ABSTRACT
Transcriptional control is critical in relaying signals mediated by Ras and Rho family small GTPases to effect gene expression. In the classical model, signaling components such as MAP kinase target sequence-specific transcription factors, which in turn recruit RNA polymerase (Pol) II holoenzyme to the promoter and activate transcription. Findings in recent years have led to the proposal of a shortcut model in which the Mediator components of the Pol II holoenzyme are regulated by signaling pathways. A very recent finding shows that an evolutionarily conserved Rho GTPase signaling pathway can directly modulate the Pol II C-terminal domain (CTD) phosphorylation by inhibiting the CTD phosphatase in yeast and Arabidopsis. This shortcut model allows direct targeting of the Pol II CTD code and thus has an advantage over the classical model in bringing about rapid, large-scale changes in gene expression.
Introduction
Regulation of gene expression is essential for growth and/or developmental processes in all eukaryotes from yeast to plants and animals. Although these organisms are separated by a billion years of evolution, they share some common mechanisms from robustly responding to dynamic internal cues and external stimuli to effecting gene expression. For more than two decades, the upstream signaling switches called Ras superfamily small GTPases, and the core of transcriptional regulation, RNA polymerase (Pol) II holoenzyme, have been intensively but separated studied to reveal the signaling mechanisms leading to the most efficient and cost-effective transcriptional control.
Ras superfamily GTPases include two signaling GTPase families, Ras and Rho. In yeast and animals, Rho family GTPases include Rho, Rac, and Cdc42.Citation1 However, plants do not possess an otholog of Ras family GTPases or any of the three subfamilies of Rho GTPases.Citation2 Instead, they have a plant-unique subfamily of Rho GTPase called ROP, with 11 members in the Arabidopsis model plant, which are believed to perform the cellular functions controlled by Ras and Rho in yeast and animals. GTPases cycle between a GDP-bound, inactive and a GTP-bound, active forms. In response to extracellular signals, GTPases can be activated by guanine nucleotide exchange factors (GEFs; promoting the exchange of bound GDP for free GTP), thus turning on various intracellular signaling pathways (). Numerous studies using loss-of-function and constitutively active (for example, G12V or Q61L that locks GTPase in the GTP-bound form) mutants have demonstrated that Ras and Rho family GTPases are critical molecular switches for a wide range of biologic processes including cell proliferation, morphogenesis, adhesion, migration and invasion.
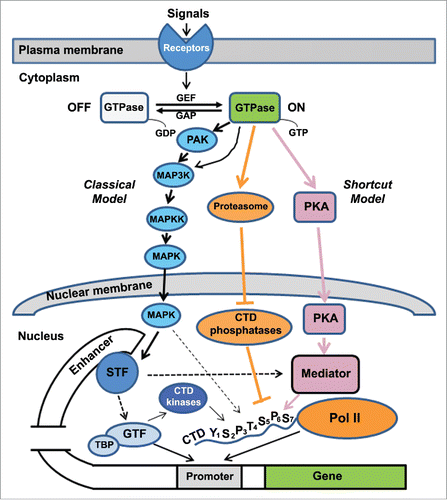
Figure 1. A simplified diagram showing two models of transcriptional control in Ras and Rho GTPase signaling. In response to extracellular signals, the GDP-bound, inactive GTPase is converted to the GTP-bound, active form via receptor-stimulated guanine nucleotide exchange factors (GEF). The GTP-bound GTPase can be converted back to the GDP-bound form after GTP hydroxylation enhanced by GTPase activating proteins (GAP). Upon activation, GTPases use either classical or shortcut models to regulate transcription. In the classical model (shown in blue), MAPK kinase cascades are activated. For Rac and Cdc42 GTPases, p21-activated kinases (PAK) are activated, leading to sequential phosphorylation of MAPK3K, MAPKK and MAPK in the JNK or p38 MAPK kinase cascades. For Ras GTPases, MAP3K (Raf) is activated, and ultimately MAPK is phosphorylated. MAPK is then translocated into the nucleus and phosphorylates a sequence-specific transcription factor (STF). Upon phosphorylation, STF recognizes and binds to the enhancer, which can be close to or far away from the core promoter region. Subsequently, the Pol II transcriptional machinery is recruited to the core promoter region by STF through its interaction with general transcription factors (GTF; such as TFIID) via TATA-binding proteins (TBP). STF also interacts with the Mediator complex that “mediates” the formation of a pre-initiation complex on the promoter through its binding with the Pol II CTD. One of general transcription factors, TFIIH, contains CTD kinase CDK7, which phosphorylates Ser5 of the CTD, leading to the promoter escape and transitioning to the elongation step. In the shortcut model involving protein kinase A (PKA) (shown in purple), upon Ras activation, PKA is activated, causing it to translocate into the nucleus where it directly phosphorylates a component of Mediator (Srb9) in yeast and thus turning on transcription. Another shortcut model involving CTD phosphatases (shown in orange) has been proposed for Rho GTPases including Arabidopsis ROP2 and yeast Cdc42. In this model, the active form of GTPase promotes the degradation of CTD phosphatases (such as Arabidopsis CPL1 and yeast Fcp1), leading to increased Ser2 and Ser5 phosphorylation in the Pol II CTD and consequently altering gene expression.
Pol II is a multi-unit holoenzyme complex critical for the transcription of almost all protein-coding and miRNA genes. The C-terminal domain (CTD) of RPB1, the largest subunit of Pol II, contains a certain number of both highly conserved heptad peptide (Y1S2P3T4S5P6S7) repeats and slightly variant repeats, ranging from a total of 29 repeats in fission yeast, 34 in Arabidopsis to 52 in humans.Citation3-8 Each of the amino acid residues in the repeats can be modified posttranslationally, such as phosphorylation of Tyr1, Ser2, Thr4, Ser5 and Ser7, isomerization at Pro3 and Pro6 and acetylation at Thr4. Thus, various modifications on the large number of the repeats theoretically would result in very complex combinations, and because of their importance in transcriptional control, these modification patterns on the CTD repeats have bene collectively dubbed the “CTD code”.Citation3-8 Accumulating evidence shows that dynamic modulation of the CTD code in Pol II along genes is essential for completing key steps of transcription, by recruiting the CTD-associated proteins to the transcribing Pol II, including several CTD kinases and CTD phosphatases. The role of Ser phosphorylation is most well-studied. When a gene needs to be transcribed in full capacity, Ser5 is highly phosphorylated (designated Ser5P), which leads the Pol II complex to dissociate from the Mediator and facilitates 5′-end capping of the nascent RNA. This process, called promoter escape, makes a transition from transcription initiation to elongation and is crucial for productive transcription. Subsequently, Ser5P decreases and concomitantly Ser2P increases to complete transcript elongation and splicing. Finally, Ser5 is dephosphorylated completely, allowing transcript cleavage, polyadenylation and termination. The role for Ser7P is less clear, but it is frequently observed at elongation and termination stages. Before entering another transcription cycle, Ser2 and Ser7 are also dephosphorylated, and thus unphosphorylated Pol II is ready for recycling.
The classical or “indirect” model of transcriptional control
In the classical molecular genetics dogma of transcriptional control (), upon the activation of Ras and Rho GTPases, the intracellular signaling pathways such as the MAPK kinase cascades are activated, leading to the translocation of MAPK into the nucleus.Citation9,10 Subsequently, MAPK phosphorylates the sequence-specific transcription factors and thus these activated transcription factors bind to a cis-regulatory DNA element called enhancer. Enhancer can be located close to or very far away from the promoter. Consequently, this sequence-specific binding recruits the Pol II transcriptional machinery to the core promoter region by either interacting with general transcription factors (GTF; such as TFIID) via TATA-binding proteins (TBP) or by interacting with the Mediator complex.Citation11,12 The Mediator itself is not involved in transcription, but through its binding with the Pol II CTD, it “mediates” the formation of a pre-initiation complex on the promoter, which is the rate limiting step in transcription initiation.Citation12,13 Another class of GTF, TFIIH, contains a CTD kinase called CDK7, which is responsible for writing the Ser5P mark. Because this commonly accepted, classical signaling pathway undergoes many steps involving distinct players, it has been referred to the indirect model of transcriptional control when a more “direct” or “shortcut” model or pathway was reported mostly in yeast studies.Citation14-16
A direct or shortcut model of transcriptional control
Ras/PKA-Pol II pathway in yeast
The shortcut or direct model was implicated in several early studies involving yeast. For example, in budding yeast glucose signaling, Snf1 kinase was suggested to target the Mediator components, although Snf1 is not a target of Ras signaling.Citation14 However, the most convincing evidence for the existence of such a direct or shortcut pathway from Ras to Pol II transcription came from another yeast genetic study.Citation16 A constitutively active Ras2 mutation was found to suppress the transcriptional defects caused by a srb9 (a Mediator subunit) mutant in a cAMP-dependent fashion. Biochemical evidence showed that Srb9 could be directly phosphorylated by the Ras signaling effector, cAMP-dependent protein kinase or protein kinase A (PKA). In Ras signaling, besides the MAPK kinase cascade, PKA pathway is also activated.Citation17 In brief, the active form of Ras stimulates adenylyl cyclase, leading to an increase of the intracellular concentrations of cAMP for consequential binding by the regulatory subunit of PKA. Such binding then activates the PKA catalytic subunit (Cα), which is then translocated into the nucleus. Therefore, it is likely that PKA-Cα phosphorylates Srb9 and thus gene expression is turned on. Because this pathway does not rely on the MAPK cascade and its transcription factor targets and instead it directly regulates the critical component of Pol II transcriptional machinery (), it was referred to as a “direct” or “bypass” model of transcriptional control.
Although PKA does not phosphorylate Pol II CTD in yeast,Citation16 there is evidence from a prior study in a rat cardiac cell cultureCitation18 that inactivation of Ras by a dominant negative mutation leads to the reduction of phosphorylation in the Pol II CTD. Consistently, the expression of a mutant form of a GTPase activating protein (GAP) that lacks the catalytic domain and thus activates Ras promotes the Pol II phosphorylation. Although it was not tested whether Ser2P, Ser5P or Ser7P was impacted because the phosphor-specific antibodies were not used at the time of the study,Citation18 it strongly indicates that the CTD phosphorylation pattern may be modulated by Ras signaling in animals. Another intriguing observation is that the Cdk7 protein level was reduced and increased in the Ras and GAP mutants, respectively. However, it remains to be tested whether Ras signaling directly targets Cdk7 protein in the CTD code modulation as the increase of Cdk7 could also be caused indirectly via transcriptional control in response to Ras activation.
An evolutionarily conserved Rho-CTD phosphatase-Pol II shortcut in yeast and Arabidopsis
Like Ras, Rho family GTPases were initially discovered to be important regulators of transcription (e.g., the identification of the JNK and the p38 MAPK kinase cascades in regulating the activity of transcription factors including c-Jun/AP1 and c-fos for Rac and Cdc42 GTPasesCitation19-21), but the majority of subsequent studies in the past two decades have mainly focused on their critical roles in the control of cytoskeletal organization.Citation1,2,22,23 Recent findings, including the abnormal expression of these GTPases and their regulators in tumor cellsCitation22-24 and the importance of CDK7-mediated transcriptional regulation in cancers,Citation25,26 have renewed the interest in the Rho signaling control of gene expression in particular in cancers.
A very recent study has demonstrated that Rho GTPase signaling can directly target the Pol II CTD code and effect large-scale gene expression.Citation27 This link from Rho signaling to Pol II transcription was first revealed in an Arabidopsis cell morphogenic study. Constitutive activation of ROP2 (designated CA-rop2; a G12V mutation) leads to the conversion of interdigitated cotyledon pavement cells to the fat, hyper-parallel, near-rectangular shape, but a recessive mutation in the CTD phosphatase gene CPL1 further enhances the shape phenotype along with the enhancement in cell size and cell number phenotypes.Citation27 CA-rop2 overexpression causes an increase in the CTD Ser2P and Ser5P levels but not Ser7P, indicating that Ser2- and Ser5-specific phosphorylation pattern is impacted. This modulation is mediated by promoting CPL1 degradation. Consistently, CPL1 overexpression reduces Ser2P and Ser5P levels, accompanied by strong suppression of the cell shape phenotype caused by CA-rop2 overexpression. Transcriptomic studies show that approximately 10% of detectable genes are affected (with a twofold difference) by CA-rop2 and cpl1 mutation. Importantly, almost half of those affected genes are commonly impacted by ROP2 and CPL1. As the transcription factor involved in plant leaf pavement cell shape formation has not been identified yet, this finding represents an important breakthrough in plant cell morphogenesis. Cell shape determination has been frequently attributed to the cytoskeletal control involving ROP2 and ROP4 that regulate distinct actin and microtubule dynamics.Citation28 Now that the Pol II CTD code has been identified as a critical target of ROP2 signaling, the shortcut model of transcriptional control will have a lasting impact in cell morphogenic process by exerting large-scale changes in gene expression.
As yeast Ras-PKA signaling was not shown to modulate the CTD Ser phosphorylation code,Citation16 a test was performed to determine whether yeast uses Rho rather than Ras family GTPases to modulate the CTD code.Citation27 Genetic analysis suggests that a Cdc42 loss-of-function mutation in fission yeast led to much lower Ser2P and Ser5P. Together with the observation that the induction of Arabidopsis CA-rop2 also caused cell shape and Ser2P and Ser5P changes, these results show that Rho GTPase signaling modulation of the CTD code is evolutionarily conserved in unicellular (yeast) and multicellular (Arabidopsis) eukaryotes. Furthermore, the promotion of the CTD phosphatase Fcp1 degradation by CA-rop2 induction is also conserved in budding yeast. The finding that fission yeast Rpb1 S2A and in particular S5A mutants could almost completely suppress the cell shape phenotype caused by CA-rop2 induction demonstrates that the CTD Ser2 and Ser5 are critical targets for Rho signaling in cell morphogenesis.
Clearly, these recent findings in both yeasts and Arabidopsis plants suggest that modulation of the Pol II CTD code by Rho GTPase signaling via the degradation of CTD phosphatases represents an evolutionarily conserved mechanism in intracellular signaling.Citation27 This shortcut model () allows cells to quickly respond to extracellular signals (such as the plant hormone auxin), leading to the activation of Rho GTPases. Subsequently, CTD phosphatases are degraded, which causes the CTD to be hyperphosphorylated at Ser5 and Ser2 sites. Such a CTD code modulation may enable the cells to quickly effect transcriptional changes in the genes involved in cell growth and shape formation.
Important questions to be addressed in the future
One of the immediate questions that need to be addressed is which proteasome pathways are involved in the degradation of the CTD phosphatases such as CPL1 in plants and Fcp1 in yeast? The use of the proteasome inhibitors lactacystin and MG132 in both in vitro and in vivo degradation experiments indicates the involvement of 20S and 26S proteasomes in CTD phosphatase degradation. Given that ROP GTPase has been reported to promote the degradation of auxin signaling-related proteins such as AUX in the nucleusCitation29 and that CPL1 does not seem to be located in the cytoplasm,Citation30 it is likely that ROP2-mediated CPL1 or Fcp1 degradation occurs in the nucleus. If this is the case, ROP2 might activate a signaling effector that is then translocated into the nucleus to stimulate the protein degradation pathway. Alternatively, ROP2 is activated in the nucleus or the active form of ROP2 is translocated into the nucleus where it acts on proteasome to degrade CPL1 or Fcp1. Although it has not been reported whether a proportion of ROP2, albeit possibly very tiny, exhibits such nuclear localization or nucleocytoplasmic shuttling, such occurrence has been reported for Rac1,Citation31 RhoACitation32 and ROP11 GTPases.Citation33
The second question is whether CTD kinases are also potentially targeted by Ras or Rho GTPase signaling. The alteration in Ser2P and Ser5P levels may also be caused by the regulation of CTD kinases. Given its critical importance in transcriptional control in cancer cells,Citation25,26 CDK7 can be a primary target for future testing. Indeed, Ras inactivation decreases the Cdk7 level in rat cells,Citation18 although whether Cdk7 reduction is responsible for the reduced CTD phosphorylation remains unanswered. Perhaps, the combined action of CTD kinases and phosphatases will enable the cells to robustly and dynamically modulate the CTD code so as to most effectively regulate transcription when urgent cues are perceived.
Another unanswered question is what specific CTD phosphorylation patterns have been modulated by Rho GTPases. In the aforementioned Arabidopsis and yeast study,Citation27 Western blots were used to detect Ser2P and Ser5P changes with the aid of phosphor-specific antibodies, but this technique did not allow to profile which repeat(s) have been phosphorylated. Theoretically, the sets of the combinations for just the Ser2 and Ser5 two sites on the large number of the CTD repeats could be extremely big.Citation34 The recently developed genetic- or ultraviolet photodissociation-based mass-spectrometric approachesCitation35-37 have the potential of revealing the exact CTD phosphorylation patterns that are modulated by GTPase signaling in response to distinct extracellular signals.
Which genes are targeted by the shortcut model of transcriptional control is another question that needs to be resolved. Given that the CTD code is targeted by Rho signaling, presumably a large number of genes will be impacted. However, it has also been argued that the CTD code, such as Thr4P or Ser7P, could target a specific group of genes.Citation4 Indeed, the Arabidopsis transcriptomic study revealed that only 10% of genes were affected by the ROP2 and CPL1 mutations.Citation27 Nevertheless, it should be noted that this study did not address distinct signals, and thus it remains quite possible that many other signal-specific response genes are targeted by the ROP2-Pol II shortcut pathway.
At last, it will be interesting to test how the classical model and the shortcut model cooperate to achieve the most efficient and cost-effective control in bringing the most productive transcription. The ERK-type MAPKs can also act as CTD kinases on Ser5,Citation38,39 raising the possibility that the MPAK kinase cascade presumably involved in the classical model of transcriptional can somehow incorporate the concept of the shortcut model, in particular, in the regulation of developmentally related genes.
Concluding remarks
Increasing evidence suggests that Pol II is regulated by distinct signaling pathways in various biologic processes including cell proliferation, cell morphogenesis and embryo development.Citation40-42 These processes are also subjected to the control by Ras or Rho GTPase signaling. The unexpected findings in two divergent model systems, yeast and Arabidopsis, link for the first time Rho GTPase signaling directly to the Pol II CTD code modulation. This evolutionarily conserved pathway has rendered a strong support for the shortcut model of transcriptional control. This shortcut model, including the Ras/PKA-Mediator pathway reported earlier, likely operates in concert with the classical transcriptional model. Further dissection on the Rho-Pol II shortcut model will significantly expand our understanding of gene expression regulation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
Z.-L.Z. was supported by NIH grant 3S06GM008225-20S1 and NSF Grant IOS-1121551.
References
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247-269; PMID:16212495; https://doi.org/10.1146/annurev.cellbio.21.020604.150721
- Craddock C, Lavagi I, Yang Z. New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol 2012; 22:492-501; PMID:22795444; https://doi.org/10.1016/j.tcb.2012.05.002
- Hsin JP, Manley JL. The RNA polymerase II CTD coordinates transcription and RNA processing. Genes Dev 2012; 26:2119-2137; PMID:23028141; https://doi.org/10.1101/gad.200303.112
- Egloff S, Dienstbier M, Murphy S. Updating the RNA polymerase CTD code: adding gene-specific layers. Trends Genet 2012; 28:333-341; PMID:22622228; https://doi.org/10.1016/j.tig.2012.03.007
- Hajheidari M, Koncz C, Eick D. Emerging roles for RNA polymerase II CTD in Arabidopsis. Trends Plant Sci 2013; 18:633-643; PMID:23910452; https://doi.org/10.1016/j.tplants.2013.07.001
- Aristizabal MJ, Kobor MS. A single flexible RNAPII-CTD integrates many different transcriptional programs. Transcription 2016; 7:50-56; PMID:26985717; https://doi.org/10.1080/21541264.2016.1163451
- Zaborowska J, Egloff S, Murphy S. The pol II CTD: new twists in the tail. Nat Struct Mol Biol 2016; 23:771-777; PMID:27605205; https://doi.org/10.1038/nsmb.3285
- Harlen KM, Churchman LS. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat Rev Mol Cell Biol 2017; 18(4):263-273; PMID:28248323; https://doi.org/10.1038/nrm.2017.10
- Marshall C. How do small GTPase signal transduction pathways regulate cell cycle entry? Curr Opin Cell Biol 1999; 11:732-736; PMID:10600705; https://doi.org/10.1016/S0955-0674(99)00044-7
- Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta 2011; 1813:1619-1633; PMID:21167873; https://doi.org/10.1016/j.bbamcr.2010.12.012
- Luse DS. The RNA polymerase II preinitiation complex: through what pathway is the complex assembled? Transcription 2013; 5:e27050; PMID:24406342; https://doi.org/10.4161/trns.27050
- Wong KH, Jin Y, Struhl K. TFIIH phosphorylation of the Pol II CTD stimulates mediator dissociation from the preinitiation complex and promoter escape. Mol Cell 2014; 54:601-612; PMID:24746699; https://doi.org/10.1016/j.molcel.2014.03.024
- Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol 2015; 16:155-166; PMID:25693131; https://doi.org/10.1038/nrm3951
- Kuchin S, Treich I, Carlson M. A regulatory shortcut between the Snf1 protein kinase and RNA polymerase II holoenzyme. Proc Natl Acad Sci U S A 2000; 97:7916-7920; PMID:10869433; https://doi.org/10.1073/pnas.140109897
- Howard SC, Budovskaya YV, Chang YW, Herman PK. The C-terminal domain of the largest subunit of RNA polymerase II is required for stationary phase entry and functionally interacts with the Ras/PKA signaling pathway. J Biol Chem 2002; 277:19488-19497; PMID:12032176; https://doi.org/10.1074/jbc.M201878200
- Chang YW, Howard SC, Herman PK. The Ras/PKA signaling pathway directly targets the Srb9 protein, a component of the general RNA polymerase II transcription apparatus. Mol Cell 2004; 15:107-116; PMID:15225552; https://doi.org/10.1016/j.molcel.2004.05.021
- Inglis DO, Sherlock G. Ras signaling gets fine-tuned: regulation of multiple pathogenic traits of Candida albicans. Eukaryotic Cell 2013; 12:1316-1325; PMID:23913542; https://doi.org/10.1128/EC.00094-13
- Abdellatif M, Packer SE, Michael LH, Zhang D, Charng MJ, Schneider MD. A Ras-dependent pathway regulates RNA polymerase II phosphorylation in cardiac myocytes: implications for cardiac hypertrophy. Mol Cell Biol 1998; 18:6729-6736; PMID:9774686; https://doi.org/10.1128/MCB.18.11.6729
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, Miki T, Gutkind JS. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell 1995; 81:1137-1146; PMID:7600581; https://doi.org/10.1016/S0092-8674(05)80018-2
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 1995; 81:1147-1157; PMID:7600582; https://doi.org/10.1016/S0092-8674(05)80019-4
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 1995; 81:1159-1170; PMID:7600583; https://doi.org/10.1016/S0092-8674(05)80020-0
- Stengel K, Zheng Y. Cdc42 in oncogenic transformation, invasion, and tumorigenesis. Cell Signal 2011; 23:1415-1423; PMID:21515363; https://doi.org/10.1016/j.cellsig.2011.04.001
- Hanna S, El-Sibai M. Signaling networks of Rho GTPases in cell motility. Cell Signal 2013; 25:1955-1961; PMID:23669310; https://doi.org/10.1016/j.cellsig.2013.04.009
- Porter AP, Papaioannou A, Malliri A. Deregulation of Rho GTPases in cancer. Small GTPases 2016; 7:123-138; PMID:27104658; https://doi.org/10.1080/21541248.2016.1173767
- Chipumuro E, Marco E, Christensen CL, Kwiatkowski N, Zhang T, Hatheway CM, Abraham BJ, Sharma B, Yeung C, Altabef A et al. CDK7 inhibition suppresses super-enhancer-linked oncogenic transcription in MYCN-driven cancer. Cell 2014; 159:1126-1139; PMID:25416950; https://doi.org/10.1016/j.cell.2014.10.024
- Wang Y, Zhang T, Kwiatkowski N, Abraham BJ, Lee TI, Xie S, Yuzugullu H, Von T, Li H, Lin Z et al. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell 2015; 163:174-186; PMID:26406377; https://doi.org/10.1016/j.cell.2015.08.063
- Zhang B, Yang G, Chen Y, Zhao Y, Gao P, Liu B, Wang H, Zheng ZL. C-terminal domain (CTD) phosphatase links Rho GTPase signaling to Pol II CTD phosphorylation in Arabidopsis and yeast. Proc Natl Acad Sci U S A 2016; 113:E8197-E8206; PMID:27911772; https://doi.org/10.1073/pnas.1605871113
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005; 120:687-700; PMID:15766531; https://doi.org/10.1016/j.cell.2004.12.026
- Tao LZ, Cheung AY, Nibau C, Wu HM. RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell 2005; 17:2369-2383; PMID:15994909; https://doi.org/10.1105/tpc.105.032987
- Koiwa H, Hausmann S, Bang WY, Ueda A, Kondo N, Hiraguri A, Fukuhara T, Bahk JD, Yun DJ, Bressan RA et al. Arabidopsis C-terminal domain phosphatase-like 1 and 2 are essential Ser-5-specific C-terminal domain phosphatases. Proc Natl Acad Sci U S A 2004; 101:14539-14544; PMID:15388846; https://doi.org/10.1073/pnas.0403174101
- Navarro-Lerida I, Pellinen T, Sanchez SA, Guadamillas MC, Wang Y, Mirtti T, Calvo E, Del Pozo MA. Rac1 nucleocytoplasmic shuttling drives nuclear shape changes and tumor invasion. Dev Cell 2015; 32:318-334; PMID:25640224; https://doi.org/10.1016/j.devcel.2014.12.019
- Staus DP, Weise-Cross L, Mangum KD, Medlin MD, Mangiante L, Taylor JM, Mack CP. Nuclear RhoA signaling regulates MRTF-dependent SMC-specific transcription. Am J Physiol Heart Circ Physiol 2014; 307:H379-H390; PMID:24906914; https://doi.org/10.1152/ajpheart.01002.2013
- Li Z, Li Z, Gao X, Chinnusamy V, Bressan R, Wang ZX, Zhu JK, Wu JW, Liu D. ROP11 GTPase negatively regulates ABA signaling by protecting ABI1 phosphatase activity from inhibition by the ABA receptor RCAR1/PYL9 in Arabidopsis. J Integr Plant Biol 2012; 54:180-188; PMID:22251383; https://doi.org/10.1111/j.1744-7909.2012.01101.x
- Schwer B, Bitton DA, Sanchez AM, Bahler J, Shuman S. Individual letters of the RNA polymerase II CTD code govern distinct gene expression programs in fission yeast. Proc Natl Acad Sci U S A 2014; 111:4185-4190; PMID:24591591; https://doi.org/10.1073/pnas.1321842111
- Schuller R, Forne I, Straub T, Schreieck A, Texier Y, Shah N, Decker TM, Cramer P, Imhof A, Eick D. Heptad-specific phosphorylation of RNA polymerase II CTD. Mol Cell 2016; 61:305-314; PMID:26799765; https://doi.org/10.1016/j.molcel.2015.12.003
- Suh H, Ficarro SB, Kang UB, Chun Y, Marto JA, Buratowski S. Direct analysis of phosphorylation sites on the Rpb1 C-terminal domain of RNA polymerase II. Mol Cell 2016; 61:297-304; PMID:26799764; https://doi.org/10.1016/j.molcel.2015.12.021
- Mayfield JE, Robinson MR, Cotham VC, Irani S, Matthews WL, Ram A, Gilmour DS, Cannon JR, Zhang YJ, Brodbelt JS. Mapping the phosphorylation pattern of drosophila melanogaster RNA polymerase II carboxyl-terminal domain using ultraviolet photodissociation mass spectrometry. ACS Chem Biol 2017; 12:153-162; PMID:28103682; https://doi.org/10.1021/acschembio.6b00729
- Bellier S, Dubois MF, Nishida E, Almouzni G, Bensaude O. Phosphorylation of the RNA polymerase II largest subunit during Xenopus laevis oocyte maturation. Mol Cell Biol 1997; 17:1434-1440; PMID:9032270; https://doi.org/10.1128/MCB.17.3.1434
- Tee WW, Shen SS, Oksuz O, Narendra V, Reinberg D. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell 2014; 156:678-690; PMID:24529373; https://doi.org/10.1016/j.cell.2014.01.009
- Danko CG, Hah N, Luo X, Martins AL, Core L, Lis JT, Siepel A, Kraus WL. Signaling pathways differentially affect RNA polymerase II initiation, pausing, and elongation rate in cells. Mol Cell 2013; 50:212-222; PMID:23523369; https://doi.org/10.1016/j.molcel.2013.02.015
- Lagha M, Bothma JP, Esposito E, Ng S, Stefanik L, Tsui C, Johnston J, Chen K, Gilmour DS, Zeitlinger J et al. Paused Pol II coordinates tissue morphogenesis in the Drosophila embryo. Cell 2013; 153:976-987; PMID:23706736; https://doi.org/10.1016/j.cell.2013.04.045
- Saunders A, Core LJ, Sutcliffe C, Lis JT, Ashe HL. Extensive polymerase pausing during Drosophila axis patterning enables high-level and pliable transcription. Genes Dev 2013; 27:1146-1158; PMID:23699410; https://doi.org/10.1101/gad.215459.113
