ABSTRACT
Rho GTPases are known to play an essential role in fundamental processes such as defining cell shape, polarity and migration. As such, the majority of Rho GTPases localize and function at, or close to, the plasma membrane. However, it is becoming increasingly clear that a number of Rho family proteins are also associated with the Golgi complex, where they not only regulate events at this organelle but also more widely across the cell. Given the central location of this organelle, and the numerous membrane trafficking pathways that connect it to both the endocytic and secretory systems of cells, it is clear that the Golgi is fundamental for maintaining cellular homoeostasis. In this review, we describe these GTPases in the context of how they regulate Golgi architecture, membrane trafficking into and away from this organelle, and cell polarity and migration. We summarize the key findings that show the growing importance of the pool of Rho GTPases associated with Golgi function, namely Cdc42, RhoA, RhoD, RhoBTB1 and RhoBTB3, and we discuss how they act in concert with other key families of molecules associated with the Golgi, including Rab GTPases and matrix proteins.
Introduction
Cells rely on a wide variety of mechanisms to achieve homoeostasis. This is particularly pertinent with respect to the endomembrane system, which needs to ensure a specific distribution of proteins, lipids, carbohydrates and signalling molecules across all its constituent compartments such that the functionality of each is maintained. The organelles of the endomembrane system are linked through trafficking pathways, which together ensure that critical processes such as endocytosis and secretion are tightly regulated. If this balance is lost, it can lead to disease states such as metabolic defects [Citation1], skeletal disorders [Citation2,Citation3] and cancer [Citation2,Citation4–6]. Considering all the organelles within the cell, the Golgi apparatus, from both a physical and functional perspective, is central. The Golgi is well known for its roles in the modification, sorting and delivery of proteins to downstream organelles in the secretory pathway, but as discussed below, it is also linked to other critical cellular processes such as signalling, cell polarity and migration. One unique feature of the Golgi is its physical structure. It consists of a number of flattened and interconnected membrane discs, called cisternae, and in many mammalian cells, these cisternae form a higher order structure termed the Golgi ribbon. The cisternae assemble into stacks, which can be subdivided into the cis, medial and trans cisternae. The cis side receives material from the endoplasmic reticulum (ER), whereas the trans side coordinates cargo flow towards the endosomal system, as well as the plasma membrane. The trans-most cisterna is a highly convoluted membrane with its own distinct functionality, and is termed the trans-Golgi network (TGN). In order to fulfil their specialized tasks, each cisterna has its own set of resident enzymes involved in specific modifications (e.g. glycosylation, phosphorylation, sulphation), and as such, disruption to the function or morphology of the Golgi can lead to disease [Citation7–9].
The Golgi, like the entire endomembrane system, is regulated by a wide variety of protein families, with the master regulators widely accepted to be the Rab family of small GTPases [Citation10]. In human cells, the Rab family consists of over 60 members, with one distinctive feature being that they each localize to specific organelles. Rab proteins cycle between an active (GTP-bound) and inactive (GDP-bound) state. Once they are active and bound to their preferred membrane compartment, they can interact with different effectors, which in turn can positively or negatively influence the membrane traffic event. The activity of the Rab proteins themselves is also strictly regulated. Rabs are typically held in an inactive state, bound to Rab guanine dissociation inhibitor (GDI) proteins, but can be activated by guanine nucleotide exchange factors (GEFs), which exchange GDP for GTP. Having then recruited their relevant effectors, they are inactivated through hydrolysis of the GTP to GDP, stimulated by the presence of GTPase activating proteins (GAPs). While Rab proteins are essential controllers of all membrane trafficking events in the cell, the high complexity of the Golgi inevitably means that a large number of Rabs localize to this compartment, with studies suggesting that as many as one third of all Rab proteins play a role either physically at the Golgi, or on pathways directly linking to the Golgi [Citation11,Citation12].
However, Rab GTPases are not the only Ras family members involved in regulation of the Golgi. In recent years, Rho GTPases have also emerged as playing a critical role. This is arguably not surprising given the diversity within their family and their links to the cytoskeleton, which itself is fundamental to the organization of the Golgi. The Rho family of small GTPases has 21 members and can be broadly categorized into two groups, namely typical and atypical Rho proteins [Citation13]. The typical Rho GTPases act as molecular switches, and in a similar manner to that described for the Rabs, they are controlled through interactions with specific GEFs, GAPs and GDIs. Many of the atypical Rho GTPases are unable to hydrolyse GTP, or they show unusual GDP-GTP exchange characteristics, such that their regulation occurs through mechanisms different from conventional nucleotide exchange. Rho family members play numerous roles in the cell, including in cell polarity and migration, organization of the cytoskeleton, regulation of the cell cycle, as well as membrane trafficking [Citation13]. Although many are localized at the plasma membrane, several family members have been shown to localize to the Golgi. Since our previous review of this subject [Citation5], a number of new studies have provided additional insight into how these Rho proteins influence a variety of functional and morphological effects at the Golgi, and it is this pool of Rho GTPases that is the focus of this review.
Rho family proteins and Golgi architecture
The importance of the microtubule cytoskeleton in maintenance of Golgi morphology is widely recognized. Due to the unique positioning of the Golgi at the centrosome, it is inevitable that many proteins associated with the microtubule cytoskeleton also concentrate at this organelle. The microtubules originating from the centrosome are polarized (contain fast and slow growing ends) and this polarization, in concert with molecular motors, allows the transport of membrane carriers towards and away from the organelle in a directional way. In addition to this, the microtubule network is also responsible for the maintenance of Golgi morphology and position during cell polarization through creation of mechanical tension and force. However, it is much less clear as to how this organelle links to the actin cytoskeleton, and what function Rho GTPases may play in this regard. Nevertheless, many proteins associated with the actin network localize to the Golgi, with evidence emerging that the actin network is also important for Golgi polarization, trafficking to and from the Golgi and maintenance of its morphology [Citation14]. One such example is the protein Coronin7, which has recently been shown to interact with Cdc42 and regulate Golgi morphology via the actin nucleator WASp [Citation15].
Indeed, Cdc42 was the first Rho protein to be localized to the Golgi [Citation16]. Subsequently, other Rho family proteins including RhoA [Citation17], RhoD [Citation18] and RhoBTB3 [Citation19] have all been localized to this compartment. In turn, several actin regulatory proteins such as Arp2/3, mDia1, WASp, WAVE, WHAMM, and many others associated with actin nucleation, have been found at the Golgi [Citation20,Citation21]. Therefore, it is not surprising that the actin network, and its associated regulatory machinery, is increasingly being found to play roles in the fundamental organization of the Golgi, at both structural and functional levels. Cdc42 itself is a prime example in this respect, as it has been found to interact with the γ-COP subunit of the coat protein complex I (COPI), a proteinaceous assembly that not only is involved in membrane traffic events into and out of the Golgi, but that plays a vital role in the physical stability of the organelle [Citation22–25]. Golgi architecture is also maintained by the activity of matrix proteins, which are typically long coiled-coil peripheral proteins that serve a key function in linking the cisternal elements and in assisting transport vesicle tethering events at the Golgi. Of these, one of the best characterized molecules is GM130, which has been shown to exist in a complex with Cdc42 and one of its GEFs, Tuba, together playing a key role in organization of the centrosome [Citation26].
Given the observed localization of several Rho GTPases to the Golgi, it is logical to assume that many of their associated GEFs will also be found there, and so much recent work has been focussed on this aspect. Rho GEFs are thought to number more than 80 in total in mammalian cells [Citation27]; however, the picture is still far from clear in terms of which GEFs activate which Rho proteins. In the case of Cdc42, several GEFs have been identified, including FGD1, Dbs, and Tuba [Citation26,Citation28,Citation29], and so understanding these relationships is essential to fully decipher Cdc42 function at the Golgi [Citation30]. In this regard, a recent study has reported a new interaction between a Cdc42 GEF and the TGN-localized golgin GCC88, providing further evidence that actin assembly plays a role in Golgi morphology [Citation31]. Previously, it was shown that GCC88 function is linked to maintenance of the Golgi ribbon, as overexpression caused fragmentation of the Golgi [Citation32]. However, the question remained open as to the mechanism by which overexpression altered Golgi morphology. Possible resolution to this was recently provided by Citation31, who identified intersectin-1 (ITSN-1) as an interactor of GCC88. ITSN-1 is a Cdc42 GEF (the long isoform contains a C-terminal GEF domain) playing a role in endocytosis, regulation of the actin cytoskeleton and signalling in physiological and disease states [Citation33–35], although ITSN-1 itself was not localized to the Golgi in these previous studies. However, Makhoul and colleagues used a proximity-dependent labelling approach to demonstrate that GCC88 can guide ITSN-1 to the TGN, thus leading to activation of Cdc42 ()). Co-immunoprecipitation experiments showed that GCC88 is an interacting partner of both isoforms of ITSN-1 at the TGN, with depletion of GCC88 leading to a drastic decrease of ITSN-1 levels at these membranes. Further evidence of GCC88 and ITSN-1-mediated changes to the Golgi ribbon was shown by the Golgi phenotypes being reversible in GCC88-overexpressing cells following RNAi-mediated depletion of ITSN-1. Importantly, it was shown that changes to the Golgi ribbon are induced by actin, involving the motor myosin IIA, rather than microtubule assembly [Citation31]. Taken together, this study provides further context to the picture that golgins are important interactors with actin cytoskeleton components [Citation14], and that Cdc42 GEFs, such as ITSN-1, are critical for maintenance of Golgi architecture.
Figure 1. Rho family proteins and Golgi architecture. (a) Overexpression of the matrix protein GCC88 results in increased recruitment of the GEF intersectin-1 (ITSN-1) to the TGN, and in turn increased activation of Cdc42. This leads to Golgi fragmentation. (b) Overexpression of MEC-17 results in increased binding to ARHGAP21, inhibiting its GAP activity on Cdc42, resulting in higher levels of activated Cdc42 and elevated levels of the matrix protein GM130 at the Golgi. This leads to Golgi fragmentation. (c) Depletion of RhoBTB1 results in a reduction in expression of the METTL7B gene, which in turn leads to Golgi fragmentation. Whether this occurs via an intermediary factor is not clear
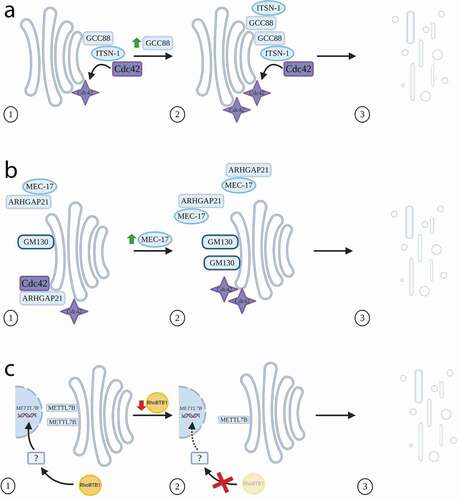
A number of other Cdc42 interactors have also been shown to influence the state of the Golgi. MEC-17 is a recently identified ɑ-tubulin acetyltransferase, and a recent study in A549 cells has shown that an increase in MEC-17 levels results in Golgi dispersion and elevated levels of GM130 protein, likely through upregulation of GM130 gene expression [Citation36] ()). MEC-17 controls Cdc42 activity through inhibiting the Golgi-localized GAP, ARHGAP10 (also known as ARHGAP21). This Rho GAP has previously been described as a key regulator of Cdc42, being recruited to the Golgi in an ADP ribosylation factor-1 (ARF1)-dependent manner, and playing a role in Golgi organization [Citation37]. In this new study, the overexpression of MEC-17 was found to result in the level of active GTP-bound Cdc42 increasing, which in turn led to the phenotypes seen on GM130 at the Golgi. Ultimately, Golgi orientation is lost, with consequences on polarity and migration (discussed below), including epithelial-mesenchymal transition (EMT) [Citation36]. This link between GM130 and Cdc42 at the Golgi is clearly crucial, with other work showing that in fact GM130 regulates Cdc42 activity by binding RasGRF2 [Citation38], a Ras GEF, which was previously shown to inhibit Cdc42 activity by competing against Cdc42 GEFs for the binding of inactive Cdc42 [Citation39]. Depletion of GM130 reduces the level of active Cdc42 at the leading edge of HeLa cells thus destroying the asymmetric distribution of Cdc42 in migrating cells. The reduction of active Cdc42 at the leading edge resulted from blocked trafficking from the Golgi to the plasma membrane [Citation38]. However, the work of Lee and colleagues leaves open the question of whether MEC-17 controls Cdc42 activity solely through inhibition of ARHGAP21, or whether by also increasing the levels of GM130, there is increased binding to RasGRF2 (thus reducing its inhibitory ability) and thereby resulting in an increase in availability of Cdc42 for activation. Together, these findings not only illustrate the role of Cdc42 in the fundamental organization of the Golgi, but critically how Cdc42 and its interactors regulate the position of the Golgi and the consequences of this with respect to cell polarity and migration (discussed further later).
In addition to the typical Rho GTPases, atypical Rho GTPases are also implicated in structural aspects of the Golgi. One such example is the RhoBTB family, consisting of three members (RhoBTB1-3). Their structure is unique in the wider family of Rho GTPases, as following their GTPase domains these proteins contain a proline-rich region and two BTB domains, involved in homo- and hetero-dimerization, as well as binding to other factors [Citation40,Citation41]. The first RhoBTB protein to be associated with the Golgi was RhoBTB3 [Citation19,Citation42], although most studies suggest that its role relates more with trafficking pathways into the Golgi (discussed below), rather than with Golgi architecture itself. Another family member, RhoBTB1, has primarily been studied in the context of tumourigenesis, with its localization suggested to be at membrane ruffles and peripheral punctate structures [Citation43]. Although RhoBTB1 does not seem to be itself localized to the Golgi, a recent study by [Citation44], showed that RhoBTB1 has an indirect effect on Golgi morphology by regulating expression of METTL7B, the gene encoding a Golgi-associated methyltransferase identified several years ago by subcellular proteomics [Citation45]. RNAi-mediated depletion of RhoBTB1 in HeLa cells decreased the expression levels of METTL7B, which led to fragmentation of the Golgi ()); a phenotype that could be reversed by ectopic expression of METTL7B [Citation44]. Experiments using a GFP-tagged version of METTL7B indicated that this protein localizes to the Golgi [Citation45]; however, this more recent study reported a localization of METTL7B to the ER. This discrepancy may be due to the use of different cell types in the two studies. Interestingly, the endogenous expression levels of RhoBTB1 were found to be comparatively lower in several cancer cell lines, with T47D cells displaying a fragmented Golgi phenotype, highly similar to that seen in the HeLa cells depleted for RhoBTB1 using RNAi. Strikingly, a conventional juxtanuclear Golgi pattern could be generated in the T47D cells by overexpression of RhoBTB1. The authors also carried out cell migration studies, revealing in these cells that reduction of RhoBTB1 leads to an invasive phenotype, thereby demonstrating an intriguing link between RhoBTB1 function as a tumour suppressor and a role in Golgi morphology [Citation44].
The common theme in all the above findings is that modulation of levels of a number of Rho proteins and their effectors can influence Golgi architecture. However, such phenotypes do not necessarily implicate the respective protein with a direct function in control of Golgi morphology. For example, modulation of membrane trafficking pathways into and out of this organelle can also result in a change in Golgi shape and composition (discussed further below). Indeed, an RNAi screen systematically targeting all Rho GTPases in HeLa cells was recently reported, in which the primary read-out was Golgi fragmentation [Citation46]. Consistent with the existing literature, many of the strongest fragmentation phenotypes were seen in cells depleted of Cdc42 and RhoA. Interestingly, depletion of both RhoBTB1 and RhoBTB3 also induced strong Golgi fragmentation, but in both of these cases, the fragmentation phenotype was attributed to alterations in membrane trafficking events into the Golgi.
Regulation of Golgi-associated membrane trafficking events by Rho proteins
The Golgi is a central organelle in the cell, and its homoeostasis is dependent on balanced transport of proteins, carbohydrates, lipids both into and out of the organelle. Cargo molecules can be either synthesized in the ER and transported through the Golgi to their destination within or outside the cell via the anterograde pathway, or can be endocytosed from the plasma membrane. In the latter case, internalized cargo is predominantly transported to endocytic compartments, however transport pathways from these to the TGN and Golgi, are critical to balance overall membrane flux in the cell. Similarly, the retrograde pathway provides a route from the Golgi back to the ER. Once again, of all of the Rho family members, it is Cdc42 that has been among the first to be shown to play a role in trafficking events at the Golgi, specifically through interactions with the γ-COP subunit of the COPI coat [Citation22–25]. Cdc42 was initially thought to only play a role in the retrograde trafficking pathway, with experiments showing that Cdc42 is localized to Golgi-associated coated and uncoated vesicles, and influencing retrograde transport of the Shiga toxin from the plasma membrane to the ER, as well as the transport of the KDEL receptor from the Golgi to the ER [Citation23,Citation47]. Moreover, Citation22, demonstrated that Cdc42 impedes the binding of the dynein motor to COPI vesicles allowing time for cargo packaging and coatomer assembly. More recently however, Cdc42 has also been shown to play a role in anterograde transport through the Golgi. The mechanism involves diminishing Golgi membrane curvature, in turn stimulating COPI formation to sort anterograde cargo into tubules. At the same time, it controls bidirectional Golgi transport by affecting COPI function in cargo sorting and carrier formation [Citation24]. Cdc42 function in membrane trafficking at the Golgi is also regulated through its GEFs. For example, inhibition of the GEF Dbs leads to reduced activity of Cdc42, and as a result, transport from the Golgi to the plasma membrane of the model secretory cargo protein, the vesicular stomatitis virus glycoprotein (VSV-G), is impaired [Citation48].
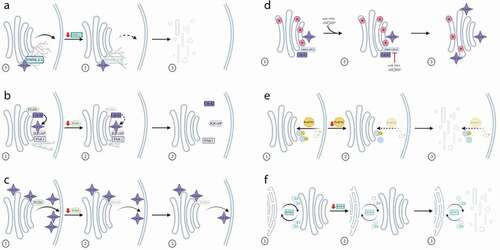
As discussed above, Cdc42 is widely considered to be the most prevalent Rho at the Golgi, where it has the ability to interact with a wide variety of actin-associated molecules. One such family of proteins utilized in the assembly of actin filaments is the formins, containing 15 members in mammals [Citation49]. Earlier studies have shown that three members of the formin family, namely mDia1, INF2 and FMNL1 isoform γ were localized to the Golgi complex [Citation50–52]. In 2017, Kage and colleagues reported two further members of the formin family, FMNL2 and FMNL3, to localize to medial and trans elements of the Golgi [Citation53]. This localization was enhanced in the presence of activated Cdc42 leading to a denser actin network around the Golgi. In contrast to the mechanism by which FMNL2 and FMNL3 localize to lamellipodia, the mechanism of localization to the Golgi was shown to be through their myristoylated N-terminus as well as through recruitment by Cdc42. RNAi and knock-out cell line models were then used to assess the functional importance of these formins at the Golgi, with both approaches consistently showing a fragmented Golgi phenotype. However, in the knock-out cells, these formins were not seen to play any role in Golgi assembly, but instead to function in anterograde trafficking from the Golgi, demonstrated by a reduction in delivery of transport carriers containing the cargo molecule VSV-G arriving at the plasma membrane [Citation53] ()). Although this work did not provide a mechanistic explanation for the role of these formins working in tandem with Cdc42 at the Golgi, the findings are entirely consistent with the proposed role for Cdc42 in regulating membrane curvature as part of the process in transport vesicle generation at the Golgi [Citation24].
Figure 2. Regulation of Golgi-associated membrane trafficking events by Rho proteins. (a) The formin family members FMNL2 and FMNL3 are recruited to the Golgi in a Cdc42-dependent mechanism. Depletion of FMNL2/3 results in reduced actin assembly at the Golgi, a reduction in transport from the Golgi to the plasma membrane, and Golgi fragmentation. (b) Depletion of the GEF FGD1 results in reduced activation of Cdc42, which working in concert with IQGAP and PAK1 leads to shorter microtubules and reduced levels of Golgi to plasma membrane trafficking. (c) Depletion of the GEF FGD1 results in a decrease of active Cdc42 at the plasma membrane, but does not affect levels of active Cdc42 at the Golgi. (d) The microRNA miR-199a inhibits the activity of the Cdc42 GAP ARHGAP21, resulting in increased activation of Cdc42 and a reduction in transport of the HSV-1 virus through the Golgi. (e) RhoBTB1 is observed on endocytic carriers. Its depletion results in reduced delivery of cargo to the Golgi and fragmentation of the Golgi. (f) RhoBTB3 is observed on membrane carriers at the ER-Golgi interface. Depletion of RhoBTB3 results in a reduction in the speed of movement of these carriers and Golgi fragmentation
Given the importance of maintaining high levels of Cdc42 at the Golgi, it seems pertinent to ask how this is achieved in cells. The complex localization pattern of Cdc42, and other Rho GTPases throughout the endomembrane system [Citation54], inevitably means that the Rho proteins must use specific binding partners at the various organelles. In the case of Cdc42, it is becoming increasingly clear that the active pool of this protein at the Golgi is controlled by many different regulators, with GEFs being potential candidates. One example is FGD1, a GEF of Cdc42 shown originally to be localized to patches underlying the plasma membrane as well as at the Golgi [Citation28]. Mutations in the FGD1 gene are linked to the rare disease faciogenital dysplasia, and it was this observation that first drew attention to its possible role in the cell [Citation55]. It was subsequently shown that the FGD1 protein localizes to the TGN and that its depletion results in reduced transport of VSV-G cargo between the TGN and cell surface. In addition, overexpression of an FGD1 mutant, which was unable to activate Cdc42, also resulted in impaired post-TGN transport of endogenous cargo in osteoblast cells [Citation56].
Additional downstream effectors of Cdc42 involved in the trafficking of these post-Golgi carriers have also been identified [Citation57]. Based on knowledge of several known Cdc42 interactors, an RNAi approach on these candidates was applied, comparing the post-Golgi trafficking phenotypes observed in these cells with those in FGD1-depleted cells. This allowed identification of IQGAP1, a Ras GTPase-activating-like protein, and the kinase PAK1, as being involved in FGD1-dependent trafficking from the TGN. Nevertheless, their overexpression could not completely recover the deficiency in post-Golgi trafficking in FGD1-depleted cells, suggesting that other FGD1/Cdc42 effectors were also abrogated as a result of the FGD1 depletion. However, perhaps the most interesting finding in this work was that depletion of either FGD1, IQGAP1 or PAK1 all resulted in suppression of microtubule growth from the TGN, thereby providing an explanation for why post-Golgi carrier formation was inhibited ()). The authors speculate that the FGD1/Cdc42 downstream signalling cascade may extend to microtubule-associated proteins such as the CLASPs [Citation57].
While evidence for the involvement of FGD1/Cdc42 in post-Golgi transport seems strong, these studies described above do not specifically address whether FGD1 is itself responsible for activation of Cdc42 at the Golgi. This important point has been studied using a biosensor approach, which allowed the distinct populations of active Cdc42 at the Golgi and plasma membrane to be assessed [Citation58]. Not only did this work reveal that active Cdc42 could be detected equally across all Golgi cisternae, and not just at the TGN, but surprisingly that RNAi-induced depletion of FGD1 decreased the level of Cdc42 at the plasma membrane while having no effect on the pool localized at the Golgi, thereby suggesting that FGD1 is not the primary GEF for Cdc42 activation at the Golgi ()). Furthermore, they went on to show that in fact either downregulation of the Cdc42 GEF, Tuba [Citation26,Citation59], or overexpression of the Cdc42-associated GAP, ARHGAP10 (ARHGAP21) [Citation37] could reduce the activity of Cdc42 at the Golgi. While this work provides new insight into one mechanism for Cdc42 control at the Golgi, it still leaves open the question of how (and why) FGD1 controls the pool of Cdc42 at the plasma membrane and not at the Golgi, despite its localization there.
What are the other implications for Cdc42-regulated control of membrane traffic at the Golgi? Given that the Golgi plays such a central role in secretion, it is inevitable that changes in Cdc42 activity at this organelle may have impacts on a wide variety of molecules requiring passage through it. One example is virus assembly. The herpes simplex virus 1 (HSV-1) uses membranes from the trans-Golgi as part of its maturation, allowing it to gain a secondary envelope. As such any changes in flux through the Golgi are likely to have an effect on this process. Indeed, a recent study observed that the synthesis of the secondary envelope of HSV-1 is inhibited at the TGN [Citation60]. On further examination of this phenotype, it was shown that this effect was caused by activation of Cdc42, which in turn was driven by decreased levels of its GAP, ARHGAP21. Downregulation of this GAP was caused by activity of a specific microRNA, miR-199a, the target of which was determined to be ARHGAP21 [Citation60]. A similar phenotype could be generated by expression of a constitutively active mutant of Cdc42, which resulted in the number of secondary envelope particles being decreased. HSV-1-infected cells either treated with miR-199a or short hairpin RNAs (shRNA) targeting ARHGAP21 resulted in reduced co-localization of the viral envelope glycoprotein gD with the trans-Golgi marker p230, and an increased co-localization with the cis-/medial-Golgi marker giantin ()). These observations are fully consistent with the notion that the synthesis of the secondary envelope is blocked in the presence of active Cdc42 at the medial Golgi cisternae, before it reaches the trans-Golgi and TGN [Citation60]. However, the specific question of how activated Cdc42 blocks secondary envelope transport within the Golgi stack remains open. Taking into account the more recently reported findings that the GEF ITSN-1 can activate Cdc42 via GCC88 at the TGN [Citation31], it would now be interesting to assess whether this Cdc42 GEF also plays a direct role in HSV-1 maturation.
As discussed in the section above, members of the RhoBTB family are also implicated at the Golgi, likely not only associated with morphology of the organelle but also in regulating membrane trafficking events. In the case of RhoBTB1, two independent studies have shown that reduced levels of RhoBTB1 result in Golgi fragmentation; however, this phenotype seems to have little impact on the transport of either VSV-G secretory cargo as it passes through the Golgi, or the p24 cargo receptors that shuttle between the ER and Golgi complex [Citation44,Citation46]. However, in the latter of these two studies, RNAi-mediated downregulation of RhoBTB1 was shown to result in a reduction of delivery of the exogenous cargo Shiga-like toxin B-chain (SLTxB) from the plasma membrane to the Golgi complex. This cargo molecule is often used as a tracer, allowing the entire retrograde pathway from cell surface to ER, via the Golgi complex, to be interrogated. In RhoBTB1-depleted cells, SLTxB accumulated in punctate structures in the cell periphery and only at extended time points after internalization did it arrive in the Golgi ()). Interestingly, RhoBTB1 was partially localized to early endosomes, marked with EEA1 and Rab5, and both knockdown and overexpression impaired endosomal and lysosomal architecture [Citation46]. However, despite the strong effects on the Golgi complex, no evidence for RhoBTB1 localizing to this organelle was seen, suggesting that the strong Golgi fragmentation phenotype was a consequence of impaired traffic into the organelle from the endosomal system. Further research is needed to understand the role of RhoBTB1 in endocytic trafficking and the relationship to Golgi morphology.
Similar to RhoBTB1, RhoBTB3 has also previously been shown to be associated with trafficking events into the Golgi complex, although in this case, RhoBTB3 seems to be directly localized to the Golgi. RhoBTB3 binds to Rab9, a Rab GTPase that controls late endosome-to-Golgi trafficking of critical cargo molecules such as the mannose-6-phosphate receptor. Its proposed role is to assist in the docking of these carriers on the Golgi membrane [Citation19]. Subsequent work also revealed an additional role for RhoBTB3 in cell cycle regulation, via a RhoBTB3-Cullin3-dependent Ring-E3 ubiquitin ligase complex at the Golgi, although it remains unclear as to whether this function is linked to its role in membrane traffic [Citation42]. Most recently, our own RNAi screening work has implicated RhoBTB3 in transport events at the ER-Golgi interface. We observed a small pool of RhoBTB3 on transport carriers containing the cargo receptor p24, and the depletion of RhoBTB3 reduced the speed of these carriers as well as inducing Golgi fragmentation [Citation46] ()). Whether RhoBTB3 also functions in docking events at the cis face of the Golgi remains to be established.
As mentioned above, the formin family member mDia1 is localized to the Golgi. mDia1 is a downstream target of RhoA and plays a role in the formation of Rab6-positive transport carriers emanating from the Golgi. Previous work has shown that overexpression of a constitutively active mutant of RhoA increases the formation of Rab6-positive carriers, whereas depletion of mDia1 suppresses their formation [Citation52]. RhoA activation in the juxtanuclear area, encompassing both Golgi elements and the endosomal recycling compartment (ERC), has also been seen following RNAi-mediated depletion of the RhoA GAP, DLC3 (deleted in liver cancer 3). In these cells, Golgi fragmentation occurred, as well as vesiculation of ERC membranes coated with the small GTPase Rab8, likely due to an imbalance in trafficking [Citation61]. These aspects of RhoA function at the Golgi have previously been reviewed extensively [Citation5], and since that time little new information about its function has emerged, although the RhoA GEF, ARHGEF10, and Rab8 have both been implicated [Citation62].
The final Rho worth consideration with respect to membrane trafficking events at the Golgi is RhoD. This family member was one of the first Rho proteins to be linked with membrane trafficking, specifically being associated with endosomal pathways [Citation63,Citation64]. RhoD has been reported to also exist in a Golgi-localized pool, predominantly co-localizing with the trans-Golgi marker GalT [Citation18]. Overexpression of either dominant negative or constitutively active RhoD point mutants resulted in defects in VSV-G transport, with this secretory cargo seemingly blocked in an early Golgi compartment. This study went on to show that this transport defect, along with observed reorganization of Golgi membranes, was likely through links to its binding partner, the actin nucleator WHAMM [Citation65]. More recently however, the same authors have published another study of RhoD, in which they focus on the pool of RhoD localizing to early endosomes and motile vesicles [Citation66]. In this latter study they propose a role for RhoD in vesicle fusion, with no mention, or indeed little evidence for RhoD at the Golgi, suggesting that there remains much to be discovered about this Rho family member.
Roles in cell polarity and migration
Perhaps the most intriguing aspect of the function of Rho proteins at the Golgi relates to their emerging roles in defining cell polarity and direction of migration. Cell motility is not only an important process during embryogenesis but also for general maintenance of tissues and organs. While it is clear that the majority of Rho proteins that drive these processes do this from a localization at or close to the plasma membrane [Citation67,Citation68], an ever increasing body of evidence implicates the Golgi pool of several Rho GTPases in the regulation of cell polarity and migration. Very early work, utilizing wound healing assays, showed that orientation of the Golgi is important for the determination of the leading edge in migrating cells [Citation69]. Similarly, wound healing assays have also been used to show that the position of the Golgi is critical in defining polarity [Citation70], with Cdc42 activity being fundamental to microtubule-dependent Golgi positioning [Citation71]. Further molecular understanding of how activity at the Golgi can regulate events at the plasma membrane continue to emerge. For example, Preisinger and colleagues showed that one regulatory mechanism of cell migration was via interaction of the Golgi matrix protein GM130 with two members of the MST family of Ste20 kinases, namely YSK1 and MST4. Through the binding to GM130, both proteins were able to associate with the Golgi and become autophosphorylated, leading to their activation. Curiously however, YSK1 and MST4 have opposite effects on cell migration. YSK1 phosphorylates the 14-3-3ζ protein and thus activates further downstream targets to activate cell migration, whereas, MST4 inhibits cell migration, suggesting that YSK1 and MST4 compete against each other for the binding to GM130 [Citation72]. Interestingly, depletion of the matrix protein GRASP65, a binding partner of GM130, along with causing a Golgi organization defect also results in impaired migratory capacity of cells, emphasizing the critical relationship between Golgi organization and events at the cell periphery [Citation73]. The molecular link with respect to all of the above is of course Cdc42 and its network of interactors. As such, it is now widely accepted that the Golgi-localized pool of Cdc42 plays roles far beyond this organelle [Citation74]. Given the important role for Golgi matrix proteins in organizing the shape and structure of this organelle, it is perhaps unsurprising that it is these proteins, particularly GM130, that also regulate Cdc42 function. Elegant work from the Farhan laboratory strongly supports the idea that GM130 controls the activity of Cdc42, via binding and release of the Ras GEF RasGRF2 [Citation38]. The proposed mechanism involves RasGRF2 competing with Cdc42 GEFs for binding to inactive Cdc42 [Citation39]. While RasGRF2 is bound to GM130, Cdc42 is available for activation by its GEFs, which in turn results in changes to Golgi polarity and ultimately activity of Cdc42 at the leading edge of cells [Citation38]. By contrast, when RasGRF2 is released, it binds Cdc42 preventing its activation and subsequent downstream effects. This mechanism fully aligns with the concept of the Golgi acting as a reservoir for Cdc42, such that Cdc42 can be rapidly transported to the leading edge plasma membrane when needed at that location.
Given the importance of the cell being able to utilize a Golgi-localized pool of Cdc42 for control of events at the plasma membrane during migration, it is inevitable that Cdc42 will come into contact with a variety of membrane trafficking machinery molecules as part of this. In this regard, the small GTPase Rab6 has recently emerged as another potential Cdc42 interactor. Rab6 is a critical regulator of transport pathways both into and away from the Golgi, using mechanisms that are independent of the COPI coat. Recently, it has been shown that depletion of Rab6 causes an increase in cell migration speed and reorganization of the actin network, likely through activation of Cdc42. Further investigation revealed that in fact Rab6 can bind Cdc42, and that the two GTPases can be visualized on transport carriers moving from the Golgi area of cells towards the cell periphery, as well as at filopodia [Citation75]. The mechanism of Cdc42 activation in this case seems to be via the protein Trio, a member of the Dbl family of GEFs [Citation76,Citation77]. Interestingly, Trio contains a putative Rab6 binding site, allowing speculation that Cdc42 activity can be regulated through a complex of Rab6, Trio, and the motor myosin II, the latter of which is controlled via its phosphorylation state. When Rab6 is silenced, myosin II phosphorylation decreases, leading to the abolishment of Trio inhibition and therefore activation of Cdc42 [Citation75] ()). These findings further strengthen the importance of cross-talk between Rho and Rab activities [Citation78–80].
Figure 3. Roles in cell polarity and migration. (a) Cdc42 and Rab6 can be found on carriers moving between the Golgi and plasma membrane. Depletion of Rab6 reduces the inhibition of Trio, allowing greater activation of Cdc42, which leads to increased cell migration. (b) Activated RhoA binds FAM65A, which in turn binds CCM3 and MST4. This complex relocates to the plasma membrane with a resulting re-orientation of the Golgi towards the leading edge of the cell. (c) The chemoattractant Activin B activates the formin mDia1, via RhoA, as well as acting on Cdc42. Together this results in Golgi re-orientation and increased cell migration
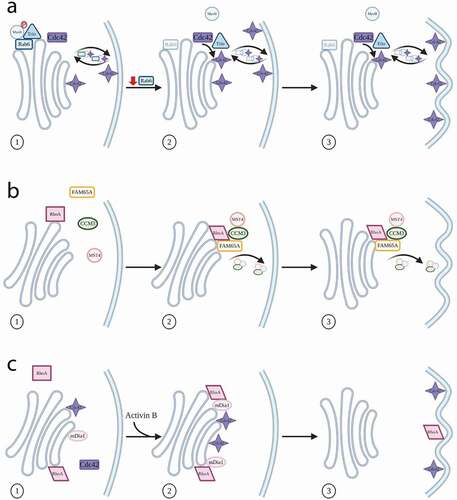
In addition to the contribution of Cdc42 to Golgi re-orientation, new evidence for involvement of other Rho proteins, specifically the RhoA sub-family, has emerged. Using a biochemical pulldown approach, a number of new interactors of RhoA were identified, including the previously uncharacterized protein FAM65A [Citation81]. Activated RhoA binds FAM65A, which in turn leads to binding to CCM3 (cerebral cavernous malformation-3 protein). CCM3 then builds a complex with the kinase MST4, resulting in this complex relocating from the Golgi to cytoplasmic vesicular structures. This relocation is essential for both membrane trafficking from the Golgi, and its orientation towards the leading edge of the cell ()). Furthermore, neither RhoA nor FAM65A affect the kinase activity of MST4. A similar mechanism of relocation of MST4 from the Golgi to the plasma membrane, involving the serine/threonine kinase LKB1 and the adaptor protein Mo25, has been shown previously in gut epithelial cells [Citation82], but it is unclear whether this relocation of MST4 influences Golgi re-orientation.
Orientation of the Golgi is clearly important for the cell to determine the direction of cell motility, and this information is potentially useful in an applied context of therapeutic design. One specific application of this can be seen with Activin B, a chemoattractant with potential as a therapy for skin wound repair [Citation83]. Treatment of rat bone marrow-derived mesenchymal stromal cells (BMSCs) with Activin B leads primarily to activation of mDia1, but also of Cdc42 [Citation84]. In these cells, there is an increase in the polarization of the Golgi, leading to its re-orientation towards the leading edge and thus increased cell migration. From a molecular perspective, these observations are interesting because Activin B appears to be able to both activate mDia1, via RhoA, thereby influencing migration, and at the same time act on Cdc42 to effect Golgi positioning [Citation84] ()). Future studies will be needed to establish the relative contributions of the Golgi- versus the plasma membrane-localized pools of Cdc42 to these effects.
Perspectives
In this review, we have attempted to reconcile a wide variety of data relating to Rho family function at the Golgi apparatus. The emerging picture is a complex one, in which several Rho members, in association with their GEFs and GAPs coordinate a number of different activities at this central organelle. While we have broadly classified these roles as relating to either Golgi architecture, membrane trafficking, or determining polarity and migration, it is evident that in fact each activity has dependency on the others, and as such should not be studied in isolation [Citation85]. What is also becoming apparent is that the Rho GTPases show cross-talk with Rab GTPases, many of which are well-established regulators of Golgi function. This is perhaps not surprising given that of the 60 or so Rabs in human cells, approximately one third either localize to or control functions associated with the Golgi [Citation11]. Wider cross-talk with other GTPase families, such as the ARFs, is also likely, and multi-domain proteins such as ARAP1 have been shown to possess both ARF GAP and Rho GAP activity, influencing diverse functions such as maintenance of Golgi integrity and cell spreading [Citation86]. The variety of activities that Rho proteins participate in the Golgi can also be considered as a consequence of their own family diversity. While the typical Rho family members are largely controlled via the classical GTPase cycle, requiring interactions with GEFs and GAPs, the atypical Rho GTPases potentially have access to a wider repertoire of regulatory mechanisms. As such, it is to be expected that more information on their interaction networks at the Golgi is still to emerge. In this context, the mechanisms by which Rho proteins are delivered to specific membranes also remain to be fully elucidated. While delivery of Rho proteins from a soluble pool to a membrane-associated pool is undoubtedly one mechanism of localization, it is also possible that discrete transfer between membranes might occur via ‘membrane contact sites’ [Citation87]. Although this has not yet been shown for Rho GTPases at the Golgi, this organelle exhibits a wide range of close contacts with many other organelles, including the ER and mitochondria.
One major challenge towards our greater appreciation of Rho function is deciphering the GTPase-GEF-GAP interaction network, and this is not a trivial proposition given that in human cells the 21 Rho family GTPases have the potential to interact with approximately 65 different GAPs and 80 GEFs [Citation27]. One exciting study, recently reported, paves the way for how this challenge may be solved. A systematic analysis of all known Rho GEFs and GAPs employed a combination of subcellular localization, FRET-based activity assays and bioinformatics tools to carefully map their interaction networks. Specific focus in this work was given to Rac1, RhoA and Cdc42, revealing their breadth of interactions, new aspects of signalling and importantly the promiscuity of GAPs compared to GEFs [Citation88]. Another curious feature of Rho function is that they can act in distinct ways at different membranes. Currently Cdc42 is the archetypal example of this, with the striking finding that its pool at the Golgi can impact events at the plasma membrane, as discussed above. Delineating the status of Rho activity at distinct membranes will therefore be key, and the use of FRET-based biosensors is one exciting development that should enable this [Citation58]. The specific challenge of course at the Golgi is one of the spatial resolution, with each cisterna only being separated by tens of nanometres, a number that is below the diffraction limit of light. In addition, given the dynamic nature of GTPase interactions with membranes, approaches that utilize live cells, thereby providing temporal information, will also be invaluable. Our picture of the role of Rho GTPases at the Golgi complex remains incomplete, however their importance is now established, and it seems likely that in the next couple of years further clarity to their role at this organelle will be achieved.
Acknowledgments
The authors gratefully acknowledge all the members of the JCS lab for comments on this article. Work in the JCS laboratory is funded by the UCD College of Science, and Science Foundation Ireland (SFI), under grants (16/RI/3745) and (13/RC/2073), and is co-funded under the European Regional Development Fund. The figures were created with BioRender.com
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Marques ARA, Saftig P. Lysosomal storage disorders - challenges, concepts and avenues for therapy: beyond rare diseases. J Cell Sci. 2019;132(2):1739.
- Egorov MV, Polishchuk RS. Emerging role of Cdc42-specific guanine nucleotide exchange factors as regulators of membrane trafficking in health and disease. Tissue Cell. 2017;49(2 Pt A):157–162.
- Morgan NE, Cutrona MB, Simpson JC. Multitasking rab proteins in autophagy and membrane trafficking: a focus on Rab33b. Int J Mol Sci. 2019;20(16):3916.
- Guadagno NA, Progida C. Rab GTPases: switching to human diseases. Cells. 2019;8(8):0909.
- Long M, Simpson JC. Rho GTPases operating at the Golgi complex: implications for membrane traffic and cancer biology. Tissue Cell. 2017;49(2 Pt A):163–169.
- Tzeng HT, Wang YC. Rab-mediated vesicle trafficking in cancer. J Biomed Sci. 2016;23(1):70.
- Bexiga MG, Simpson JC. Human diseases associated with form and function of the Golgi complex. Int J Mol Sci. 2013;14(9):18670–18681.
- Makhoul C, Gosavi P, Gleeson PA. Golgi dynamics: the morphology of the mammalian Golgi apparatus in health and disease. Front Cell Dev Biol. 2019a;7112. DOI:https://doi.org/10.3389/fcell.2019.00112
- Ungar D. Golgi linked protein glycosylation and associated diseases. Semin Cell Dev Biol. 2009;20(7):762–769.
- Zhen Y, Stenmark H. Cellular functions of Rab GTPases at a glance. J Cell Sci. 2015;128(17):3171–3176.
- Galea G, Simpson JC. High-content analysis of Rab protein function at the ER-Golgi interface. Bioarchitecture. 2015;5(3–4):44–53.
- Goud B, Liu S, Storrie B. Rab proteins as major determinants of the Golgi complex structure. Small GTPases. 2018;9(1–2):66–75.
- Hodge RG, Ridley AJ. Regulating Rho GTPases and their regulators. Nat Rev Mol Cell Biol. 2016;17(8):496–510.
- Ravichandran Y, Goud B, Manneville JB. The Golgi apparatus and cell polarity: roles of the cytoskeleton, the Golgi matrix, and Golgi membranes. Curr Opin Cell Biol. 2020;62104–62113. DOI:https://doi.org/10.1016/j.ceb.2019.10.003
- Bhattacharya K, Swaminathan K, Peche VS, et al. Novel Coronin7 interactions with Cdc42 and N-WASP regulate actin organization and Golgi morphology. Sci Rep. 2016;625411. DOI:https://doi.org/10.1038/srep25411
- Erickson JW, Zhang C, Kahn RA, et al. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J Biol Chem. 1996;271(43):26850–26854.
- Quassollo G, Wojnacki J, Salas DA, et al. A RhoA signaling pathway regulates dendritic Golgi outpost formation. Curr Biol. 2015;25(8):971–982.
- Blom M, Reis K, Nehru V, et al. RhoD is a Golgi component with a role in anterograde protein transport from the ER to the plasma membrane. Exp Cell Res. 2015;333(2):208–219.
- Espinosa EJ, Calero M, Sridevi K, et al. RhoBTB3: a Rho GTPase-family ATPase required for endosome to Golgi transport. Cell. 2009;137(5):938–948.
- Egea G, Serra-Peinado C, Salcedo-Sicilia L, et al. Actin acting at the Golgi. Histochem Cell Biol. 2013;140(3):347–360.
- Matas OB, Martínez-Menárguez JA, Egea G. Association of Cdc42/N-WASP/Arp2/3 signaling pathway with Golgi membranes. Traffic. 2004;5(11):838–846.
- Chen JL, Fucini RV, Lacomis L, et al. Coatomer-bound Cdc42 regulates dynein recruitment to COPI vesicles. J Cell Biol. 2005;169(3):383–389.
- Luna A, Matas OB, Martínez-Menárguez JA, et al. Regulation of protein transport from the Golgi complex to the endoplasmic reticulum by CDC42 and N-WASP. Mol Biol Cell. 2002;13(3):866–879.
- Park SY, Yang JS, Schmider AB, et al. Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature. 2015;521(7553):529–532.
- Wu WJ, Erickson JW, Lin R, et al. The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature. 2000;405(6788):800–804.
- Kodani A, Kristensen I, Huang L, et al. GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization. Mol Biol Cell. 2009;20(4):1192–1200.
- Van Buul JD, Geerts D, Huveneers S. Rho GAPs and GEFs: controling switches in endothelial cell adhesion. Cell Adh Migr. 2014;8(2):108–124.
- Estrada L, Caron E, Gorski JL. Fgd1, the Cdc42 guanine nucleotide exchange factor responsible for faciogenital dysplasia, is localized to the subcortical actin cytoskeleton and Golgi membrane. Hum Mol Genet. 2001;10(5):485–495.
- Liu Z, Adams HC 3rd, Whitehead IP. The rho-specific guanine nucleotide exchange factor Dbs regulates breast cancer cell migration. J Biol Chem. 2009;284(23):15771–15780.
- Reinhard NR, Van Der Niet S, Chertkova A, et al. Identification of guanine nucleotide exchange factors that increase Cdc42 activity in primary human endothelial cells. Small GTPases. 2019;1–15. DOI:https://doi.org/10.1080/21541248.2019.1658509
- Makhoul C, Gosavi P, Duffield R, et al. Intersectin-1 interacts with the golgin GCC88 to couple the actin network and Golgi architecture. Mol Biol Cell. 2019b;30(3):370–386.
- Gosavi P, Houghton FJ, Mcmillan PJ, et al. The Golgi ribbon in mammalian cells negatively regulates autophagy by modulating mTOR activity. J Cell Sci. 2018;131(3):1987.
- Herrero-Garcia E, O’bryan JP. Intersectin scaffold proteins and their role in cell signaling and endocytosis. Biochim Biophys Acta Mol Cell Res. 2017;1864(1):23–30.
- Hunter MP, Russo A, O’bryan JP. Emerging roles for intersectin (ITSN) in regulating signaling and disease pathways. Int J Mol Sci. 2013;14(4):7829–7852.
- Hussain NK, Jenna S, Glogauer M, et al. Endocytic protein intersectin-l regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3(10):927–932.
- Lee CC, Cheng YC, Chang CY, et al. Alpha-tubulin acetyltransferase/MEC-17 regulates cancer cell migration and invasion through epithelial-mesenchymal transition suppression and cell polarity disruption. Sci Rep. 2018;8(1):17477.
- Dubois T, Paléotti O, Mironov AA, et al. Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat Cell Biol. 2005;7(4):353–364.
- Baschieri F, Confalonieri S, Bertalot G, et al. Spatial control of Cdc42 signalling by a GM130-RasGRF complex regulates polarity and tumorigenesis. Nat Commun. 2014;54839. DOI:https://doi.org/10.1038/ncomms5839
- Calvo F, Sanz-Moreno V, Agudo-Ibáñez L, et al. RasGRF suppresses Cdc42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat Cell Biol. 2011;13(7):819–826.
- Berthold J, Schenkova K, Rivero F. Rho GTPases of the RhoBTB subfamily and tumorigenesis. Acta Pharmacol Sin. 2008;29(3):285–295.
- Ji W, Rivero F. Atypical Rho GTPases of the RhoBTB Subfamily: roles in vesicle trafficking and tumorigenesis. Cells. 2016;5(2):28.
- Lu A, Pfeffer SR. Golgi-associated RhoBTB3 targets cyclin E for ubiquitylation and promotes cell cycle progression. J Cell Biol. 2013;203(2):233–250.
- Haga RB, Garg R, Collu F, et al. RhoBTB1 interacts with ROCKs and inhibits invasion. Biochem J. 2019;476(17):2499–2514.
- Mckinnon CM, Mellor H. The tumor suppressor RhoBTB1 controls Golgi integrity and breast cancer cell invasion through METTL7B. BMC Cancer. 2017;17(1):145.
- Wu CC, Maccoss MJ, Mardones G, et al. Organellar proteomics reveals Golgi arginine dimethylation. Mol Biol Cell. 2004;15(6):2907–2919.
- Long M, Kranjc T, Mysior MM, et al. RNA interference screening identifies novel roles for RhoBTB1 and RhoBTB3 in membrane trafficking events in mammalian cells. Cells. 2020;9(5):1089.
- Hehnly H, Longhini KM, Chen JL, et al. Retrograde Shiga toxin trafficking is regulated by ARHGAP21 and Cdc42. Mol Biol Cell. 2009;20(20):4303–4312.
- Fitzpatrick ER, Hu T, Ciccarelli BT, et al. Regulation of vesicle transport and cell motility by Golgi-localized Dbs. Small GTPases. 2014;5(4):1–12.
- Higgs HN, Peterson KJ. Phylogenetic analysis of the formin homology 2 domain. Mol Biol Cell. 2005;16(1):1–13.
- Colón-Franco JM, Gomez TS, Billadeau DD. Dynamic remodeling of the actin cytoskeleton by FMNL1γ is required for structural maintenance of the Golgi complex. J Cell Sci. 2011;124(Pt 18):3118–3126.
- Ramabhadran V, Korobova F, Rahme GJ, et al. Splice variant-specific cellular function of the formin INF2 in maintenance of Golgi architecture. Mol Biol Cell. 2011;22(24):4822–4833.
- Zilberman Y, Alieva NO, Miserey-Lenkei S, et al. Involvement of the Rho-mDia1 pathway in the regulation of Golgi complex architecture and dynamics. Mol Biol Cell. 2011;22(16):2900–2911.
- Kage F, Steffen A, Ellinger A, et al. FMNL2 and −3 regulate Golgi architecture and anterograde transport downstream of Cdc42. Sci Rep. 2017;7(1):9791.
- Phuyal S, Farhan H. Multifaceted Rho GTPase SIGNALING at the endomembranes. Front Cell Dev Biol. 2019;7127. DOI:https://doi.org/10.3389/fcell.2019.00127
- Pasteris NG, Gorski JL. An intragenic TaqI polymorphism in the faciogenital dysplasia (FGD1) locus, the gene responsible for aarskog syndrome. Hum Genet. 1995;96(4):494.
- Egorov MV, Capestrano M, Vorontsova OA, et al. Faciogenital dysplasia protein (FGD1) regulates export of cargo proteins from the Golgi complex via Cdc42 activation. Mol Biol Cell. 2009;20(9):2413–2427.
- Egorov M, Polishchuk R. Identification of CDC42 effectors operating in FGD1-dependent trafficking at the Golgi. Front Cell Dev Biol. 2019;77. DOI:https://doi.org/10.3389/fcell.2019.00007
- Herrington KA, Trinh AL, Dang C, et al. Spatial analysis of Cdc42 activity reveals a role for plasma membrane-associated Cdc42 in centrosome regulation. Mol Biol Cell. 2017;28(15):2135–2145.
- Salazar MA, Kwiatkowski AV, Pellegrini L, et al. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J Biol Chem. 2003;278(49):49031–49043.
- Kobayashi K, Suemasa F, Sagara H, et al. MiR-199a Inhibits secondary envelopment of herpes simplex virus-1 through the downregulation of Cdc42-specific GTPase activating protein localized in Golgi apparatus. Sci Rep. 2017;7(1):6650.
- Braun AC, Hendrick J, Eisler SA, et al. The Rho-specific GAP protein DLC3 coordinates endocytic membrane trafficking. J Cell Sci. 2015;128(7):1386–1399.
- Shibata S, Kawanai T, Hara T, et al. ARHGEF10 directs the localization of Rab8 to Rab6-positive executive vesicles. J Cell Sci. 2016;129(19):3620–3634.
- Gasman S, Kalaidzidis Y, Zerial M. RhoD regulates endosome dynamics through diaphanous-related formin and Src tyrosine kinase. Nat Cell Biol. 2003;5(3):195–204.
- Murphy C, Saffrich R, Grummt M, et al. Endosome dynamics regulated by a Rho protein. Nature. 1996;384(6608):427–432.
- Gad AK, Nehru V, Ruusala A, et al. RhoD regulates cytoskeletal dynamics via the actin nucleation-promoting factor WASp homologue associated with actin Golgi membranes and microtubules. Mol Biol Cell. 2012;23(24):4807–4819.
- Blom M, Reis K, Aspenström P. RhoD localization and function is dependent on its GTP/GDP-bound state and unique N-terminal motif. Eur J Cell Biol. 2018;97(6):393–401.
- Aspenström P. Fast-cycling Rho GTPases. Small GTPases. 2018. DOI:https://doi.org/10.1080/21541248.2017.1391365
- Warner H, Wilson BJ, Caswell PT. Control of adhesion and protrusion in cell migration by Rho GTPases. Curr Opin Cell Biol. 2019;5664–5670. DOI:https://doi.org/10.1016/j.ceb.2018.09.003
- Kupfer A, Louvard D, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center in cultured fibroblasts at the edge of an experimental wound. Proc Natl Acad Sci U S A. 1982;79(8):2603–2607.
- Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity, and wound healing. Mol Biol Cell. 2009;20(6):1728–1736.
- Hehnly H, Xu W, Chen JL, et al. Cdc42 regulates microtubule-dependent Golgi positioning. Traffic. 2010;11(8):1067–1078.
- Preisinger C, Short B, De Corte V, et al. YSK1 is activated by the Golgi matrix protein GM130 and plays a role in cell migration through its substrate 14-3-3zeta. J Cell Biol. 2004;164(7):1009–1020.
- Ahat E, Xiang Y, Zhang X, et al. GRASP depletion-mediated Golgi destruction decreases cell adhesion and migration via the reduction of α5β1 integrin. Mol Biol Cell. 2019;30(6):766–777.
- Farhan H, Hsu VW. Cdc42 and cellular polarity: emerging roles at the Golgi. Trends Cell Biol. 2016;26(4):241–248.
- Vestre K, Kjos I, Guadagno NA, et al. Rab6 regulates cell migration and invasion by recruiting Cdc42 and modulating its activity. Cell Mol Life Sci. 2019;76(13):2593–2614.
- Lee CS, Choi CK, Shin EY, et al. Myosin II directly binds and inhibits Dbl family guanine nucleotide exchange factors: a possible link to Rho family GTPases. J Cell Biol. 2010;190(4):663–674.
- Peurois F, Veyron S, Ferrandez Y, et al. Characterization of the activation of small GTPases by their GEFs on membranes using artificial membrane tethering. Biochem J. 2017;474(7):1259–1272.
- Borg M, Bakke O, Progida C. A novel interaction between Rab7b and actomyosin reveals a dual role in intracellular transport and cell migration. J Cell Sci. 2014;127(Pt 22):4927–4939.
- Kjos I, Vestre K, Guadagno NA, et al. Rab and Arf proteins at the crossroad between membrane transport and cytoskeleton dynamics. Biochim Biophys Acta Mol Cell Res. 2018;1865(10):1397–1409.
- Palamidessi A, Frittoli E, Garré M, et al. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134(1):135–147.
- Mardakheh FK, Self A, Marshall CJ. RHO binding to FAM65A regulates Golgi reorientation during cell migration. J Cell Sci. 2016;129(24):4466–4479.
- Ten Klooster JP, Jansen M, Yuan J, et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev Cell. 2009;16(4):551–562.
- Zhang M, Sun L, Wang X, et al. Activin B promotes BMSC-mediated cutaneous wound healing by regulating cell migration via the JNK-ERK signaling pathway. Cell Transplant. 2014;23(9):1061–1073.
- Wang X, Tang P, Guo F, et al. mDia1 and Cdc42 regulate activin b-induced migration of bone marrow-derived mesenchymal stromal cells. Stem Cells. 2019;37(1):150–162.
- Vaidžiulytė K, Coppey M, Schauer K. Intracellular organization in cell polarity - placing organelles into the polarity loop. J Cell Sci. 2019;132(24):995.
- Miura K, Jacques KM, Stauffer S, et al. ARAP1: a point of convergence for Arf and Rho signaling. Mol Cell. 2002;9(1):109–119.
- Silva BSC, Digiovanni L, Kumar R, et al. Maintaining social contacts: the physiological relevance of organelle interactions. Biochim Biophys Acta Mol Cell Res. 2020;1867(11):118800.
- Müller PM, Rademacher J, Bagshaw RD, et al. Systems analysis of RhoGEF and RhoGAP regulatory proteins reveals spatially organized RAC1 signalling from integrin adhesions. Nat Cell Biol. 2020;22(4):498–511.
