ABSTRACT
Through actin-binding proteins such as the neural Wiskott-Aldrich syndrome protein (N-WASP) and WASP-interacting protein (WIP), the Rho family GTPases RhoA, Rac1 and Cdc42 are major modulators of the cytoskeleton. (N-)WASP and WIP control Rho GTPase activity in various cell types, either by direct WIP/(N-)WASP/Cdc42 or potential WIP/RhoA binding, or through secondary links that regulate GTPase distribution and/or transcription levels. WIP helps to regulate filopodium generation and participates in the Rac1-mediated ruffle formation that determines cell motility. In neurons, lack of WIP increases dendritic spine size and filamentous actin content in a RhoA-dependent manner. In contrast, WIP deficiency in an adenocarcinoma cell line significantly reduces RhoA levels. These data support a role for WIP in the GTPase-mediated regulation of numerous actin-related cell functions; we discuss the possibility that this WIP effect is linked to cell proliferative status.
Introduction
In this review, we will summarize some of the most recent data that describe the link between WIP (WASP-interacting protein) and some classical GTPases and how they regulate several general cell processes such as migration, proliferation and differentiation.
Rho GTPase family members
From the pioneer report by Ridley and Hall, who demonstrated that active Rho stimulates stress fiber formationCitation1, the Rho GTPase family has expanded to 20 members. They are usually classified in two major groups, the canonical (Rho, Rac, Cdc42, RhoD/F) and the atypical (Rnd, RhoU/V, RhoH, RhoBTB). Many reports describe the activation of canonical Rho-family members through membrane receptors and cell adhesion molecules which leads to activation of the Arp2/3 (actin-related protein) complex and actin assembly. Studies on less well characterized atypical members of the Rho-family support new levels of complexity and inter-connectivity in Rho-family GTPase signaling during cell migration.Citation2,Citation3 In response to extra- and intracellular stimuli that modulate their many roles, including regulation of the actin cytoskeleton and cell signaling, most Rho family canonical GTPases and some atypical members cycle between an active GTP-bound form and an inactive GDP-bound form. This itinerant status is governed by three sets of proteins, the activating guanine nucleotide-exchange factors (GEF), the inhibitory GTPase-activating proteins (GAP), and the inhibitory guanine nucleotide-dissociation inhibitors (GDI).Citation4 When bound to GTP, the GTPases can interact with and activate downstream effector proteins, and thus stimulate a plethora of cell processes (migration, division and adhesion, neuron development, final organ morphogenesis). This capacity can affect cytoskeletal structures, modifying not only actin-based activities but also microtubule dynamics, in almost all cell lineages. An essential set of information arose from analysis of the canonical elements Rho, Rac, Cdc42, and their direct control of filamentous actin (F-actin) regulation.Citation5 Cdc42 has a conserved role in regulating cell polarity and stimulating filopodium induction, whereas Rac proteins generate lamellipodia that regulate membrane ruffle formation and induce membrane extension. In contrast, the three homologous Rho isoforms —RhoA, RhoB and RhoC— all induce stress fiber formation when overexpressed in fibroblasts.
The atypical GTPases Rnd1, Rnd2 and Rnd3 are always bound to GTP and, therefore, are not regulated by the same kind of effectors than the canonical ones. Some reports have provided important insights into the mechanisms that control the function of Rnd proteins; rather than by the GDP/GTP switch, their activity is regulated by their expression, localization and phosphorylation. Interestingly, there are common players between canonical and atypical forms as Rnd1 and Rnd3 antagonize RhoA-mediated actin remodeling during cell migration through localized recruitment of p190RhoGAP.Citation6
The generation of GTPase-regulated cytoskeletal structures usually requires the action of several actin-related proteins that provide the final link between the GTPases and the regulation of F-actin formation. The active/inactive status of each GTPase can control some central elements of polymerization, such as the Arp2/3 complex, as well as a broader group of proteins that transduce overall signals into specific actions or territories; some of these are GTPase-specific, such as cofilin, rhotekin, NADPH oxidase, citron kinase, profilin, and Par3/Par6. Alternatively they can trigger both actin polymerization and force generation through activation of the formin mDia.Citation2
Wiskott-Aldrich syndrome protein (WASP) family proteins
Among the best-characterized GTPase effectors, two of the six mammalian WASP family members stand out; these are WASP and neural WASP (N-WASP), whose main function is to act as nucleation-promoting factors (NPF) for actin polymerization.Citation7 (N-)WASP-mediated activation of the Arp2/3 complex allows 70° angle nucleation over an existing “mother” actin filament. This generates a branched cytoskeletal mesh that supports cell protrusions such as the lamellipodium, which is involved in processes such as cell adhesion or migration.
The multidomain protein structure of (N-)WASP is ideal for integrating multiple input signals to coordinate appropriate final changes in the overall actin cytoskeleton.Citation8 In several cell systems, the interaction of active Cdc42-GTP with the (N-)WASP GTPase binding domain (GBD) promotes ‘opening’ of the (N-)WASP structure and increases its nucleation activity.Citation9-Citation11 An in vitro pyrene-actin polymerization assay using recombinant proteins demonstrated that (N-)WASP activation by Cdc42 is also regulated by WIP binding, which promotes the (N-)WASP inactive conformation.Citation12 Together with cell membrane-bound Cdc42-GTP, WIP is also involved in (N-)WASP recruitment to specific subcellular locations.Citation13-Citation15 These interactions recruit (N-)WASP to the cell region at which actin must polymerize, and contribute to the indispensable spatial and temporal control of this process. WIP also mediates actin tail formation for vaccinia virus motility; after its Nck-dependent recruitment, (N-)WASP engages with the Cdc42 GEF intersectin-1 to activate the GTPase and maintain sustained actin polymerization.Citation16,Citation17 The WIP/(N-)WASP role is conserved in evolution, as reported for D-WIP (Drosophila-WIP); the Arp2/3 WASP/WIP complex, located at the actin caplets during spermatogenesis in flies, is needed to ensure correct spermatid release from the head cyst cell.Citation18 In addition to Cdc42 and the cortical actin cytoskeleton, (N-)WASP also binds curved anionic membranes in lipid rafts, thought to be induced by glycosylphosphatidylinositol-anchored proteins.Citation19
WASP-interacting protein (WIP)
WIP was first identified as a partner of the hematopoietic-specific WASP in lymphocytes,Citation20,Citation21 and then as an N-WASP partner in many other cell types (e.g., fibroblasts, neurons, epithelial cells), as both WIP and N-WASP are expressed ubiquitously.Citation22 WIP can also exert its activity independently of its (N-)WASP interaction.Citation23,Citation24
-WIP relation with Rho GTPases
Twenty years ago, WIP was shown to bind the WASP N-terminal region at a site distinct from the GBD, which interacts with Cdc42, weakly with Rac, and not with Rho.Citation9-Citation11,Citation20 From the outset, WIP was therefore linked indirectly to GTPases, and the lack of any detectable GBD in its sequence suggested that direct WIP/GTPase interaction is unlikely. A recent publication reports a pull-down experiment using mixed lysates containing tagged overexpressed proteins (HA-RhoA or WIP-His) and speculate on direct WIP binding to Rho.Citation25 In vitro assays using purified recombinant proteins (full-length and deletion mutants), alone or with (N-)WASP, would nonetheless be needed to determine the precise nature of this WIP-RhoA interaction. Of great interest for GTPase/cytoskeletal studies would also be the confirmation of endogenous WIP and Rho protein interaction in physiological resting and/or stimulation conditions.
WIP and small GTPases are also linked through the direct, WASP-independent binding of GEF DOCK8 (dedicator of cytokinesis 8) to WIP.Citation24 DOCK8 associates constitutively with the WIP/WASP complex in resting primary T cells and this multi-complex persists after T cell receptor (TCR)-mediated stimulation. The spatial proximity of DOCK8, WASP, and actin in this complex ensures that Cdc42 (activated by DOCK8 following TCR binding) drives WASP-mediated actin polymerization. This control of the subcortical actin cytoskeleton regulates immune synapse formation, mechanotransduction, T cell transendothelial migration, and homing to lymph nodes, all of which also depend on WASP.
There is much less information on the connection between GTPases and other verprolin/WIP family members such as CR16 (corticosteroids and regional expression 16) or WICH/WIRE (WIP-CR16 homologous/WIP-related).Citation26 The few available studies on these proteins report that Cdc42 regulates IRSp53-WIRE interaction as well as localization of this complex to the plasma membrane to generate filopodia,Citation27 whereas Toca-1 (transducer of Cdc42-dependent actin assembly) promotes actin nucleation by activating the (N-)WASP-WIP/CR16 complex.Citation28
-WIP as a regulator of actin-rich cellular components
At the functional level, WIP activity is associated with the generation of specific actin-rich structures such as filopodia, lamellipodia and stress fibers, as a result of actin cytoskeleton reorganization mediated by Cdc42, Rac, and Rho activation, respectively. In 3T3 murine fibroblasts, WIP regulates N-WASP-induced actin nucleation and contributes significantly to the formation of actin-containing microspikes promoted by bradykinin and Cdc42-GTP; WIP microinjection in murine fibroblasts induces Cdc42-GTP/N-WASP-dependent filopodia, whereas anti-WIP microinjection prevents their generation.Citation12
In this same cell system, WIP also participates in Rac-mediated actin reorganization and dorsal/circular ruffle formation induced by PDGF (platelet-derived growth factor), a chemotactic factor for fibroblasts.Citation29 WIP overexpression enhances dorsal/circular ruffle formation in response to PDGF and, conversely, microinjection of anti-WIP antibody (or lack of WIP in knocked-down primary murine fibroblasts) leads to decreased ruffle formation in response to PDGF. In this setting, the WIP effect depends on its ability to interact directly with actin, as overexpression of a shortened WIP form that lacks the actin-binding site prevents PDGF-induced membrane ruffling.
Rac-regulated circular ruffling is commonly associated with macropinocytosis (internalization of solutes and membrane components), a process that takes place prior to cell movement, as transient ruffling contributes to establishment of polarity in motile cells. The reduced ability of WIP-deficient murine fibroblasts to form circular ruffles suggested a role for WIP in fibroblast movement, which was later confirmed by demonstration that both mesenchymal and amoeboid motility depend on WIP levels.Citation30 The use of lentivirally reconstituted WIP-deficient murine fibroblasts broadened our knowledge of the requirement for WIP interaction with N-WASP and the adaptor Nck for efficient dorsal ruffle formation; it also identified the need for WIP-Nck binding for efficient fibroblast chemotaxis to PDGF-AA but not to stimuli such as lysophosphatidic acid, epidermal growth factor or fibroblast growth factor. In addition, WIP participates in the amoeboid form of B lymphocyte motility in response to the B cell-specific chemokine CXCL13 (C-X-C motif chemokine 13) by controlling lamellipodium formation and cell polarization. In both types of migration, mesenchymal and amoeboid, WIP regulates the directional persistence of cell movement, whereas cell speed is only affected in amoeboid B lymphocytes.
-WIP participates in invasiveness and oncogenic activities
A recent functional connection was identified between the oncogenic effect of WIP and Rac activity in astrocytes and glioblastoma (GB) cells.Citation31 As WIP overexpression in cultured primary human astrocytes increases cell survival by stabilizing the transcriptional co-activators YAP/TAZ (Yes-associated protein/transcriptional coactivator with PDZ- binding motif), screening of molecules that produce cell death in this system allows identification of pathway components able to impair WIP-mediated survival. Incubation of cells with the Rac inhibitor NSC23766 reversed WIP ability to stabilize YAP/TAZ, which was unaffected by Cdc42 (casin) or RhoA (Y16) inhibitors in astrocytes and GB. Conversely, YAP/TAZ stability, which is notably reduced in WIP knocked-down cells, was unaffected by the presence of a constitutive active Rac mutant (Rac-V12), which suggests a downstream GTPase effect. WIP knockdown in GB also reduced phosphorylation of PAK, a Rac substrate in many processes. These findings support strong dependence on Rac/PAK activation for the WIP oncogenic effect in astrocytes, as well as in breast cancer cells.
Several recent reports highlight WIP as an important regulator of cell invasion, proliferation and anchorage-independent growth in various tumor types. WIP expression is upregulated in human GB explants and in invasive breast cancer cell lines,Citation31 in ameloblastoma,Citation32 in highly metastatic A5-RT3 cells (Ras-transformed keratinocytes) vs. non-metastatic parental HaCaT cells, in cancerous A549 cells vs. non-cancerous human small airway epithelial cells (H-SAEC),Citation25 and in human papillary thyroid tumors.Citation33
In ameloblastoma, the most commonly diagnosed odontogenic epithelial neoplasm, WIP is upregulated significantly along the tumor invasive front compared to tumor centers; it is the most widely expressed invadopodial protein in this tumor.Citation32 Invadopodia are F-actin-rich membrane protrusions that concentrate and secrete metalloproteases, which facilitate extracellular matrix (ECM) degradation.Citation34 Invadopodia attach to the ECM via their ring-shaped adhesion domain, which confines the actin core and contains GTPases. After the adhesion stage and the receptor-mediated signaling event, cytoskeleton activators are recruited to the membrane, which leads to actin polymerization in invadopodium cores mediated by a Rho-family small GTPase-regulated process.Citation35 This mainly Cdc42-dependent mechanism can activate the (N-)WASP/WIP complex directly and produce a sustained invasive protuberance and promotes its penetration of the ECM. Rac1 involvement in invadopodium formation was recently identified in melanoma cells; decreased expression of wild-type Rac1 reduces invadopodium-dependent matrix degradation, in contrast to decreased expression of a hyperactive Rac1 mutant that enhances invadopodium function.Citation35 GTPases have a central role not only in invadopodium generation and maturation, but also in forming functional podosomes, degradative structures closely related to invadopodia, in which Cdc42 and RhoA control actin reorganization.Citation36
WIP localizes to the tip of the invadopodium; its expression is necessary for invadopodium formation and ECM degradation by basal breast cancer cells, but is not sufficient to induce invasiveness in luminal cells.Citation37,Citation38 The identification of WIP as a potential biomarker that correlates directly with tumor aggressiveness in ameloblastoma, GB, breast cancer and thyroid cancer is of considerable clinical relevance. Due to its differential distribution at the invasive front in ameloblastoma, WIP might also be a promising target for the development of patient-tailored treatment strategies.
- WIP exerts opposite roles in neuronal differentiation and glioma proliferation
The lack of WIP in hippocampal neurons co-cultured with astrocytes leads to increased dendritic spine size and F-actin levels.Citation39,Citation40 Identification of the molecular mechanism underlying this phenotype showed direct involvement of increasing amounts of the RhoA GTPase but not of Cdc42 or Rac1, whose levels appear to be normal. Absence of WIP produces a three-fold increase in RhoA levels as well as consistently higher RhoA activity and membrane-associated distribution. Levels of the two RhoA downstream effectors ROCK (Rho-associated protein kinase) and profilin IIa are thus increased three-fold in WIP-deficient synaptosomes compared to controls. In this way, WIP deficiency facilitates activation of the RhoA-ROCK-profilin IIa pathway and contributes to increased F-actin levels in dendritic spines that lack WIP. This RhoA increase is the result of the WIP-dependent translational upregulation of neutral sphingomyelinase (NSM), whose activity reduces sphingomyelin (SM) levels at synapses. This alteration in membrane lipid composition enhances RhoA membrane binding, raft partitioning, and activation in steady state, but prevents changes in these RhoA features in response to stimuli. WIP thus has an essential role in connecting actin cytoskeleton and synaptic membrane lipid composition.Citation41 The distinct physiological effects should be noted of local RhoA activation, which leads to increased actin filaments in dendritic spines, and general RhoA activation, which can cause dendritic retraction and growth cone collapse.Citation42,Citation43
In contrast to the effect in neurons, in which lack of WIP increases RhoA levels, silencing WIP expression reduces RhoA levels and attenuates the tumorigenic and metastatic abilities of A549 lung adenocarcinoma cells. WIP overexpression enhances cell invasion and proliferation as well as their anchorage-dependent growth.Citation25 In this human epithelial cell system, and at difference from WIP-deficient neurons, WIP reduction does not affect RhoA mRNA expression or RhoA levels downstream of effectors such as ROCK-II and mDia1, or other Rho GTPases such as Cdc42 and Rac1. The overexpression effect is counteracted when cells are treated with the proteasome inhibitor MG132, which produces a time-dependent increase in RhoA expression.Citation25 This WIP protective capacity against proteasomal degradation is not restricted to RhoA, as WIP levels correlate with those of WASP, Syk.Citation44 or YAP/TAZ.Citation31 in a proteasome-dependent manner. We recently reported that loss of proliferation, anchorage-dependent growth and cell invasion in glioma cells are due to decreased WIP expression,Citation45 which leads to reduced RhoA levels (unpublished data), a result of p53 silencing.
Some GTPases might regulate WIP levels. We observed that expression of constitutive active Rac-V12, by increasing WIP levels, can rescue proliferation of WIP-deficient gliomas.Citation31 This strongly suggests Rac-WIP crosstalk, and requires further analysis. Data differ with respect to Ras, however; complementary RNA-sequencing analysis identified increased WIP expression in Ras-transformed human keratinocytes compared to controls,Citation25 whereas we found that direct transformation of an astrocytoma with Ras-V12 increased the proliferation rate, but not WIP levels (our unpublished data).
Future perspectives
The picture of how WIP participates in GTPase regulation is still far from complete. Nonetheless, research in recent years has begun to show that WIP is not a simple structural bystander, but has relevant roles in GTPase protein stability and subcellular distribution, as well as transcriptional control. As a key component of postsynaptic membranes, WIP modifies some lipid content.
At the neuron level, lack of WIP leads to altered levels of SM and its catabolic enzyme NSM at the synaptic membranes, which might affect trafficking and membrane components. All these lipid and protein arrangements could alter neuron synaptic functions and plasticity. Our data from WIP-deficient murine neurons clarify a role in neuron differentiation for WIP, which, by modulating F-actin levels, has an important regulatory function in dendritic spines. We observed dysfunction of some Akt downstream elements in WIP-deficient neurons, although further work is needed to link mTORC1 dysfunction, F-actin polymerization and RhoA in these cells.Citation39-Citation41
Figure 1. Scheme summarizing several pathways that control cell proliferation during glioma progression, including the recently identified position of WIP. Representative groups are shown of membrane receptors such as tyrosine kinase receptors (IGFR, GFR) or seven-transmembrane receptors (such as Wnt receptor). Through various mechanisms, these receptors can trigger generic PI3K-Akt signaling, which controls WIP activity. In many tumor cell types, certain proto-oncogenic proteins, such as the mutant versions of p53, enhance these membrane receptor activities. The oncogenic function of WIP operates by controlling YAP/TAZ stability/degradation. In some tumor cell types, YAP/TAZ can work together with beta-catenin; this collaboration enhances the relevance of this regulatory pathway, as an abundance of genes could be upregulated to ensure proliferation and survival. Levels of active GTPases such as RhoA are modified in some WIP-deficient cells, whereas in other cases Rac activity can compensate WIP-deficient function and/or WIP levels.
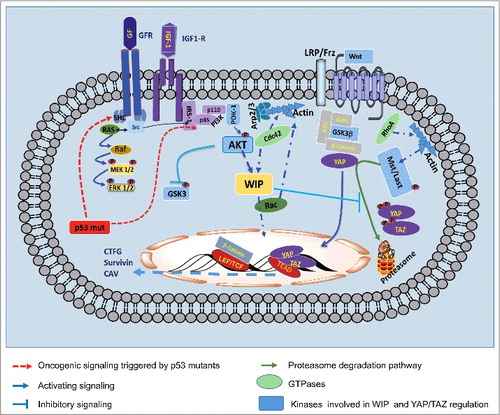
Many data support an essential role for WIP and GTPases, not only in tumor cell migration but also in cancer stem cell proliferation. These data allow the proposal of a molecular mechanism involved in cell proliferation, differentiation and actin cytoskeleton dynamics, all instrumental features in cell transformation and invasiveness. Our working hypothesis is that, as seen in gliomas, in other tumors WIP is under the control of mutant p53; through Akt activity, WIP regulates protein stability and subcellular distribution of regulatory proteins such as the co-transcription factors YAP/TAZ ().
These findings highlight WIP versatility, which enables it to modulate GTPase-dependent actin cytoskeleton reorganization in different ways depending on cell type, status and site, as well as the partners with whom it associates.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
All authors wrote and revised the manuscript.
Acknowledgments
We are grateful to all members of Lab 16 at the Centro Nacional de Biotecnología and of Lab 206 at the Centro de Biología Molecular Severo Ochoa (CBM-SO) for thoughtful discussions during the preparation of this review, and C. Mark for editorial assistance.
Additional information
Funding
References
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi:10.1016/0092-8674(92)90163-7.
- Sadok A, Marshall CJ. Rho GTPases: Masters of cell migration. Small GTPases. 2014;5:e29710. doi:10.4161/sgtp.29710.
- Ridley AJ. Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–112. doi:10.1016/j.ceb.2015.08.005.
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies, Nature reviews. Molecular cell biology. 2008;9:690–701.
- Jaffe AB, Hall A. Rho GTPases: Biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi:10.1146/annurev.cellbio.21.020604.150721.
- Chardin P. Function and regulation of Rnd proteins. Nat Rev Mol Cell Biol. 2006;7:54–62. doi:10.1038/nrm1788.
- Tyler JJ, Allwood EG, Ayscough KR. WASP family proteins, more than Arp2/3 activators. Biochem Soc Trans. 2016;44:1339–1345. doi:10.1042/BST20160176.
- Massaad MJ, Ramesh N, Geha RS. Wiskott-Aldrich syndrome: A comprehensive review. Ann N Y Acad Sci. 2013;1285:26–43. doi:10.1111/nyas.12049.
- Aspenstrom P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. doi:10.1016/S0960-9822(02)00423-2.
- Derry JM, Ochs HD, Francke U. Isolation of a novel gene mutated in Wiskott-Aldrich syndrome. Cell. 1994;78:635–644. doi:10.1016/0092-8674(94)90528-2.
- Kolluri R, Tolias KF, Carpenter CL, Rosen FS, Kirchhausen T. Direct interaction of the Wiskott-Aldrich syndrome protein with the GTPase Cdc42. Proc Natl Acad Sci U S A. 1996;93:5615–5618. doi:10.1073/pnas.93.11.5615.
- Martinez-Quiles N, Rohatgi R, Anton IM, Medina M, Saville SP, Miki H, Yamaguchi H, Takenawa T, Hartwig JH, Geha RS, Ramesh N. WIP regulates N-WASP-mediated actin polymerization and filopodium formation. Nat Cell Biol. 2001;3:484–491. doi:10.1038/35074551.
- Ditlev JA, Michalski PJ, Huber G, Rivera GM, Mohler WA, Loew LM, Mayer BJ. Stoichiometry of Nck-dependent actin polymerization in living cells. J Cell Biol. 2012;197:643–658. doi:10.1083/jcb.201111113.
- Chou HC, Anton IM, Holt MR, Curcio C, Lanzardo S, Worth A, Burns S, Thrasher AJ, Jones GE, Calle Y. WIP regulates the stability and localization of WASP to podosomes in migrating dendritic cells. Curr Biol. 2006;16:2337–2344. doi:10.1016/j.cub.2006.10.037.
- Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N, Geha RS. Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol Cell. 2002;10:1269–1281. doi:10.1016/S1097-2765(02)00728-1.
- Humphries AC, Donnelly SK, Way M. Cdc42 and the Rho GEF intersectin-1 collaborate with Nck to promote N-WASP-dependent actin polymerisation. J Cell Sci. 2014;127:673–685. doi:10.1242/jcs.141366.
- Donnelly SK, Weisswange I, Zettl M, Way M. WIP provides an essential link between Nck and N-WASP during Arp2/3-dependent actin polymerization. Curr Biol. 2013;23:999–1006. doi:10.1016/j.cub.2013.04.051.
- Dubey P, Shirolikar S, Ray K, Localized, Reactive F-Actin Dynamics Prevents Abnormal Somatic Cell Penetration by Mature Spermatids. Dev Cell. 2016;38:507–521. doi:10.1016/j.devcel.2016.07.001.
- Sharonov GV, Balatskaya MN, Tkachuk VA, Glycosylphosphatidylinositol-Anchored Proteins as Regulators of Cortical Cytoskeleton. Biochemistry (Mosc). 2016;81:636–650. doi:10.1134/S0006297916060110.
- Ramesh N, Anton IM, Hartwig JH, Geha RS, WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells. Proc Natl Acad Sci U S A. 1997;94:14671–14676. doi:10.1073/pnas.94.26.14671.
- Ramesh N, Anton IM, Martinez-Quiles N, Geha RS, Waltzing with WASP. Trends Cell Biol. 1999;9:15–19. doi:10.1016/S0962-8924(98)01411-1.
- Miki H, Miura K, Takenawa T, N-WASP, a novel actin-depolymerizing protein, regulates the cortical cytoskeletal rearrangement in a PIP2-dependent manner downstream of tyrosine kinases. EMBO J. 1996;15:5326–5335.
- Anton IM, Jones GE. WIP: A multifunctional protein involved in actin cytoskeleton regulation. Eur J Cell Biol. 2006;85:295–304. doi:10.1016/j.ejcb.2005.08.004.
- Janssen E, Tohme M, Hedayat M, Leick M, Kumari S, Ramesh N, Massaad MJ, Ullas S, Azcutia V, Goodnow CC, et al. A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton. J Clin Invest. 2016;126:3837–3851. doi:10.1172/JCI85774.
- Salvi A, Thanabalu T, WIP promotes in-vitro invasion ability, anchorage independent growth and EMT progression of A549 lung adenocarcinoma cells by regulating RhoA levels. Biochem Biophys Res Commun. 2017;482:1353–1359. doi:10.1016/j.bbrc.2016.12.040.
- Garcia E, Jones GE, Machesky LM, Anton IM. WIP: WASP-interacting proteins at invadopodia and podosomes. Eur J Cell Biol. 2012;91:869–877. doi:10.1016/j.ejcb.2012.06.002.
- Misra A, Rajmohan R, Lim RP, Bhattacharyya S, Thanabalu T, The mammalian verprolin, WIRE induces filopodia independent of N-WASP through IRSp53. Exp Cell Res. 2010;316:2810–2824. doi:10.1016/j.yexcr.2010.07.015.
- Ho HY, Rohatgi R, Lebensohn AM, Le M, Li J, Gygi SP, Kirschner MW. Toca-1 mediates Cdc42-dependent actin nucleation by activating the N-WASP-WIP complex. Cell. 2004;118:203–216. doi:10.1016/j.cell.2004.06.027.
- Anton IM, de la Fuente MA, Sims TN, Freeman S, Ramesh N, Hartwig JH, Dustin ML, Geha RS, WIP deficiency reveals a differential role for WIP and the actin cytoskeleton in T and B cell activation. Immunity. 2002;16:193–204. doi:10.1016/S1074-7613(02)00268-6.
- Banon-Rodriguez I, Saez de Guinoa J, Bernardini A, Ragazzini C, Fernandez E, Carrasco YR, Jones GE, Wandosell F, Anton IM, WIP regulates persistence of cell migration and ruffle formation in both mesenchymal and amoeboid modes of motility. PLoS One. 2013;8:e70364. doi:10.1371/journal.pone.0070364.
- Gargini R, Escoll M, Garcia E, Garcia-Escudero R, Wandosell F, Anton IM. WIP Drives Tumor Progression through YAP/TAZ-Dependent Autonomous Cell Growth. Cell Rep. 2016;17:1962–1977. doi:10.1016/j.celrep.2016.10.064.
- Siar CH, Rahman ZA, Tsujigiwa H, Mohamed Om Alblazi K, Nagatsuka H, Ng KH, Invadopodia proteins, cortactin, N-WASP and WIP differentially promote local invasiveness in ameloblastoma. J Oral Pathol Med. 2016;45:591–598. doi:10.1111/jop.12417.
- Zhang T, Shen X, Liu R, Zhu G, Bishop J, Xing M, Epigenetically upregulated WIPF1 plays a major role in BRAF V600E-promoted papillary thyroid cancer aggressiveness. Oncotarget. 2016;8:900–914.
- Linder S, The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–117. doi:10.1016/j.tcb.2007.01.002.
- Revach OY, Winograd-Katz SE, Samuels Y, Geiger B, The involvement of mutant Rac1 in the formation of invadopodia in cultured melanoma cells. Exp Cell Res. 2016;343:82–88. doi:10.1016/j.yexcr.2016.02.003.
- Moreau V, Tatin F, Varon C, Genot E, Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Mol Cell Biol. 2003;23:6809–6822. doi:10.1128/MCB.23.19.6809-6822.2003.
- Garcia E, Machesky LM, Jones GE, Anton IM, WIP is necessary for matrix invasion by breast cancer cells. Eur J Cell Biol. 2014;93:413–423. doi:10.1016/j.ejcb.2014.07.008.
- Garcia E, Ragazzini C, Yu X, Cuesta-Garcia E, Bernardino de la Serna J, Zech T, Sarrio D, Machesky LM, Anton IM, WIP and WICH/WIRE co-ordinately control invadopodium formation and maturation in human breast cancer cell invasion. Sci Rep. 2016;6:23590. doi:10.1038/srep23590.
- Franco A, Knafo S, Banon-Rodriguez I, Merino-Serrais P, Fernaud-Espinosa I, Nieto M, Garrido JJ, Esteban JA, Wandosell F, Anton IM, WIP is a negative regulator of neuronal maturation and synaptic activity. Cereb Cortex. 2012;22:1191–1202. doi:10.1093/cercor/bhr199.
- Franco-Villanueva A, Wandosell F, Anton IM, Neuritic complexity of hippocampal neurons depends on WIP-mediated mTORC1 and Abl family kinases activities. Brain Behav. 2015;5:e00359. doi:10.1002/brb3.359.
- Franco-Villanueva A, Fernandez-Lopez E, Gabande-Rodriguez E, Banon-Rodriguez I, Esteban JA, Anton IM, Ledesma MD. WIP modulates dendritic spine actin cytoskeleton by transcriptional control of lipid metabolic enzymes. Hum Mol Genet. 2014;23:4383–4395. doi:10.1093/hmg/ddu155.
- Sayas CL, Avila J, Wandosell F. Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J Neurosci. 2002;22:6863–6875.
- Sayas CL, Avila J, Wandosell F, Regulation of neuronal cytoskeleton by lysophosphatidic acid: Role of GSK-3. Biochim Biophys Acta. 2002;1582:144–153. doi:10.1016/S1388-1981(02)00149-X.
- Kettner A, Kumar L, Anton IM, Sasahara Y, de la Fuente M, Pivniouk VI, Falet H, Hartwig JH, Geha RS. WIP regulates signaling via the high affinity receptor for immunoglobulin E in mast cells. J Exp Med. 2004;199:357–368. doi:10.1084/jem.20030652.
- Escoll M, Gargini R, Cuadrado A, Anton IM, Wandosell F. Mutant p53 oncogenic functions in cancer stem cells are regulated by WIP through YAP/TAZ. Oncogene. 2017:36(25):3515–3527. doi:10.1038/onc.2016.518.
