Rab6 effectors varies significantly and hence as the expression of wild-type or GTP-locked Rab6 is increased the importance of relatively weak or minor pathways is over emphasized. If so, this would suggest that the effector(s) prompting retrograde trafficking to the ER have comparatively low binding constants. To date the determination of effector has been a qualitative, yes or no, experiment. Comparative quantitative data on effector binding might well make possible a systems biology approach to the prediction of the various pathways as Rab6 is titered.
- Conclusions and perspectives
The roughly 20 Rab GTPases discussed here relative to the regulation and maintenance of Golgi complex structure all share certain common features such as the underlying biochemistry of the GTPase cycle and shared protein sequence and folding features (). Initially, these common features were very helpful in the search for new family members, an important stage in the development of the field. However, as should be apparent from our enumeration of the roles and functions of varied Golgi-associated Rab proteins, the challenge today is different. A major aspect is to understand how individual Rab proteins modulate the activity of distinct and context-sensitive protein machines while at the same time giving attention to how multiple machines can be integrated to give functionality within the cell, for example, to determine Golgi complex structure. To understand the even simplified individuality apparent and summarized in , we suggest that research efforts must go to both “drilling down” at the level of individual process and biochemistry and integrating through a systems biology approach quantification determinations and modeling to reveal patterns of action and how they change with increased or decreased Rab protein levels. Hence, we suggest in the previous section of this review that Rab6 and an individual context sensitive process such as myosin II dependent vesicle formation may be an attractive example for the drill down approach. On the other hand, the question of how Rab6 overexpression leads to Golgi protein redistribution to the ER rather than transport to the plasma membrane, endosomes or lysosomes likely will require an integrative systems biology approach. We suggest in this case that weaker interactions are “ratched up” and determinative. This preferential ratching up then leads to redistribution to Golgi proteins to the ER. We note that today that there is no quantitative data to indicate the existence of weaker or stronger interactions that might be preferentially modulation in the overexpression case.
In sum, today we can only give the reader the qualitative answer that the role of Rab proteins in Golgi structure relies on their role in trafficking. We project that an understanding of specific, locality-sensitive roles of Golgi-associated Rabs will be a step towards transforming this situation. We suggest that the Rab6/myosin II interaction at the trans-Golgi/TGN is apt to be an illustrative example. Furthermore, an integrated understanding of the relative role of Rabs and in particular Rab effectors is likely in comparison to require quantitative data from which model(s) of predictive value regarding Golgi phenotype could be made. Again, this would be a transformative step. In conclusion, we predict in 10 years that a review such as this will be a quantitative statement of how an individual Rab protein through multiple effectors produces an integrated Golgi phenotype. Considering the magnitude of the task, that outcome will likely require the effort of multiple laboratories.
Acknowledgements
B. Goud is supported by grants from the CNRS, the Institut Curie and the ERC (European Research Council) (project 339847 'MYODYN'). B. Storrie and S. Liu are supported by grants from the National Institutes of Health (NIH), R01 GM092960 and U54 GM105814 to BS.
Additional information
Funding
References
- Aizawa M, Fukuda M. Small GTPase Rab2B and Its Specific Binding Protein Golgi-associated Rab2B Interactor-like 4 (GARI-L4) regulate golgi morphology. J Biol Chem. 2015;290(36):22250–61. doi:10.1074/jbc.M115.669242. PMID:26209634.
- Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion. Science. 2000;289(5478):444–8. doi:10.1126/science.289.5478.444. PMID:10903204.
- Alshammari MJ, Al-Otaibi L, Alkuraya FS. Mutation in RAB33B, which encodes a regulator of retrograde Golgi transport, defines a second Dyggve–Melchior–Clausen locus. J Med Genet. 2012;49(7):455–61. doi:10.1136/jmedgenet-2011-100666. PMID:22652534.
- Alvarez C, Garcia-Mata R, Brandon E, Sztul E. COPI recruitment is modulated by a Rab1b-dependent mechanism. Mol Biol Cell. 2003;14(5):2116–27. doi:10.1091/mbc.E02-09-0625. PMID:12802079.
- Bard F, Casano L, Mallabiabarrena A, Wallace E, Saito K, Kitayama H, Guizzunti G, Hu Y, Wendler F, Dasgupta R, et al. Functional genomics reveals genes involved in protein secretion and Golgi organization. Nature. 2006;439(7076):604–7. doi:10.1038/nature04377. PMID:16452979.
- Bardin S, Miserey-Lenkei S, Hurbain I, Garcia-Castillo D, Raposo G, Goud B. Phenotypic characterisation of RAB6A knockout mouse embryonic fibroblasts. Biol Cell. 2015;107(12):427–39. doi:10.1111/boc.201400083. PMID:26304202.
- Barnekow A, Thyrock A, Kessler D. Chapter 5: rab proteins and their interaction partners. Int Rev Cell Mol Biol. 2009;274:235–74. doi:10.1016/S1937-6448(08)02005-4. PMID:19349039.
- Barr FA. A novel Rab6-interacting domain defines a family of Golgi-targeted coiled-coil proteins. Curr Biol. 1999;9(7):381–4. doi:10.1016/S0960-9822(99)80167-5. PMID:10209123.
- Bhuin T, Roy JK. Rab proteins: the key regulators of intracellular vesicle transport. Exp Cell Res. 2014;328(1):1–19. doi:10.1016/j.yexcr.2014.07.027. PMID:25088255.
- Burguete AS, Fenn TD, Brunger AT, Pfeffer SR. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell. 2008;132(2):286–98. doi:10.1016/j.cell.2007.11.048. PMID:18243103.
- Cheung PY, Limouse C, Mabuchi H, Pfeffer SR. Protein flexibility is required for vesicle tethering at the Golgi. Elife. 2015;4:e12790. doi:10.7554/eLife.12790. PMID:26653856.
- Cox JV, Kansal R, Whitt MA. Rab43 regulates the sorting of a subset of membrane protein cargo through the medial Golgi. Mol Biol Cell. 2016;27(11):1834–44. doi:10.1091/mbc.E15-03-0123. PMID:27053659.
- Cuif MH, Possmayer F, Zander H, Bordes N, Jollivet F, Couedel-Courteille A, Janoueix-Lerosey I, Langsley G, Bornens M, Goud B. Characterization of GAPCenA, a GTPase activating protein for Rab6, part of which associates with the centrosome. EMBO J. 1999;18(7):1772–82. doi:10.1093/emboj/18.7.1772. PMID:10202141.
- Dejgaard SY, Murshid A, Erman A, Kizilay O, Verbich D, Lodge R, Dejgaard K, Ly-Hartig TB, Pepperkok R, Simpson JC, et al. Rab18 and Rab43 have key roles in ER-Golgi trafficking. J Cell Sci. 2008;121(Pt 16):2768–81. doi:10.1242/jcs.021808. PMID:18664496.
- Diao A, Rahman D, Pappin DJ, Lucocq J, Lowe M. The coiled-coil membrane protein golgin-84 is a novel rab effector required for Golgi ribbon formation. J Cell Biol. 2003;160(2):201–12. doi:10.1083/jcb.200207045. PMID:12538640.
- Distefano MB, Kjos I, Bakke O, Progida C. Rab7b at the intersection of intracellular trafficking and cell migration. Commun Integr Biol. 2015;8(6):e1023492. doi:10.1080/19420889.2015.1023492. PMID:27066171.
- Doring T, Prange R. Rab33B and its autophagic Atg5/12/16L1 effector assist in hepatitis B virus naked capsid formation and release. Cell Microbiol. 2015;17(5):747–64. doi:10.1111/cmi.12398. PMID:25439980.
- Dupuis N, Lebon S, Kumar M, Drunat S, Graul-Neumann LM, Gressens P, El Ghouzzi V. A novel RAB33B mutation in Smith-McCort dysplasia. Hum Mutat. 2013;34(2):283–6. doi:10.1002/humu.22235. PMID:23042644.
- Echard A, Jollivet F, Martinez O, Lacapère JJ, Rousselet A, Janoueix-Lerosey I, Goud B. Interaction of a Golgi-associated kinesin-like protein with Rab6. Science. 1998;279(5350):580–5. doi:10.1126/science.279.5350.580. PMID:9438855.
- Echard A, Opdam FJ, de Leeuw HJ, Jollivet F, Savelkoul P, Hendriks W, Voorberg J, Goud B, Fransen JA. Alternative splicing of the human Rab6A gene generates two close but functionally different isoforms. Mol Biol Cell. 2000;11(11):3819–33. doi:10.1091/mbc.11.11.3819. PMID:11071909.
- Fernandes H, Franklin E, Recacha R, Houdusse A, Goud B, Khan AR. Structural aspects of Rab6-effector complexes. Biochem Soc Trans. 2009;37(Pt 5):1037–41. doi:10.1042/BST0371037. PMID:19754447.
- Fridmann-Sirkis Y, Siniossoglou S, Pelham HR. TMF is a golgin that binds Rab6 and influences Golgi morphology. BMC Cell Biol. 2004;5:18. doi:10.1186/1471-2121-5-18. PMID:15128430.
- Fuchs E, Haas, AK, Spooner RK, Yoshimura S, Lord JM, Barr FA. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J Cell Biol. 2007;177:1133–43. doi:10.1083/jcb.200612068. PMID:17562788.
- Fukuda M, Itoh T. Direct link between Atg protein and small GTPase Rab: Atg16L functions as a potential Rab33 effector in mammals. Autophagy. 2008;4(6):824–6. doi:10.4161/auto.6542. PMID:18670194.
- Galea G, Simpson JC. High-content analysis of Rab protein function at the ER-Golgi interface. Bioarchitecture. 2015;5(3–4):44–53. doi:10.1080/19490992.2015.1102826. PMID:26693811.
- Gerondopoulos A, Bastos RN, Yoshimura S, Anderson R, Carpanini S, Aligianis I, Handley MT, Barr FA. Rab18 and a Rab18 GEF complex are required for normal ER structure. J Cell Biol. 2014;205(5):707–20. doi:10.1083/jcb.201403026. PMID:24891604.
- Giannandrea M, Bianchi V, Mignogna ML, Sirri A, Carrabino S, D'Elia E, Vecellio M, Russo S, Cogliati F, Larizza L, et al. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet. 2010;86(2):185–95. doi:10.1016/j.ajhg.2010.01.011. PMID:20159109.
- Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, et al. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127(6):1265–81. doi:10.1016/j.cell.2006.10.036. PMID:17174899.
- Goud B, Zahraoui A, Tavitian A, Saraste J. Small GTP-binding protein associated with Golgi cisternae. Nature. 1990;345(6275):553–6. doi:10.1038/345553a0. PMID:2112230.
- Goud B, Akhmanova A. Rab6 GTPase. In: Guangpu L and Segev N, editors. Rab GTPases and membrane trafficking; 2012. p. 34–46. ebooks.benthamscience.com/.
- Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, et al. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13(2):305–14. doi:10.1016/j.devcel.2007.06.010. PMID:17681140.
- Haas AK, Yoshimura S, Stephens DJ, Preisinger C, Fuchs E, Barr FA. Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J Cell Sci. 2007;120(Pt 17):2997–3010. doi:10.1242/jcs.014225. PMID:17684057.
- Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell. 2009;20(1):209–17. doi:10.1091/mbc.E08-07-0740. PMID:18946081.
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–49. doi:10.1152/physrev.00059.2009. PMID:21248164.
- Ishida M, Ohbayashi N, Fukuda M. Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP. Sci Rep. 2015;5:8238. doi:10.1038/srep08238. PMID:25649263.
- Jiang S, Storrie B. Cisternal rab proteins regulate Golgi apparatus redistribution in response to hypotonic stress. Mol Biol Cell. 2005;16(5):2586–96. doi:10.1091/mbc.E04-10-0861. PMID:15758030.
- Kamena F, Diefenbacher M, Kilchert C, Schwarz H, Spang A. Ypt1p is essential for retrograde Golgi-ER transport and for Golgi maintenance in S. cerevisiae. J Cell Sci. 2008;121(Pt 8):1293–302. doi:10.1242/jcs.016998. PMID:18388317.
- Kelly EE, Giordano F, Horgan CP, Jollivet F, Raposo G, McCaffrey MW. Rab30 is required for the morphological integrity of the Golgi apparatus. Biol Cell. 2012;104(2):84–101. doi:10.1111/boc.201100080. PMID:22188167.
- Lee PL, Ohlson MB, Pfeffer SR. Rab6 regulation of the kinesin family KIF1C motor domain contributes to Golgi tethering. Elife. 2015;4. doi:10.7554/eLife.06029.
- Levivier E, Goud B, Souchet M, Calmels TP, Mornon JP, Callebaut I. uDENN, DENN, and dDENN: indissociable domains in Rab and MAP kinases signaling pathways. Biochem Biophys Res Commun. 2001;287(3):688–95. doi:10.1006/bbrc.2001.5652. PMID:11563850.
- Liewen H, Meinhold-Heerlein I, Oliveira V, Schwarzenbacher R, Luo G, Wadle A, Jung M, Pfreundschuh M, Stenner-Liewen F. Characterization of the human GARP (Golgi associated retrograde protein) complex. Exp Cell Res. 2005;306(1):24–34. doi:10.1016/j.yexcr.2005.01.022. PMID:15878329.
- Liu S, Storrie B. Are Rab proteins the link between Golgi organization and membrane trafficking? Cell Mol Life Sci. 2012;69(24):4093–106. doi:10.1007/s00018-012-1021-6. PMID:22581368.
- Liu S, Hunt L, Storrie B. Rab41 is a novel regulator of Golgi apparatus organization that is needed for ER-to-Golgi trafficking and cell growth. PLoS One. 2013;8(8):e71886. doi:10.1371/journal.pone.0071886. PMID:23936529.
- Liu S, Storrie B. How Rab proteins determine Golgi structure. Int Rev Cell Mol Biol. 2015;315:1–22. doi:10.1016/bs.ircmb.2014.12.002. PMID:25708460.
- Liu S, Majeed W, Kudlyk T, Lupashin V, Storrie B. Identification of Rab41/6d effectors provides an explanation for the differential effects of Rab41/6d and Rab6a/a' on golgi organization. Front Cell Dev Biol. 2016;4:13. doi:10.3389/fcell.2016.00013. PMID:26973836.
- Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156(4):653–64. doi:10.1083/jcb.200110081. PMID:11839770.
- Martin S, Driessen K, Nixon SJ, Zerial M, Parton RG. Regulated localization of Rab18 to lipid droplets. J Biol Chem. 2005;280:42325–35. doi:10.1074/jbc.M506651200. PMID:16207721.
- Martinez O, Schmidt A, Salaméro J, Hoflack B, Roa M, Goud B. The small GTP-binding protein rab6 functions in intra-Golgi transport. J Cell Biol. 1994;127(6 Pt 1):1575–88. doi:10.1083/jcb.127.6.1575. PMID:7798313.
- Martinez O, Antony C, Pehau-Arnaudet G, Berger EG, Salamero J, Goud B. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1997;94(5):1828–33. doi:10.1073/pnas.94.5.1828. PMID:9050864.
- Matanis T, Akhmanova A, Wulf P, Del Nery E, Weide T, Stepanova T, Galjart N, Grosveld F, Goud B, De Zeeuw CI, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol. 2002;4(12):986–92. doi:10.1038/ncb891. PMID:12447383.
- Matsui T, Ohbayashi N, Fukuda M. The Rab interacting lysosomal protein (RILP) homology domain functions as a novel effector domain for small GTPase Rab36: Rab36 regulates retrograde melanosome transport in melanocytes. J Biol Chem. 2012;287(34):28619–31. doi:10.1074/jbc.M112.370544. PMID:22740695.
- Mignogna ML, Giannandrea M, Gurgone A, Fanelli F, Raimondi F, Mapelli L, Bassani S, Fang H, Van Anken E, Alessio M, et al. The intellectual disability protein RAB39B selectively regulates GluA2 trafficking to determine synaptic AMPAR composition. Nat Commun. 2015;6:6504. doi:10.1038/ncomms7504. PMID:25784538.
- Miserey-Lenkei S, Chalancon G, Bardin S, Formstecher E, Goud B, Echard A. Rab and actomyosin-dependent fission of transport vesicles at the Golgi complex. Nat Cell Biol. 2010;12(7):645–54. doi:10.1038/ncb2067. PMID:20562865.
- Miserey-Lenkei S, Dimitrov A, Bardin A, Pylypenko O, Bressanelli G, Fraisier V, Houdusse A, Echard A, Goud B. Coupling generation and exit of RAB6 vesicles at Golgi fission hot spots through kinesin-myosin interactions. Nat Commun. 2017;8(1):1254. doi:10.1038/s41467-017-01266-0
- Mizuno-Yamasaki E, Rivera-Molina F, Novick P. GTPase networks in membrane traffic. Annu Rev Biochem. 2012;81:637–59. doi:10.1146/annurev-biochem-052810-093700. PMID:22463690.
- Monetta P, Slavin I, Romero N, Alvarez C. Rab1b interacts with GBF1 and modulates both ARF1 dynamics and COPI association. Mol Biol Cell. 2007;18(7):2400–10. doi:10.1091/mbc.E06-11-1005. PMID:17429068.
- Monier S, Jollivet F, Janoueix-Lerosey I, Johannes L, Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3(4):289–97. doi:10.1034/j.1600-0854.2002.030406.x. PMID:11929610.
- Moyer BD, Allan BB, Balch WE. Rab1 interaction with a GM130 effector complex regulates COPII vesicle cis–Golgi tethering. Traffic. 2001;2(4):268–76. doi:10.1034/j.1600-0854.2001.1o007.x. PMID:11285137.
- Ng EL, Wang Y, Tang BL. Rab22B's role in trans-Golgi network membrane dynamics. Biochem Biophys Res Commun. 2007;361(3):751–7. doi:10.1016/j.bbrc.2007.07.076. PMID:17678623.
- Ng EL, Gan BQ, Ng F, Tang BL. Rab GTPases regulating receptor trafficking at the late endosome-lysosome membranes. Cell Biochem Funct. 2012;30(6):515–23. doi:10.1002/cbf.2827. PMID:22473705.
- Nuoffer C, Davidson HW, Matteson J, Meinkoth J, Balch WE. A GDP-bound of rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 1994;125(2):225–37. doi:10.1083/jcb.125.2.225. PMID:8163542.
- Opdam FJ, Echard A, Croes HJ, van den Hurk JA, van de Vorstenbosch RA, Ginsel LA, Goud B, Fransen JA. The small GTPase Rab6B, a novel Rab6 subfamily member, is cell-type specifically expressed and localised to the Golgi apparatus. J Cell Sci. 2000;113(Pt 15):2725–35. PMID:10893188.
- Ozeki S, Cheng J, Tauchi-Sato K, Hatano N, Taniguchi H, Fujimoto T. Rab18 localizes to lipid droplets and induces their close apposition to the endoplasmic reticulum-derived membrane. J Cell Sci. 2005;118(Pt 12):2601–11. doi:10.1242/jcs.02401. PMID:15914536.
- Papanikou E, Glick BS. Golgi compartmentation and identity. Curr Opin Cell Biol. 2014;29:74–81. doi:10.1016/j.ceb.2014.04.010. PMID:24840895.
- Pfeffer SR. How the Golgi works: a cisternal progenitor model. Proc Natl Acad Sci U S A. 2010;107(46):19614–8. doi:10.1073/pnas.1011016107. PMID:21045128.
- Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, Der CJ, Balch WE. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115(1):31–43. doi:10.1083/jcb.115.1.31. PMID:1918138.
- Romero N, Dumur CI, Martinez H, García IA, Monetta P, Slavin I, Sampieri L, Koritschoner N, Mironov AA, De Matteis MA, Alvarez C. Rab1b overexpression modifies Golgi size and gene expression in HeLa cells and modulates the thyrotrophin response in thyroid cells in culture. Mol Biol Cell. 2013;24(5):617–32. doi:10.1091/mbc.E12-07-0530. PMID:23325787.
- Rosing M, Ossendorf E, Rak A, Barnekow A. Giantin interacts with both the small GTPase Rab6 and Rab1. Exp Cell Res. 2007;313(11):2318–25. doi:10.1016/j.yexcr.2007.03.031. PMID:17475246.
- Russo AJ, Mathiowetz AJ, Hong S, Welch MD, Campellone KG. Rab1 recruits WHAMM during membrane remodeling but limits actin nucleation. Mol Biol Cell. 2016;27(6):967–78. doi:10.1091/mbc.E15-07-0508. PMID:26823012.
- Salian S, Cho TJ, Phadke SR, Gownshankar K, Bhavani GS, Shukla A, Jagadeesh S, Kim OH, Nishimura G, Ginsha KM. Aditional three patients with Smith-McCort dysplasia due to novel RAB33B mutations. Am J Med Genet A. 2017;173(3):588–595. doi:10.1002/ajmg.a.38064. PMID:28127940.
- Sannerud R, Marie M, Nizak C, Dale HA, Pernet-Gallay K, Perez F, Goud B, Saraste J. Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery. Mol Biol Cell. 2006;17(4):1514–26. doi:10.1091/mbc.E05-08-0792. PMID:16421253.
- Saraste J, Lahtinen U, Goud B. Localization of the small GTP-binding protein rab1p to early compartments of the secretory pathway. J Cell Sci. 1995;108 (Pt 4):1541–52. PMID:7615674.
- Saraste J. Spatial and Functional Aspects of ER-Golgi Rabs and Tethers. Front Cell Dev Biol. 2016;4:28. doi:10.3389/fcell.2016.00028. PMID:27148530.
- Sato K, Roboti P, Mironov AA, Lowe M. Coupling of vesicle tethering and Rab binding is required for in vivo functionality of the golgin GMAP-210. Mol Biol Cell. 2015;26(3):537–53. doi:10.1091/mbc.E14-10-1450. PMID:25473115.
- Sengupta P, Satpute-Krishnan P, Seo AY, Burnette DT, Patterson GH, Lippincott-Schwartz J. ER trapping reveals Golgi enzymes continually revisit the ER through a recycling pathway that controls Golgi organization. Proc Natl Acad Sci U S A. 2015;112(49):E6752–61. doi:10.1073/pnas.1520957112. PMID:26598700.
- Schlager MA, Kapitein LC, Grigoriev I, Burzynski GM, Wulf PS, Keijzer N, de Graaff E, Fukuda M, Shepherd IT, Akhmanova A, et al. Pericentrosomal targeting of Rab6 secretory vesicles by Bicaudal-D-related protein 1 (BICDR-1) regulates neuritogenesis. EMBO J. 2010;29(10):1637–51. doi:10.1038/emboj.2010.51. PMID:20360680.
- Shafaq-Zadah M, Gomes-Santos CS, Bardin S, Maiuri P, Maurin M, Iranzo J, Gautreau A, Lamaze C, Caswell P, Goud B, et al. Persistent cell migration and adhesion rely on retrograde transport of β(1) integrin. Nat Cell Biol. 2016;18(1):54–64. doi:10.1038/ncb3287. PMID:26641717.
- Short B, Preisinger C, Körner R, Kopajtich R, Byron O, Barr FA. A GRASP55-rab2 effector complex linking Golgi structure to membrane traffic. J Cell Biol. 2001;155(6):877–83. doi:10.1083/jcb.200108079. PMID:11739401.
- Short B, Haas A, Barr FA. Golgins and GTPases, giving identity and structure to the Golgi apparatus. Biochim Biophys Acta. 2005;1744(3):383–95. doi:10.1016/j.bbamcr.2005.02.001. PMID:15979508.
- Sinka R, Gillingham AK, Kondylis V, Munro S. Golgi coiled-coil proteins contain multiple binding sites for Rab family G proteins. J Cell Biol. 2008;183(4):607–15. doi:10.1083/jcb.200808018. PMID:19001129.
- Starr T, Sun Y, Wilkins N, Storrie B. Rab33b and Rab6 are functionally overlapping regulators of Golgi homeostasis and trafficking. Traffic. 2010;11(5):626–36. doi:10.1111/j.1600-0854.2010.01051.x. PMID:20163571.
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–25. doi:10.1038/nrm2728. PMID:19603039.
- Storrie B, White J, Röttger S, Stelzer EH, Suganuma T, Nilsson T. Recycling of golgi-resident glycosyltransferases through the ER reveals a novel pathway and provides an explanation for nocodazole-induced Golgi scattering. J Cell Biol. 1998;143(6):1505–21. doi:10.1083/jcb.143.6.1505. PMID:9852147.
- Storrie B, Micaroni M, Morgan GP, Jones N, Kamykowski JA, Wilkins N, Pan TH, Marsh BJ. Electron tomography reveals Rab6 is essential to the trafficking of trans-Golgi clathrin and COPI-coated vesicles and the maintenance of Golgi cisternal number. Traffic. 2012;13(5):727–44. doi:10.1111/j.1600-0854.2012.01343.x. PMID:22335553.
- Sun Y, Shestakova A, Hunt L, Sehgal S, Lupashin V, Storrie B. Rab6 regulates both ZW10/RINT-1 and conserved oligomeric Golgi complex-dependent Golgi trafficking and homeostasis. Mol Biol Cell. 2007;18(10):4129–42. doi:10.1091/mbc.E07-01-0080. PMID:17699596.
- Teber I, Nagano F, Kremerskothen J, Bilbilis K, Goud B, Barnekow A. Rab6 interacts with the mint3 adaptor protein. Biol Chem. 2005;386(7):671–7. doi:10.1515/BC.2005.078. PMID:16207088.
- Tisdale EJ, Artalejo CR. A GAPDH mutant defective in Src-dependent tyrosine phosphorylation impedes Rab2-mediated events. Traffic. Biol Chem. 2007;8(6):733–41.
- Tisdale EJ, Bourne JR, Khosravi-Far R, Der CJ, Balch WE. GTP-binding mutants of rab1 and rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J Cell Biol. 1992;119(4):749–61. doi:10.1083/jcb.119.4.749. PMID:1429835.
- Tisdale EJ. Rab2 interacts directly with atypical protein kinase C (aPKC) iota/lambda and inhibits aPKCiota/lambda-dependent glyceraldehyde-3-phosphate dehydrogenase phosphorylation. J Biol Chem. 2003;278(52):52524–30. doi:10.1074/jbc.M309343200. PMID:14570876.
- Valente C, Polishchuk R, De Matteis MA. Rab6 and myosin II at the cutting edge of membrane fission. Nat Cell Biol. 2010;12(7):635–8. doi:10.1038/ncb0710-635. PMID:20596045.
- Valsdottir R, Hashimoto H, Ashman K, Koda T, Storrie B, Nilsson T. Identification of rabaptin-5, rabex-5, and GM130 as putative effectors of rab33b, a regulator of retrograde traffic between the Golgi apparatus and ER. FEBS Lett. 2001;508(2):201–9. doi:10.1016/S0014-5793(01)02993-3. PMID:11718716.
- Wang S, Ma Z, Xu X, Wang Z, Sun L, Zhou Y, Lin X, Hong W, Wang T. A role of Rab29 in the integrity of the trans-Golgi network and retrograde trafficking of mannose-6-phosphate receptor. PLoS One. 2014;9(5):e96242. doi:10.1371/journal.pone.0096242. PMID:24788816.
- Wanschers B, van de Vorstenbosch R, Wijers M, Wieringa B, King SM, Fransen J. Rab6 family proteins interact with the dynein light chain protein DYNLRB1. Cell Motil Cytoskeleton. 2008;65(3):183–96. doi:10.1002/cm.20254. PMID:18044744.
- Weide T, Bayer M, Köster M, Siebrasse JP, Peters R, Barnekow A. The Golgi matrix protein GM130: a specific interacting partner of the small GTPase rab1b. EMBO Rep. 2001;2(4):336–41. doi:10.1093/embo-reports/kve065. PMID:11306556.
- White J, Johannes L, Mallard F, Girod A, Grill S, Reinsch S, Keller P, Tzschaschel B, Echard A, Goud B, et al. Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J Cell Biol. 1999;147(4):743–60. doi:10.1083/jcb.147.4.743. PMID:10562278.
- Wilson BS, Nuoffer C, Meinkoth JL, McCaffery M, Feramisco JR, Balch WE, Farquhar MG. A Rab1 mutant affecting guanine nucleotide exchange promotes disassembly of the Golgi apparatus. J Cell Biol. 1994;125(3):557–71. doi:10.1083/jcb.125.3.557. PMID:8175881.
- Wong M, Munro S. Membrane trafficking. The specificity of vesicle traffic to the Golgi is encoded in the golgin coiled-coil proteins. Science. 2014;346(6209):1256898. doi:10.1126/science.1256898. PMID:25359980.
- Young J, Stauber T, del Nery E, Vernos I, Pepperkok R, Nilsson T. Regulation of microtubule-dependent recycling at the trans-Golgi network by Rab6A and Rab6A'. Mol Biol Cell. 2005;16(1):162–77. doi:10.1091/mbc.E04-03-0260. PMID:15483056.
- Young J, Ménétrey J, Goud B. RAB6C is a retrogene that encodes a centrosomal protein involved in cell cycle progression. J Mol Biol. 2010;397(1):69–88. doi:10.1016/j.jmb.2010.01.009. PMID:20064528.
- Zahraoui A, Touchot N, Chardin P, Tavitian A. The human Rab genes encode a family of GTP-binding proteins related to yeast YPT1 and SEC4 products involved in secretion. J Biol Chem. 1989;264(21):12394–401. PMID:2501306.
- Zheng JY, Koda T, Fujiwara T, Kishi M, Ikehara Y, Kakinuma M. A novel Rab GTPase, Rab33B, is ubiquitously expressed and localized to the medial Golgi cisternae. J Cell Sci. 1998;111(Pt 8):1061–69. PMID:9512502.
