ABSTRACT
Membrane trafficking establishes and maintains epithelial polarity. Rab22a has a polarized distribution in activated T-cells, but its role in epithelial polarity has not been investigated. We showed previously that Rab14 acts upstream of Arf6 to establish the apical membrane initiation site (AMIS), but its interaction with Rab22a is unknown. Here we show that Rab14 and Rab22a colocalize in endosomes of both unpolarized and polarized MDCK cells and Rab22a localizes to the cell:cell interface of polarizing cell pairs. Knockdown of Rab22a results in a multi-lumen phenotype in three-dimensional culture. Further, overexpression of Rab22a in Rab14 knockdown cells rescues the multi-lumen phenotype observed with Rab14 knockdown, suggesting that Rab22a is downstream of Rab14. Because of the relationship between Rab14 and Arf6, we investigated the effect of Rab22a knockdown on Arf6. We find that Rab22a knockdown results in decreased active Arf6 and that Rab22a co-immunoprecipitates with the Arf6 GEF EFA6. In addition, EFA6 is retained in intracellular puncta in Rab22a KD cells. These results suggest that Rab22a acts downstream of Rab14 to traffic EFA6 to the AMIS to regulate Arf6 in the establishment of polarity.
Introduction:
The conversion of a non-polarized cell to a polarized cell requires the asymmetric distribution of both proteins and lipids. In epithelia, the generation of polarity is controlled by interactions with the extracellular matrix [Citation1–4], the actions of lipid kinases and phosphatases to generate lipid domains [Citation5–7], and active membrane trafficking [Citation8]. While many small GTPases traffic to specific membrane domains after the establishment of polarity, a subset of these have been implicated in the early generation of polarity, including Arf6, Rab8, Rab11, Rab14, and Rab35 [Citation9–17]. During the establishment of epithelial polarity, vesicles traffic from the cell periphery to the forming apical membrane to form the apical membrane initiation site or AMIS [Citation9]. Further, recent results have suggested that the site of midbody formation during cytokinesis serves as a marker for polarity, and several Rabs, including Rabs 8, 11, 14 and 35, as well as Arf6, are localized to this structure [Citation10,Citation18–23].
Previously, we reported that Rab14 mediates trafficking of a subset of proteins to the apical domain of epithelial cells [Citation24] and that knockdown of Rab14 in three-dimensional culture results in the formation of multiple lumens and misplacement of the polarity proteins aPKC, Par3, and Cdc42 [Citation11,Citation25,Citation26]. Rab14 acts upstream of Arf6 which has been shown to play a role in apical polarity [Citation12,Citation17]. Rab35 is also essential for polarity, as knockdown of Rab35 leads to polarity and cytokinetic defects [Citation10,Citation13,Citation27,Citation28]. This effect is in part through regulation of lipid domains, as Rab35 binds to OCRL, a PtdIns4,5P2 phosphatase, and regulates Arf6 activity through the Arf6 GTPase activating protein (GAP) ACAP2 [Citation27,Citation29–32]. By activating both ACAP2 and OCRL, Rab35 works to decrease the amount of PtdIns4,5P2 at the membrane [Citation33]. In addition to its role in recruiting effectors that modify the lipid composition of the plasma membrane, Rab35 also directs the trafficking of podocalyxin, an apical glycoprotein that is required to establish polarity in MDCK cells [Citation10,Citation13].
Rab22a is an endosomal GTPase that is important for clathrin-independent endocytic cargo recycling and sorting, immune cell function, regulation of nerve growth factor (NGF) signalling and neurite cell differentiation [Citation34–41]. Rab22a and Arf6 have a polarized distribution in activated T-cells, and normal activity is required for formation of the immunological synapse [Citation35]. Further, Rab22a interacts with the Rab5 guanine nucleotide exchange factor (GEF) Rabex-5 and EEA1 and thus modulates the activity of Rab5 [Citation42].
In this work, we examined the role of Rab22a in apical membrane specification. Rab22a colocalizes with Rab14 in polarized and non-polarized cells. Further, Rab22a localizes to the cell:cell interface in cell pairs, and knockdown of Rab22a results in multi-lumen formation. Importantly, we found an interaction between Rab22a and the Arf6 GEF EFA6 and propose that Rab22a delivers EFA6 to the forming cell:cell interface to activate Arf6. However, we do not observe colocalization of Rab22a and podocalyxin, suggesting that Rab22a does not impact polarity through trafficking of this protein. Our results are consistent with a model in which Rab35 negatively regulates Arf6 at the AMIS as a negative regulator of the Arf6 GAP ACAP2. In contrast, Rab22a promotes increased activity of Arf6.
Materials and methods
Reagents, antibodies and plasmids
The following cell culture reagents were purchased from Corning Life Sciences: DMEM, Opti-MEM, Trypsin and Matrigel matrix (CB40230). Puromycin dihydrochloride was purchased from Fisher Scientific (ICN19453910). The following primary antibodies were used: mouse anti-podocalyxin was a gift from Dr. Karl Matlin (University of Chicago), rabbit anti-Rab22a (Abcam, EPR9486), rabbit anti-Rab14 (Sigma, R0656-200UL), mouse anti-Rab5 (BD Biosciences, 610,725), rabbit anti-Rab6 (Cell Signalling Technology, 9625), rabbit anti-Rab7 (Cell Signalling Technologies, 9367), rabbit anti-Rab11 (Cell Signalling Technology, 5589). Secondary antibodies and Alexa Fluor 568 Phalloidin (A12380) were purchased from Thermo-Fischer. The Rab22a-WT-GFP and Rab22a-Q64 L-GFP plasmids were gifts from Dr. Julie Donaldson (National Institutes of Health). Rab14-GFP and Rab14-RFP were created as described previously [Citation24]. EFA6-FLAG and ARNO-Myc were generous gifts of James Casanova (University of Virginia).
Biochemical analysis
Cells were lysed using RIPA buffer (20 mM Tris, 100 mM NaCl, 1% Triton X-100, 1% Na-deoxycholate, 0.1% SDS) that contained protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN) as well as calyculin A (Sigma-Aldrich) and sodium orthovanadate (Sigma-Aldrich). The cells were scraped and passed through a 27-gauge needle and incubated on ice for 30 minutes. After centrifuging the sample at 16,000 g for 10 minutes at 4ºC, the supernatant was retained, and placed on a 10% SDS-PAGE gel. After transferring the protein to nitrocellulose, 5% milk in 1XTBST was used to block, and primary and secondary antibodies were also diluted in 5% milk/1XTBST.
The Arf6 pull down assay for quantification of active Arf6 in Rab22a knockdown cells was performed using an Arf6 activation Assay (STA-407-6; Cell Biolabs, Inc, San Diego, CA), using the manufacturer’s protocol.
Immunoprecipitation
HEK cells were transfected and grown for 48 hours to 90% confluence in 10 cm dishes. Cells were lysed in an NP-40 lysis buffer as described [Citation43]. Lysates were precleared with Dynabeads™ Protein G (cat. 1003D; ThermoFisher) for 1 hr at 4°C. Protein G beads were incubated in 200ul of binding buffer with 5ug anti-Flag or anti-Myc antibody for 30 min at room temperature. Beads bound with antibody were washed and then precleared lysate was added. Lysate and antibody-bound beads were incubated overnight at 4°C with mixing. Beads were washed 3X with lysis buffer and proteins eluted in SDS sample buffer.
Cell culture
MDCK and HEK cells were cultured in high-glucose DMEM with Sodium Pyruvate and supplemented with 10% foetal bovine serum, 1% non-essential amino acids, and 1% Penicillin Streptomycin Solution 100X (30-002-Cl, Corning). Cells were incubated at 37ºC with 5% CO2. Transfections with MDCK cells were completed with Lipofectamine 2000 (Life Technologies) following the manufacturers protocol. Transfections with HEK cells were completed with PEI (Polysciences) at a PEI:DNA ratio of 3:1. The Rab14 knockdown cell line was previously created by the lab [Citation26]. The Rab22a knockdown cell line was created using lentivirus constructs from the Broad Institute, Harvard Medical School using the methods as described previously [Citation26]. The following sequence was used to knockdown Rab22:
Primer Forward 5ʹ – TGCGATAAACATAAATGAACT – 3ʹ
The following sequence was used to create the Rab22a shRNA resistant plasmid:
Primer Forward 5ʹ – AACGCAATCAATATCAACGAG – 3ʹ
Rab22 and Rab14 MDCK shRNA cells were selected and maintained in DMEM containing 2g/mL puromycin after lentivirus transduction. The pLKO.1 puro construct was used as a control. Knockdown quantified by immunoblotting using anti-Rab14 and anti-Rab22a antibodies, respectively.
Cyst culture
MDCK cells were grown to 60% confluency and transfected with Lipofectamine 2000. After 24 hours of incubation, transfected cells were then typsinzed and broken up into single cells. Cells were embedded in 100% Matrigel (BD Biosciences)-coated, glass-bottom, eight-well chambers (155,409; Lab-Tek) and cultured in DMEM with 2% Matrigel. Cell pairs were fixed and labelled 16 hours after plating. For lumen quantification, cysts were fixed and labelled 24 or 48 hours after plating.
Immunofluorescence labelling
Cells on glass coverslips were immunolabeled by rinsing in 1XPBS before fixation in 4% paraformaldehyde in PBS for 20 minutes. The cells were then washed in 1XPBS and incubated in 50 mM ammonium chloride in sterile water for 10 minutes to quench free aldehydes. After rinsing with 1XPBS, the cells were blocked and permeabilized in 0.2% saponin with 10% FBS in 1XPBS for 30 minutes, followed by incubation in primary antibody in 0.2% saponin with 10% PBS in 1XPBS for 2 hours. After rinsing, the cells were incubated in secondary antibody in 0.2% saponin with 10% FBS in 1XPBS for 1 hour in the dark. Once rinsed in 1XPBS, the cells were washed briefly in ddH2O and mounted using Prolong Diamond Antifade Mountant with DAPI (Invitrogen).
Cysts and cell pairs were fixed by washing once with warm 1XPBS + (1 mM CaCl2, 0.5 mM MgCl2) and fixing in 4% paraformaldehyde for 20 minutes. Cysts and cell pairs were permeabilized and blocked with 0.2% saponin with 10% FBS in 1XPBS. They were then incubated overnight in primary antibody in 1XPBS, 0.2% saponin, and 10% FBS at 4ºC. After washing with 1XPBS, they were incubated overnight in secondary antibody at 4ºC. Once washed, they were mounted with 40 ml Prolong Diamond Antifade Mountant with DAPI. 1XPBS was placed in the chambers to prevent dehydration of the cells.
Immunofluorescence microscopy
Images were obtained with an Olympus FluoView FV1200 laser-scanning confocal microscope (Olympus, Tokyo, Japan) with a 60X Plan Apo 1.42 NA oil immersion objective. The same imaging parameters were used within a single experiment. Images were processed and merged using Adobe Photoshop software (Adobe, San Jose, CA). Quantification of podocalyxin internalization was done by counting the number of cell pairs where podocalyxin was completely at the interface, where it was completely on the outside of the cell, and where the podocalyxin was in both locations. Quantification of lumen formation was done by counting the number of cell cysts with multi-lumen and the number of cell cysts with single lumens.
Colocalization analysis of Rab22a and PDCX
Fluorescence microscopy images were acquired using a 60X Plan Apo 1.40 NA Nikon objective on a Nikon Ti-E microscope equipped with a Hamamatsu ORCA-Flash V2 cMOS camera and motorized stage. To determine colocalization of Rab22-GFP and PDCX in MDCK cells, a binary mask on GFP+ cells were generated manually using the Binary Draw Object Tool in NIS Elements (Nikon) on the phalloidin channel. The Manual Segmentation tool was used to ensure the separation of single-cell binary masks. Single-cell binary masks were converted to ROIs using the Binary>ROI Reference Function and the Automate Measurements Module was used to calculated the Pearson’s correlation coefficient of Rab22-GFP and PDCX signal per cell.
Quantification of EFA6 distribution using binary area fraction analysis
Fluorescence microscopy images were acquired as described above. To quantify diffuse or punctate localization of EFA6-Flag in MDCK cells expressing control or Rab22 shRNA, first, a binary mask of the cell body was generated using the Binary Draw Object Tool in NIS Elements (Nikon) on the phalloidin channel. Second, the Thresholding Module was used to generate a new binary layer to isolate cell nuclei. Using the Binary Operations Module, a new binary layer was generated by subtracting the nuclei layer from the cell body layer, resulting in a cytoplasmic binary layer. The Manual Segmentation tool was used to ensure separation of single cells and the cytoplasmic binary layer was converted to single-cell regions of interest (ROIs) using the Binary>ROI Reference Function in NIS Elements. Lastly, a threshold mask was generated on the EFA6-Flag channel at 3X above background. Background signal was determined per image by quantifying the average background fluorescence intensity in the EFA6-Flag channel. The binary area fraction was calculated automatically using the Automated Measurement Results Module, by dividing the area (µm2) of the EFA6-Flag binary mask by the ROI area (µm2) per cell.
Statistical analysis
Podocalyxin localization in Rab22a shRNA cell pairs was repeated independently four times. Statistical comparisons for this experiment were made using Excel (Microsoft) and a Student t test at a 95% confidence interval. Rab22a knockdown cell cyst experiment was repeated three times. Rab14 single lumen rescue cell cyst experiment was completed twice. Rab22a knockdown verification had 6 technical replicates and a t test was used to determine statistical significance. Rab22a expression levels in Rab14KD cells was quantified across three independent experiments with three replicates per experiment. All bar graphs include standard error of measure. P-value < 0.05 = *, p-value < 0.01 = **, p-value < 0.005 = ***.
Results
Rab22a localizes to Rab14-positive and Rab14-negative vesicular structures
Rab14 is involved in trafficking between the trans-golgi network (TGN), early endosomes, and the plasma membrane, and regulates trafficking of growth factors, adhesion molecules, and immune factors as well as modulating the formation of the phagosome membrane [Citation26,Citation44–50]. We previously reported that Rab14 localizes to apical endosomes and controls trafficking of a subset of apical proteins. This trafficking contributes to the establishment of the AMIS [Citation11,Citation24,Citation25]. Rab22a also localizes to endosomes and polarizes to the immune synapse [Citation35,Citation36,Citation38,Citation41]. To test if these Rabs could be functioning from a common endosomal compartment, we transfected MDCK cells grown on glass coverslips with plasmids encoding Rab22a-GFP and Rab14-RFP. Imaging of these cells demonstrated that Rab22a-GFP and Rab14-RFP colocalize in enlarged vesicular structures in the perinuclear region. In addition, there were small Rab22a-GFP puncta that did not colocalize with Rab14-RFP (). As previously reported [Citation36], these enlarged endosomal structures form as a result of overexpression of Rab22a, as expression of Rab22a alone also resulted in large vesicles (Supplemental ). To determine if these structures also contained other Rab proteins, we co-transfected cells with Rab22a-GFP and Rab14-RFP followed by labelling with antibodies against the Rab5 (early endosomes), Rab6 (golgi), Rab7 (late endosomes), and Rab11 (recycling endosomes). As shown in Supplemental , there is no colocalization of the Rab22a-GFP with these Rabs.
Figure 1. Rab22a-GFP and Rab14-RFP localization in non-polarized MDCK cells. MDCK cells were plated on glass coverslips and transfected with Rab22a-GFP (green) and Rab14-RFP (red). DAPI labels the nucleus (blue). Enlarged vesicles in the perinuclear region are both Rab22a and Rab14 positive (arrows). Scale bar, 20 μm. Lower panel is an enlargement of the inset outlined in the upper panel. Rab22a-GFP positive puncta in the cytoplasm do not contain Rab14 (arrowheads). Scale bar, 5 μm
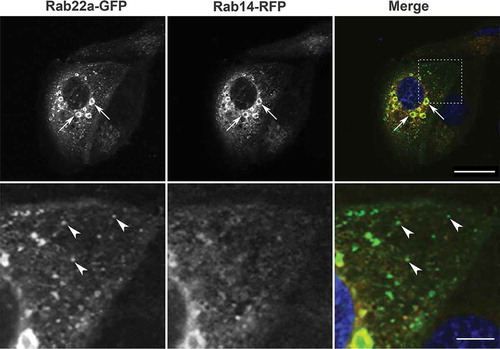
To test if Rab22a and Rab14 are essential for the localization of the other Rab, we overexpressed Rab22a-GFP in MDCK cells that had been knocked down for Rab14 or expressed Rab14-GFP in cells that had been knocked down for Rab22a. Rab22a knockdown was achieved by transducing MDCK cells with a lentivirus expressing shRNA directed at Rab22a or control shRNA followed by selection in puromycin to establish stable cell lines (Supplemental ). There was no obvious change in the distribution of either Rab under these conditions, suggesting that neither controls the localization of the other (Supplemental ). Further, we tested whether or not knockdown of Rab14 changed the expression of Rab22a protein. Immunoblotting shows that there is no change in expression of Rab22a after knockdown of Rab14 (Supplemental ).
Figure 2. Rab22 localizes to the cell:cell interface in MDCK cell pairs. Cell pairs in three-dimensional culture expressing Rab22a-WT-GFP or constitutively active (Rab22a-Q64 L-GFP) together with Rab14-WT-RFP. Transfected cells were embedded in Matrigel into a chamber slide and fixed after 16 hours. Rab22a-GFP localizes to the cell:cell interface (arrows) and to puncta in the cytoplasm (arrowheads). Rab14-RFP and Rab22a-WT-GFP and Rab22a-Q64 L-GFP colocalize in the cytoplasmic puncta (arrowheads). Scale bar, 10 μm
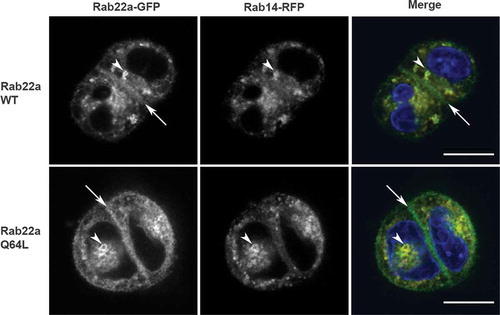
Rab22a-GFP localizes to the cell:cell interface
Because Rab22a and Rab14 colocalize in 2D cultures, we then asked whether they would colocalize in three-dimensional cultures. We expressed Rab22a-WT-GFP or Rab22a-Q64L-GFP together with Rab14-RFP in MDCK cells, embedded them as single cells in three-dimensional culture, and imaged samples 16 hours-post plating. As shown in , Rab22a-WT-GFP and Rab22a-Q64L-GFP colocalize with Rab14-RFP in vesicular puncta in the cytoplasm. Interestingly, while Rab14 does not localize to the cell:cell interface, both Rab22a-WT and Rab22a-Q64L are localized to this domain ().
Rab22a knockdown results in multi-lumen phenotype
Our previous work demonstrated that Rab14 contributes to the formation of the AMIS in MDCK cells [Citation11]. Since Rab22a is localized at the AMIS, we wondered if it could be active in the establishment of polarity. To test this, we embedded Rab22a knockdown or control MDCK cells in Matrigel and incubated them for 48 h, followed by labelling for podocalyxin (PDCX) to image the apical domain. As shown in , knockdown of Rab22a results in impaired single lumen formation. This appears to be due to Rab22a knockdown, as expression of a Rab22a shRNA resistant plasmid rescues single lumen formation ().
Figure 3. Knockdown of Rab22a results in multi-lumen formation and overexpression of Rab22a rescues Rab14 knockout. (a) MDCK Rab22a shRNA cells were embedded in Matrigel on a chamber slide and fixed after 48 hours. Podocalyxin (PDCX, red) was labelled to identify the forming apical domains. DAPI labels the nucleus (blue). Knockdown of Rab22a results in the formation of multiple lumens. Scale bar, 10 μm (b) Quantification of single lumen formation. The average percent of cysts with single lumen was recorded for shRNA control (n = 1050) and Rab22 knockdown (n = 817) cysts, across three independent repetitions with four replicates per plating. Rescue of single lumen with Rab22a shRNA resistant plasmid in cysts. MDCK cells that were transduced with Rab22a shRNA were transfected with Rab22a plasmid that was engineered to be resistant to the shRNA. Single cells were embedded in Matrigel and grown for 24 hours and the formation of single lumen cysts was quantified. The average percent of cysts with single lumen was recorded for shRNA control (n = 513), Rab22a knockdown (n = 606) and Rab22a shRNA with resistant plasmid (n = 190) cysts, across two independent repetitions with four replicates per plating. Expression of the Rab22a shRNA resistant plasmid rescues single lumen formation. (c) Rescue of single lumen formation in Rab14 KD cells. Rab14 KD cells were transfected with Rab22a-WT-GFP and Rab22a-Q67 L-GRP and single lumen formation was quantified. The average percent of cysts with single lumen was recorded for shRNA control (n = 304), Rab14 shRNA (n = 483), Rab14 shRNA with Rab22a-WT-GFP (n = 125), and Rab14 shRNA with Rab22a-Q64 L-GFP (n = 149) cysts across two independent repetitions with four replicates per plating
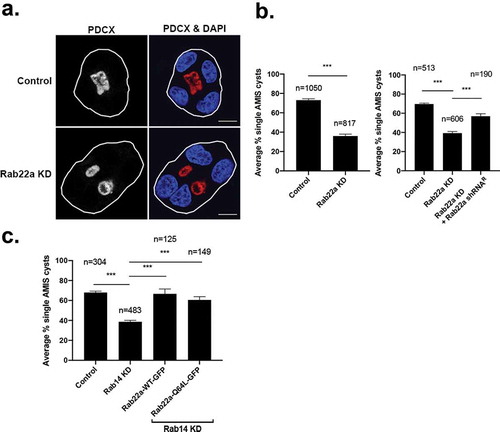
Interestingly, the degree of multi-lumen formation observed with Rab22a knockdown is similar to that observed when Rab14 is knocked down, suggesting that they could act in the same pathway [Citation11]. Therefore, we next tested whether or not overexpression of Rab22a could rescue the Rab14 KD multi-lumen phenotype. MDCK cells that were knocked down for Rab14 were transfected with Rab22a-WT-GFP or Rab22a-Q64L-GFP and single cells were embedded in Matrigel and grown for 24 hours, followed by labelling for podocalyxin. As shown in , both forms of Rab22a rescue the single lumen phenotype when Rab14 is knocked down, suggesting that Rab22a is acting downstream of Rab14.
Rab22a does not colocalize with podocalyxin.
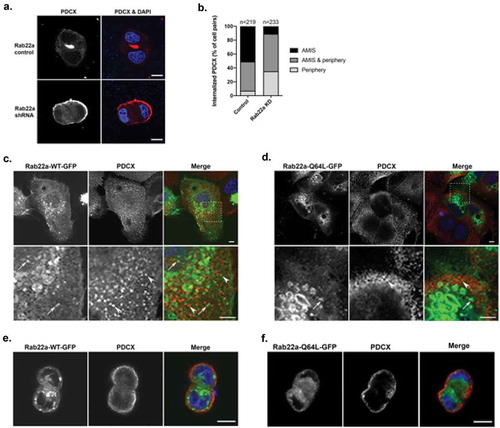
Apical lumen formation has been shown to be mediated by the trafficking of PDCX to the cell:cell interface through interaction with Rab35 [Citation10,Citation13]. To determine if depletion of Rab22a is impacting PDCX trafficking, we quantified the distribution of PDCX in cell pairs in Rab22a knockdown cells after 16 h incubation. As illustrated in , knockdown of Rab22a results in delayed internalization of PDCX from the peripheral membrane. This is similar to the phenotype observed with Rab14 knock down [Citation11], as well as Rab35 knockdown [Citation10,Citation13]. In the case of Rab35, extensive colocalization of Rab35 and PDCX is observed, and trafficking of these molecules impacts the localization of polarity determinants such as aPKC and Par3 [Citation10]. To determine if PDCX is trafficking through Rab22a-positive compartments, we labelled cells transfected with Rab22a-GFP with antibodies against PDCX. As shown in and as previously reported [Citation51], PDCX-positive puncta are largely distinct from Rab22a-GFP puncta, and quantification resulted in an average Pearson’s correlation co-efficient of 0.31, indicating minimal colocalization. Further, quantification of colocalization of Rab22a-Q64L and PDCX was also very low (Pearson’s correlation coefficient, 0.12) (). This suggests that Rab22a does not mediate the internalization or transport of PDCX and that the effects of Rab22a knockdown on PDCX trafficking are indirect and may be due to effects on other trafficking mediators. To analyse whether Rab22a and PDCX localization is impacted by cell polarity, Rab22a-WT-GFP and Rab22a-Q64L-GFP were transfected into MDCK cells and the cells were plated into Matrigel and fixed after 16 hours of incubation. As shown in , Rab22a-WT-GFP and PDCX do not colocalize in 3D polarized cells. Similarly, Rab22a-Q64L-GFP and PDCX also do not colocalize in 3D polarized cells.
Figure 4. Rab22a does not colocalize with PDCX, but knockdown of Rab22a delays its trafficking to the apical membrane. (a) Rab22a shRNA and control cells were embedded in Matrigel. Cells were fixed after 16 hours and labelled for PDCX (red). Rab22a KD results in delayed trafficking of PDCX from the periphery to the forming lumen, DAPI labels the nucleus (blue). Scale bar, 10 μm (b) Quantification of localization of PDCX in cell pairs of Rab22a shRNA (n = 233) and control cells (n = 219). PDCX location was determined as either completely within the cell at the interface, completely on the peripheral cell membrane, or both. The percentage of cell pairs represents the average percentages across the three independent repetitions of the experiment. (c) WT cells were transfected with Rab22a-GFP and plated on glass coverslips. Cells were fixed and labelled for PDCX (red). The distribution of Rab22a-GFP and PDCX are distinct. (d) WT cells were transfected with Rab22a-Q64 L-GFP and plated on glass coverslips. Cells were fixed and labelled for PDCX (red). The distribution of Rab22a-Q64 L-GFP and PDCX are distinct. Lower panels are an enlargement of the inset outlined in the upper panel. Distinct puncta that are only Rab22a-GFP positive (arrow) or PDCX positive (arrowhead) are seen and colocalization is not observed. Average Pearson’s correlation coefficient = 0.31 for Rab22a-WT-GFP, and average Pearson’s correlation coefficient = 0.12 for Rab22a-Q64 L-GFP. Scale bar (upper panels, 5 μm, lower panels, 5 m). (e) WT MDCK cells were transfected with Rab22a-WT-GFP and plated in Matrigel. Cells were fixed after 16 hours and labelled for PDCX (red). Scale bar, 10 μm. (f) WT MDCK cells were transfected with Rab22a-Q64 L-GFP and plated in Matrigel. Cells were fixed after 16 hours and labelled for PDCX (red). Scale bar, 10 μm
Rab22a affects Arf6 activity and co-immunoprecipitates with EFA6
Previously, we found that knockdown of Rab14 resulted in decreased PtdIns4,5P2 at the cell:cell interface and that overexpression of Arf6 or the lipid kinase PI(4)P5 K corrected the multi-lumen phenotype in Rab14 knockdown cells [Citation11]. Since our data suggests that Rab22a is acting downstream of Rab14, we wondered if Rab22a could be modulating Arf6 activity. To test this, we performed Arf6 activation assays using GGA-glutathione beads to pull down active Arf6 in control cells or after Rab22a knockdown. We compared active Arf6 to total levels of Arf6 under both conditions. As shown in , Rab22a KD results in decreased Arf6 activity.
Figure 5. Rab22a knockdown results in decreased Arf6 activity and Rab22a interacts with EFA6. (a) Rab22a shRNA and control cells were lysed and subjected to pull down to assess levels of active Arf6. (b) Quantification of the active-Arf6 pull down. Pull down experiments were performed in three independent experiments. (c) Rab22a co-immunoprecipitates with EFA6 but not ARNO. HEK cells expressing EFA6-Flag and either Rab22a-WT-GFP or Rab22a-Q64L-GFP or ARNO-myc and Rab22a-Q64L-GFP were precipitated with antibodies against the Flag or myc tag and blotted for Rab22a and Flag/myc
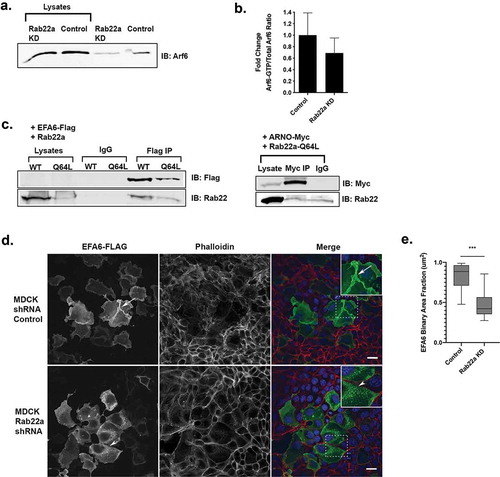
Since Rab22a knockdown decreased Arf6 activity, we postulated that Rab22a may deliver an Arf6 GEF to activate Arf6 at the cell:cell interface in a Rab/Arf cascade [Citation52,Citation53]. Two endosomal Arf6 GEFs are ARNO and EFA6 [Citation54,Citation55], and Arf6 and EFA6 have been demonstrated to be important for the establishment of polarity in MDCK cells [Citation56,Citation57]. To test for an interaction between Rab22a and EFA6, cells were transfected with EFA6-Flag and Rab22a-WT-GFP or Rab22a-Q64L-GFP followed by co-immunoprecipitation. As shown in , Rab22a-WT-GFP and Rab22-Q64L-GFP both co-immunoprecipitate EFA6-Flag. However, under the same conditions with ARNO-Myc and Rab22a-Q64L-GFP, there was no specific co-immunoprecipitation.
To further test for a role for Rab22a in EFA6 trafficking, we expressed EFA6-FLAG in control and Rab22a knockdown cells and imaged the distribution of EFA6 using anti-FLAG antibodies. As shown in and as reported previously [Citation54], EFA6 is localized primarily to the cell surface and membrane ruffles, presumably through activation of Arf6 [Citation58,Citation59]. However, after Rab22a knockdown, there are multiple EFA6-positive puncta present in the cytoplasm and limited plasma membrane EFA6.
To quantify the change in distribution of EFA6, we calculated the ratio of the cytoplasmic EFA6-Flag signal area and the total cytoplasmic area of the same cell. When the EFA6-Flag signal is localized in a more diffuse pattern and fills more of the cytoplasm, the binary area fraction approaches 1. However, as the EFA6-Flag localization becomes more punctate, the binary area fraction is closer to 0, due to the consolidation of EFA6-Flag signal within the cytoplasm. These results are quantified in . These results suggest that Rab22a plays a role in the delivery EFA6 to the plasma membrane. We attempted to test the effect of Rab22a KD on EFA6 localization in cell pairs by transfecting cells with EFA6 followed by plating in Matrigel, but the cells were not viable under these experimental conditions.
Discussion
Membrane trafficking and control of membrane composition have an essential role in the establishment and maintenance of epithelial polarity, and multiple small GTPases have been demonstrated to control trafficking and assembly of the apical plasma membrane domain [Citation60–62]. We have shown previously that Rab14, a Rab associated with endosomes and the Trans-Golgi Network [Citation24,Citation44], is upstream of Arf6 and generates a phosphatidylinositol 4,5-bisphosphate rich membrane domain at the AMIS, allowing for recruitment of Cdc42 and subsequent polarization cues that result in the single lumen formation [Citation11]. Our results reported here indicate that Rab22a, another endosomal Rab, is also important in this pathway. Our results suggest that Rab22a acts to establish polarity by delivering the Arf6 GEF EFA6 to Arf6 at the cell interface to alter lipid composition and promote formation of the AMIS.
Polarity is established through membrane trafficking, regulation of lipid composition, and recruitment of polarity proteins and scaffolding molecules. Cytokinesis also requires polarized membrane trafficking to the midbody [Citation18Citation63–66], and recent results have implicated the midbody as an early cue in the establishment of the apical domain [Citation19,Citation67,Citation68]. Many small GTPases, including Arf6, Rab11, Rab14, and Rab35 have been localized to this structure [Citation18,Citation63,Citation64,Citation67]. Interestingly, while Rab22a has not been localized to the midbody, knockdown of Rab22 in C. elegans results in embryonic lethality and germline cytokinesis defects [Citation69].
Our results show that Rab22a is required for normal polarity in epithelial cells. Rab22a is an endosomal Rab that associates with clathrin-independent trafficking pathways [Citation41]. Rab22a polarizes to the immune synapse after T cell activation, and expression of the dominant negative form of Rab22a limits the formation of the immune synapse [Citation35]. Interestingly, Rab35 is also essential for formation of the immune synapse [Citation70], suggesting that these two molecules could antagonize each other in multiple cell types to establish polarity. This effect is likely through an impact on phospholipid domains, as this and another study examining Rab22a in MDCK cells found that Rab22a does not associate with PDCX-positive vesicles [Citation51] and thus Rab22a is unlikely to impact polarity through trafficking of this molecule.
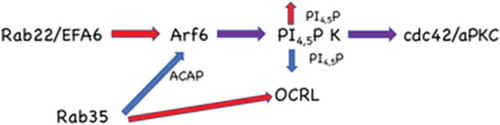
Rab14 depletion results in decreased phosphatidylinositol 4,5-bisphosphate (PtdIns4,5P2) at the cell interface in polarizing MDCK cells [Citation11], and this decreased phospholipid was thought to be a result of decreased Arf6 stimulation of phosphatidylinositol 4-phosphate 5-kinase, since overexpression of this lipid kinase rescued the Rab14KD phenotype [Citation11]. Here we show that Rab14 and Rab22a colocalize in endosomes but that Rab22 also localizes to the cell interface. While it is possible that these Rab proteins are acting in parallel pathways, since Rab22a knockdown results in decreased Arf6 activity and intracellular accumulation of EFA6, this points to a model in which Rab22a delivers EFA6 to the interface, activating Arf6, resulting in increased phosphatidylinositol 4-phosphate 5-kinase activity. Interestingly, Rab35 negatively regulates Arf6 activity through binding to the Arf6 GAP ACAP2 and also binds to the phosphatidylinositol 4,5-bisphosphatase, OCRL [Citation13,Citation29,Citation32]. We thus propose a model () that these small GTPases act in opposition to ensure that the lipid composition of the forming apical membrane is tightly regulated.
Figure 6. Model for Rab22a function in the establishment of polarity. Rab22a delivers EFA6 to the forming apical membrane, activating Arf6, resulting in increased phosphatidylinositol 4-phosphate 5-kinase activity and thus PtdIns4,5P2. Rab35 negatively regulates Arf6 activity through the Arf6 GAP ACAP2. Rab35 also regulates the phosphatidylinositol 4,5-bisphosphatase, OCRL. Both of these Rab35 regulated activities would decrease PtdIns4,5P2 at the plasma membrane. This tight regulation of the lipid composition allows the appropriate recruitment of polarity proteins such as Cdc42 and aPKC
Supplemental Material
Download MS Word (7.4 MB)Acknowledgments
We would like to thank Julie Donaldson (National Institutes of Health) for her kind gift of Rab22a-WT-GFP, and Rab22a-Q64L-GFP, and James Casanova (University of Virginia) for providing EFA6-FLAG and ARNO-Myc plasmids and helpful discussions. We thank Ruifeng Lu for help with three-dimensional cyst culture and the Wilson lab for helpful discussions. This work was funded by the National Institutes of Health RO1 DK109701 (J.M.W.), The University of Arizona Honors College Spirit of Inquiry fellowship (I.R.B.), a Science Education Award from the Howard Hughes Medical Institute to Macalester College (C.B.H.), and The University of Arizona Cancer Center (National Cancer Institute Cancer Center Support Grant P30 CA023074).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Akhtar N, Streuli CH. An integrin-ILK-microtubule network orients cell polarity and lumen formation in glandular epithelium. Nat Cell Biol. 2013;15:17–27.
- Bryant DM, Roignot J, Datta A, et al. A molecular switch for the orientation of epithelial cell polarization. Dev Cell. 2014;31:171–187.
- Liu KD, Datta A, Yu W, et al. Rac1 is required for reorientation of polarity and lumen formation through a PI 3-kinase-dependent pathway. Am J Physiol Renal Physiol. 2007;293:F1633–1640.
- Yu W, Shewan AM, Brakeman P, et al. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 2008;9:923–929.
- Feng W, Wu H, Chan LN, et al. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008;283:23440–23449.
- Martin-Belmonte F, Gassama A, Datta A, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397.
- Peng J, Awad A, Sar S, et al. Phosphoinositide 3-kinase p110delta promotes lumen formation through the enhancement of apico-basal polarity and basal membrane organization. Nat Commun. 2015;6:5937.
- Jewett CE, Prekeris R. Insane in the apical membrane: trafficking events mediating apicobasal epithelial polarity during tube morphogenesis. Traffic. 2018;19:666–678.
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045.
- Klinkert K, Rocancourt M, Houdusse A, et al. Rab35 GTPase couples cell division with initiation of epithelial apico-basal polarity and lumen opening. Nat Commun. 2016;7:11166.
- Lu R, Wilson JM. Rab14 specifies the apical membrane through Arf6-mediated regulation of lipid domains and Cdc42. Sci Rep. 2016;6:38249.
- Monteleon CL, Sedgwick A, Hartsell A, et al. Establishing epithelial glandular polarity: interlinked roles for ARF6, Rac1, and the matrix microenvironment. Mol Biol Cell. 2012;23:4495–4505.
- Mrozowska PS, Fukuda M. Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures. J Cell Biol. 2016b;213:355–369.
- Osmani N, Peglion F, Chavrier P, et al. Cdc42 localization and cell polarity depend on membrane traffic. J Cell Biol. 2010;191:1261–1269.
- Roland JT, Bryant DM, Datta A, et al. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci U S A. 2011;108:2789–2794.
- Sakamori R, Das S, Yu S, et al. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J Clin Invest. 2012;122:1052–1065.
- Tushir JS, Clancy J, Warren A, et al. Unregulated ARF6 activation in epithelial cysts generates hyperactive signaling endosomes and disrupts morphogenesis. Mol Biol Cell. 2010;21:2355–2366.
- Kelly EE, Horgan CP, Adams C, et al. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol Cell. 2010;102:51–62.
- Li D, Mangan A, Cicchini L, et al. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep. 2014;15:428–437.
- Peterman E, Prekeris R. The postmitotic midbody: regulating polarity, stemness, and proliferation. J Cell Biol. 2019;218:3903–3911.
- Schluter MA, Pfarr CS, Pieczynski J, et al. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–4663.
- Ueda T, Hanai A, Takei T, et al. EFA6 activates Arf6 and participates in its targeting to the Flemming body during cytokinesis. FEBS Lett. 2013;587:1617–1623.
- Wang T, Yanger K, Stanger BZ, et al. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J Cell Sci. 2014;127:2483–2492.
- Kitt KN, Hernandez-Deviez D, Ballantyne SD, et al. Rab14 regulates apical targeting in polarized epithelial cells. Traffic. 2008;9:1218–1231.
- Lu R, Dalgalan D, Mandell EK, et al. PKCiota interacts with Rab14 and modulates epithelial barrier function through regulation of claudin-2 levels. Mol Biol Cell. 2015;26:1523–1531.
- Lu R, Johnson DL, Stewart L, et al. Rab14 regulation of claudin-2 trafficking modulates epithelial permeability and lumen morphogenesis. Mol Biol Cell. 2014;25:1744–1754.
- Dambournet D, Machicoane M, Chesneau L, et al. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat Cell Biol. 2011;13:981–988.
- Kouranti I, Sachse M, Arouche N, et al. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–1725.
- Allaire PD, Seyed Sadr M, Chaineau M, et al. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci. 2013;126:722–731.
- Chesneau L, Dambournet D, Machicoane M, et al. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr Biol. 2012;22:147–153.
- Kobayashi H, Fukuda M. Rab35 regulates Arf6 activity through centaurin-beta2 (ACAP2) during neurite outgrowth. J Cell Sci. 2012;125:2235–2243.
- Miyamoto Y, Yamamori N, Torii T, et al. Rab35, acting through ACAP2 switching off Arf6, negatively regulates oligodendrocyte differentiation and myelination. Mol Biol Cell. 2014;25:1532–1542.
- Cauvin C, Rosendale M, Gupta-Rossi N, et al. Rab35 GTPase triggers switch-like recruitment of the lowe syndrome lipid phosphatase OCRL on newborn endosomes. Curr Biol. 2016;26:120–128.
- Cebrian I, Croce C, Guerrero NA, et al. Rab22a controls MHC-I intracellular trafficking and antigen cross-presentation by dendritic cells. EMBO Rep. 2016;17:1753–1765.
- Johnson DL, Wayt J, Wilson JM, et al. Arf6 and Rab22 mediate T cell conjugate formation by regulating clathrin-independent endosomal membrane trafficking. J Cell Sci. 2017;130:2405–2415.
- Kauppi M, Simonsen A, Bremnes B, et al. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J Cell Sci. 2002;115:899–911.
- Mayorga LS, Cebrian I. Rab22a: A novel regulator of immune functions. Mol Immunol. 2019;113:87–92.
- Mesa R, Salomon C, Roggero M, et al. Rab22a affects the morphology and function of the endocytic pathway. J Cell Sci. 2001;114:4041–4049.
- Shakya S, Sharma P, Bhatt AM, et al. Rab22A recruits BLOC-1 and BLOC-2 to promote the biogenesis of recycling endosomes. EMBO Rep. 2018;19. DOI:10.15252/embr.201845918
- Wang L, Liang Z, Li G. Rab22 controls NGF signaling and neurite outgrowth in PC12 cells. Mol Biol Cell. 2011;22:3853–3860.
- Weigert R, Yeung AC, Li J, et al. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell. 2004;15:3758–3770.
- Zhu H, Liang Z, Li G. Rabex-5 is a Rab22 effector and mediates a Rab22-Rab5 signaling cascade in endocytosis. Mol Biol Cell. 2009;20:4720–4729.
- Cox CM, Mandell EK, Stewart L, et al. Endosomal regulation of contact inhibition through the AMOT:YAP pathway. Mol Biol Cell. 2015;26:2673–2684.
- Junutula JR, De Maziere AM, Peden AA, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell. 2004;15:2218–2229.
- Kyei GB, Vergne I, Chua J, et al. Rab14 is critical for maintenance of Mycobacterium tuberculosis phagosome maturation arrest. Embo J. 2006;25:5250–5259.
- Linford A, Yoshimura S, Nunes Bastos R, et al. Rab14 and its exchange factor FAM116 link endocytic recycling and adherens junction stability in migrating cells. Dev Cell. 2012;22:952–966.
- Okai B, Lyall N, Gow NA, et al. Rab14 regulates maturation of macrophage phagosomes containing the fungal pathogen Candida albicans and outcome of the host-pathogen interaction. Infect Immun. 2015;83:1523–1535.
- Reed SE, Hodgson LR, Song S, et al. A role for Rab14 in the endocytic trafficking of GLUT4 in 3T3-L1 adipocytes. J Cell Sci. 2013;126:1931–1941.
- Ueno H, Huang X, Tanaka Y, et al. KIF16B/Rab14 molecular motor complex is critical for early embryonic development by transporting FGF receptor. Dev Cell. 2011;20:60–71.
- Weimershaus M, Maschalidi S, Sepulveda F, et al. Conventional dendritic cells require IRAP-Rab14 endosomes for efficient cross-presentation. J Immunol. 2012;188:1840–1846.
- Mrozowska PS, Fukuda M. Regulation of podocalyxin trafficking by Rab small GTPases in 2D and 3D epithelial cell cultures. J Cell Biol. 2016a;213:355–369.
- Jones S, Jedd G, Kahn RA, et al. Genetic interactions in yeast between Ypt GTPases and Arf guanine nucleotide exchangers. Genetics. 1999;152:1543–1556.
- Pfeffer SR. Rab GTPases: master regulators that establish the secretory and endocytic pathways. Mol Biol Cell. 2017;28:712–715.
- Franco M, Peters PJ, Boretto J, et al. EFA6, a sec7 domain-containing exchange factor for ARF6, coordinates membrane recycling and actin cytoskeleton organization. Embo J. 1999;18:1480–1491.
- Frank S, Upender S, Hansen SH, et al. ARNO is a guanine nucleotide exchange factor for ADP-ribosylation factor 6. J Biol Chem. 1998;273:23–27.
- Milanini J, Fayad R, Partisani M, et al. EFA6 proteins regulate lumen formation through alpha-actinin 1. J Cell Sci. 2018;131:jcs209361.
- Shultz T, Nash-Livni N, Shmuel M, et al. EFA6 regulates endosomal trafficking and affects early endosomes in polarized MDCK cells. Biochem Biophys Res Commun. 2006;351:106–112.
- Macia E, Partisani M, Favard C, et al. The pleckstrin homology domain of the Arf6-specific exchange factor EFA6 localizes to the plasma membrane by interacting with phosphatidylinositol 4,5-bisphosphate and F-actin. J Biol Chem. 2008;283:19836–19844.
- Radhakrishna H, Al-Awar O, Khachikian Z, et al. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112(Pt 6):855–866.
- Klinkert K, Echard A. Rab35 GTPase: a central regulator of phosphoinositides and F-actin in endocytic recycling and beyond. Traffic. 2016;17:1063–1077.
- Parker SS, Cox C, Wilson JM. Rabs set the stage for polarity. Small GTPases. 2018;9:116–129.
- Roman-Fernandez A, Bryant DM. Complex polarity: building multicellular tissues through apical membrane traffic. Traffic. 2016;17:1244–1261.
- Fielding AB, Schonteich E, Matheson J, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. Embo J. 2005;24:3389–3399.
- Kaplan A, Reiner O. Linking cytoplasmic dynein and transport of Rab8 vesicles to the midbody during cytokinesis by the doublecortin domain-containing 5 protein. J Cell Sci. 2011;124:3989–4000.
- Montagnac G, Echard A, Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr Opin Cell Biol. 2008;20:454–461.
- Yu X, Prekeris R, Gould GW. Role of endosomal Rab GTPases in cytokinesis. Eur J Cell Biol. 2007;86:25–35.
- Fremont S, Echard A. Membrane traffic in the late steps of cytokinesis. Curr Biol. 2018;28:R458–R470.
- Mangan AJ, Sietsema DV, Li D, et al. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat Commun. 2016;7:12426.
- Skop AR, Liu H, Yates III J, et al. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66.
- Patino-Lopez G, Dong X, Ben-Aissa K, et al. Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J Biol Chem. 2008;283:18323–18330.
