ABSTRACT
Objective
Up to 90% of antimicrobials globally are prescribed and dispensed in ambulatory care. However, there are considerable gaps regarding the extent and rationale for their use especially in low- and middle-income countries such as South Africa. Point prevalent surveys (PPS) are useful to determine current prescribing patterns, identify targets for quality improvement and evaluate the effectiveness of antimicrobial stewardship programmes (ASPs) within institutions. Consequently, the objective of this study was to undertake a PPS within community healthcare centers (CHCs) in South Africa given their importance to the public healthcare system. The findings will be used to provide guidance on future interventions to improve antimicrobial use in South Africa and wider.
Methods
A PPS of antimicrobial consumption was undertaken among patients attending 18 CHCs in South Africa. A web-based application was used to record the utilization data, with utilization assessed against World Health Organization (WHO) and South African guidelines.
Results
The overall prevalence of antimicrobial use amongst patients attending the CHCs was 21.5% (420 of 1958 patients). This included one or more antimicrobials per patient. The most frequently prescribed antimicrobials were amoxicillin (32.9%), isoniazide (11.3%) and a combination of rifampicin, isoniazid, pyrazinamide and ethambutol (Rifafour®) (10.5%), with the majority from the WHO Access list of antibiotics. There was high adherence to guidelines (93.4%). The most common indication for antibiotics were ear, nose and throat infections (22.8%), with no culture results recorded in patients’ files.
Conclusions
It’s encouraging to see high adherence to South African guidelines when antimicrobials were prescribed, with the majority taken from the WHO Access list. However, there were concerns with appreciable prescribing of antimicrobials for upper respiratory tract infections that are essentially viral in origin, and a lack of microbiological testing. The establishment of ASPs can help address identified concerns through designing and implementing appropriate interventions.
1. Introduction
In ambulatory care, especially among low- and middle-income countries (LMICs), oral antimicrobials are consistently in the top therapeutic classes of medicines by frequency of use [Citation1,Citation2]. Alongside this, there are concerns with high inappropriate prescribing among ambulatory care healthcare professionals (HCPs), enhanced by the time pressures on them combined with pressures from patients [Citation1,Citation3,Citation4]. A considerable proportion of antimicrobials are prescribed and dispensed for acute respiratory tract infections which are essentially viral in origin [Citation1,Citation5,Citation6]. Such activities have been exacerbated during the current COVID-19 pandemic with high use despite only limited bacterial or fungal co-infections [Citation7].
Of concern is that antimicrobial utilization rates are increasing among LMICs [Citation8]; however, this is often unnecessary due to a lack of regulations and their monitoring, concerns with training of HCPs and considerable informal use [Citation8]. High and unnecessary use of antimicrobials enhances antimicrobial resistance (AMR) with its associated impact on morbidity, mortality and costs [Citation9–11]. Growing rates of AMR across countries, and the consequences, led to the development of national action plans (NAPs) to reduce AMR, building on the World Health Organization’s initiative [Citation12,Citation13]. South Africa is no exception [Citation14]. However, there is currently limited data nationally on non-hospital (community/ ambulatory care) antimicrobial use across countries, which is exacerbated by the considerable purchasing of antibiotics without a prescription especially among LMICs [Citation1,Citation15].
A key element of NAPs is the documentation of current antimicrobial usage patterns across sectors including both hospital and ambulatory care [Citation14,Citation16].
South Africa is a LMIC of 60.14 million people, with approximately two thirds living in urban areas [Citation17,Citation18]. In South Africa, ambulatory care in the public healthcare system is principally provided through a nurse-based, doctor-supported system consisting of over 3500 community healthcare centers (CHCs) and primary healthcare clinics (PHCs), which should be available within 5 km to over 90% of the population, and free at the point of use [Citation19,Citation20]. CHCs are the most visited healthcare facility among patients in South Africa. Their main function is to deliver most ambulatory care services to the South African population, especially those residing in rural areas. Services include advice on hygiene, vaccinations and health education as well as antenatal care and safe child birth delivery. CHCs also provide examinations for screening purposes, treatments and referrals [Citation21].
We are aware of a number of studies conducted across South Africa investigating the utilization of antimicrobials in ambulatory care [Citation22–25] combined with studies assessing total antimicrobial utilization in South Africa [Citation26]. Alongside this, point prevalence surveys (PPS) have been undertaken among hospitals in South Africa to document their current utilization patterns [Citation27–30]. However, we were unaware of any study undertaken to date to assess current antimicrobial prescribing within CHCs in South Africa. Consequently, we instigated this study to address this information gap considering previous concerns with inappropriate prescribing of antimicrobials within ambulatory care facilities in South Africa [Citation20,Citation24]. This is in line with the goals of the South African NAP on AMR including greater knowledge of current antimicrobial utilization rates coupled with programmes to reduce unnecessary antimicrobial prescribing exception [Citation14,Citation16].
2. Materials and methods
2.1 Study design
This was a PPS study to determine antimicrobial utilization patterns among 18 CHCs across South Africa using a web-based application (APP) [Citation27]. We have used this approach before to assess current antimicrobial utilization patterns among the pediatric and adult populations in public sector hospitals in South Africa [Citation29,Citation31].
2.2 Study sites
We randomly selected 18 CHCs across South Africa from a total of 233 CHCs throughout the country. They comprised two CHCs from each of the nine provinces in South Africa, with prior selection before randomization. This was based on their proximity to an academic or tertiary hospital that was used for the referral of patients in the province as well as the availability of personnel to conduct the PPS study (convenient sampling).
2.3 Data collection tool and variables recorded
As before, we used an APP combined with a built-in paper-based data collection tool in order to reduce the time taken for data collection [Citation27].The data collection tool was based on the Global PPS and European Center for Disease Prevention and Control study forms, which was subsequently adapted to include highly prevalent co-morbid conditions found in Sub-Saharan Africa, which includes the human immunodeficiency virus (HIV), malaria, malnutrition and tuberculosis (TB) [Citation27,Citation30,Citation32–37]. In view of this, we included antimicrobials to treat patients with TB in this PPS study, and did not separate out antimicrobials prescribed for patients with TB versus those without TB, which included linezolid and quinolones. This is in line with our previous PPS studies in South Africa [Citation29,Citation30].
The data that was collected included the name of the CHC based on the South African National Department of Health (NDoH) classification [Citation29,Citation38]. The patient level data that was collected and recorded included the age of patients alongside their gender, the extent of any intubation, and the extent of any readmission as well as their antimicrobial history and any hospitalization during the last 90 days. Furthermore, the extent of any co-morbidities especially any HIV, TB and malaria were recorded.
Antimicrobial data with corresponding indications and route of administration were recorded for each patient. In addition, whether they were given for prophylaxis or for treatment. Antimicrobial utilization was analyzed according to different age groups of patients. This included neonates: 0 to 28 days; infants: 1 to 11 months; children: 1 to 11 years; adolescents: 12 to 17 years; younger adults: 18 to 35 years; middle age adults: 36 to 55 years; and older adults: 56 years and older, alongside the different ward categories. Antimicrobials were categorized on the basis of their WHO Anatomical Therapeutic Chemical (ATC) 3rd level classification system (2019) [Citation39]. These included J01A, C, D, E, F, G, M and X; J02A; J04A; J05AB and P01A antimicrobials.
We also used the AWaRe classification as a quality indicator for antimicrobial prescribing especially in children [Citation40–43]. Antibiotics from the Access list are considered as first-line or second-line treatments for key infections, and should be routinely available for appropriate prescribing and dispensing within countries especially LMICs [Citation41]. There should be limited prescribing of antibiotics in the ‘Watch’ group as these are considered to have a higher resistance potential and toxicity, alongside limited prescribing of antibiotics in the ‘Reserve’ group, which are considered as a last resort. Antibiotics in both the ‘Watch’ and ‘Reserve’ group should be prioritized for antimicrobial stewardship programmes (ASPs) where their prescribing is a concern [Citation42,Citation44,Citation45].
2.4 Patient selection and data collection
The files of all patients seen at each selected CHC on the day prior to the day of data collection were included in the study. All the relevant files were kept aside by clinic staff for the data collector to review the following day.
In order to calculate the point prevalence of antimicrobial use within each CHC, all the patients that were seen at the CHC on the day before the data collection day became the denominator, whether they were prescribed an antimicrobial or not. Data were collected over a period of five months between 1 March 2018 and 31 July 2018, with one full day spent at each of the CHCs. The CHC and the referring hospital data were collected collaboratively within the same week by the designated data collectors. The numerator included all patients who were subsequently prescribed an antimicrobial. The only exclusion criteria were patients who attended accident and emergency units, if such units were available with the CHC [Citation29,Citation30,Citation36]. Data collection took place only on weekdays to optimize representation, with the data collectors spending one day at each CHC.
The utilization data were collected only by hospital and academic pharmacists that had received extensive training on how to conduct a PPS prior to data collection. This included a demonstration and a practice session on the use of the purposely developed APP, similar to the training provided in previous PPS studies conducted in South Africa and wider [Citation29,Citation30,Citation36,Citation37,Citation46].
2.5 Quality indicators
We used a number of indicators to assess the quality of antimicrobial prescribing, which were based on previous studies [Citation33,Citation36,Citation42,Citation47–49]. The indicators used included current prevalence rates for the prescribing of antimicrobials as well as the number prescribed per patient. Alongside this, whether the indication for the antimicrobial being prescribing was recorded in the patient’s notes. We also reviewed whether microbiological culture results were recorded in the patients’ files.
We also assessed the ATC class of antimicrobial prescribed, the route of administration and whether the antimicrobials were being prescribed for prophylaxis or treatment [Citation33,Citation36,Citation47,Citation50]. Surgical prophylaxis is typically defined as the administration of antibiotics before, during, or after a surgical procedure to help prevent infectious complications, with medical prophylaxis defined as the prevention of infections in non-surgical situations [Citation36,Citation46,Citation47]. In addition, we assessed the proportion of antimicrobials prescribed in each of the three AWaRe categories as the total number of antimicrobials prescribed in the respective Access, Watch, or Reserve groups as a percentage of the total number of antimicrobials prescribed among the participating CHCs [Citation43,Citation44,Citation51].
Finally, we also assessed whether the antimicrobials prescribed followed the NDoH Essential Medicines List and Standard Treatment Guidelines (EML-STG) [Citation33]. This is because there have been concerns with adherence rates to published guidelines in South Africa and among other African countries [Citation20,Citation52–54].
2.6 Data management and statistical analysis
The APP feeds directly into an Excel® database. The data are subsequently imported into SAS (version 9.4 for Windows) for analysis in consultation with a statistician. Before analysis, the data was cleaned and validated by ensuring that all the data required for subsequent analysis was present, that the correct units were used and entered, and that there were no errors or duplications among the submitted data. If errors were found, retracing was performed to try correct the anomaly or the data was removed if there was no accountability.
All patients aged below 18 years were regarded as pediatric patients, and broken down by specific age groups. Those aged 18 years and above were viewed as adults, and again broken down by specific age groups, as previously described. Situations where an antimicrobial was prescribed but the condition was not an infection were recorded as not applicable.
As mentioned, we assessed antimicrobial utilization as percentages (proportional use) by indication (prophylactic or therapeutic), age category and by AWaRe classification based on the 2017 WHO EML Access, Watch, Reserve grouping [Citation43]. However, because some of the antimicrobials had not yet been classified, we included these as an unclassified group.
We used the Chi square (χ2) test with a p-value <0.05 for significance to assess the relationship between the categorical variables. Cramer’s V or phi coefficient ≥0.50 was considered a strong association for interpretation, 0.30–0.49 as a moderate association, 0.10–0.29 as a weak association and <0.10 limited if any association.
2.7 Ethical approval
Data collection commenced after receiving ethical approval from the Sefako Makgatho University Research Ethics Committee (SMUREC/P/36/2018: PG) and permissions from the various study sites. Patient and hospital confidentiality was maintained at all times by applying unique study identification numbers for hospitals and patients. Alongside this, no personal identifiers were recorded for patients in order to maintain anonymity.
No patients, parents or guardians were approached for consent since this was a retrospective study based on data collected from patients’ medical records, with no direct contact with patients, children, their parents, or guardians. This is similar to previous PPS studies performed by the coauthors [Citation30,Citation49,Citation51,Citation55–57].
3. Results
Overall, 1958 patients were reviewed across the 18 CHCs. The majority (84.8%;1661/1958) were adults while 15.2% were pediatric patients. The median (IQR) range for age was 41 (Citation27–41) years and there were more females (64.0%; 1253/1958) than males. Of the 1661 adults, the majority were female (66.6%;1107/1661) whilst pediatric patients were almost equally distributed with 49.2% (146/297) females.
Antimicrobials were prescribed for 21.5% (420/1958) of patients among the 18 participating CHCs. Pediatric patients consumed more antimicrobials than adults at 25.3% (75/297) versus 20.8% (345/1661) for adults. However, due to majority of the population being adults in the study, most of the antimicrobials were prescribed for the adults. Overall, 19 antimicrobials were prescribed a total of 486 times, with a minority receiving more than one antimicrobial. Adults contributed 83.3% (405/486) of total antimicrobial prescribing, with the majority prescribed among adults aged 18–35 years (36.0%; 175/486) and those aged 36–55 years (31.9%; 155/486). provides a further breakdown by the designated age groups.
Table 1. Overall antimicrobial consumption by patient demographics.
Antimicrobials were prescribed the most for indications of upper respiratory tract infections (URTIs), e.g., infections of the ear, nose, throat, larynx and mouth but excluding the bronchus (16.3%; 79/486), followed by TB (8.4%; 41/486) and subsequently soft tissue infections including cellulitis, wound and deep soft tissue, but excluding bone (5.1%; 25/486) (). On 50 occasions (10.3%) antimicrobials were prescribed but this was not an applicable indication for antimicrobial use. According to the South African CDC, this is when the indication is not an infection but antimicrobials are prescribed.
Table 2. Indications along with the antimicrobials prescribed for treatment.
Out of the 420 patients on antimicrobials, 55 (13.1%) patients were prescribed more than one antimicrobial at the time of the survey whilst 365 (86.9%) received a single antimicrobial.
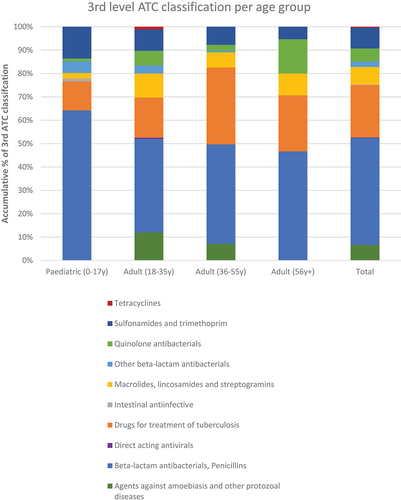
The beta-lactam antibacterials including the penicillins, were the most frequently prescribed class of antimicrobials (45.9%; 223/486), followed by antimicrobials for treating TB (22.4%; 109/486) and sulfonamides and trimethoprim (8.8%; 43/486) ( and ).
Figure 1. 3rd level ATC Classification of antimicrobials by age groups.
Considering specific antimicrobials, amoxicillin was the most frequently prescribed antimicrobial (32.9%; 160/486) followed by isoniazid (11.3%; 55/485) and a combination of rifampicin, isoniazid, pyrazinamide and ethambutol (Rifafour®) (10.5%; 51/486) ().
Table 3. Antimicrobials prescribed according to the top five antimicrobial classes.
illustrates the different antimicrobial classes prescribed by age group.
Most of the antimicrobials prescribed were for oral administration (97.5%; 474/486) with the remainder administered parenteral (2.5%) (). None of the files reviewed contained any culture results in them. Consequently, all antimicrobials in this study were prescribed empirically.
Table 4. Quality indicator summary.
Overall compliance with the current South African EML-STG for the antimicrobials prescribed was 93.4% (454/486), however, only 69.5% (338/486) were prescribed by their generic name (International nonproprietary name [INN]) (). More than half (62.1% 302/486) of the antimicrobials prescribed belonged to the Access category and 15% (73/486) were from the Watch category, with similar groupings across the age groups. Encouragingly, no Reserve antimicrobials were prescribed among the reviewed patients in the different age groups (). However, 22.8% of the antimicrobials prescribed could not be classified under the current AWaRe system ().
Antimicrobial prescriptions were mostly issued by medical officers (60.7% 295/486), followed by nurses (36.2% 176/486), with specialists only accounting for a minority 3.1% of the prescribed antimicrobials ().
4. Discussion
We believe this is the first study to fully assess antimicrobial prescribing among CHCs in South Africa as the first step to improving their future prescribing. Overall, 21.5% of the admitted patients to CHCs in South Africa received at least one antimicrobial. The pediatric population had an antimicrobial consumption rate of 25.3% and this rate mirrors Fink et al. (2020), who ascertained a rate of 24.5% among young children attending healthcare facilities in LMICs [Citation58]. Encouragingly, the overall rate of 21.5% in our study was appreciably lower than seen among public healthcare facilities (PHCs) in Ghana (59.9%), Pakistan (57.2%), India (49%), Thailand (46.9%), Nepal (44.7%), Botswana (42.7%) and Cameroon (36.7%) [Citation2,Citation59–64]. However, it was higher than that seen in the study of Ab Rahman et al. (2016) who found a rate of 6.8% among patients attending PHCs in Malaysia [Citation65].
The prescribing rates among the CHCs in South Africa were also lower than a pooled prescribing prevalence rate of 51.5% to 52.0% among PHCs across Africa and other LMICs in the studies of Ofori-Asenso et al. (2015) and Sulis et al. (2020) [Citation66,Citation67]. The difference between the findings of Fink et al. (2020) and Sulis et al. (2020) may reflect differences in the nature of the PHCs and the ages of the populations studied. In addition, there is a potential mix of private and public healthcare facilities among the included publications. This is because we know that different incentives can influence antimicrobial prescribing habits in private versus public clinics [Citation68,Citation69]. In Malaysia, Ab Rahman et al. documented a prescribing rate of antibiotics at 30.8% of patients attending private clinics versus only 6.8% among those attending public clinics [Citation65]. There was also high use of antimicrobials among private physicians in Botswana treating patients with upper respiratory tract infections (URTIs) enhanced by patient pressure, as well as a higher use of antibiotics and medicines administered by injection among the same physicians treating patients in private versus public clinics in Iran [Citation69,Citation70]. The lower rate of antimicrobial prescribing among the 18 CHCs in South Africa could be due to a number of factors. These include the establishment of ASPs within hospitals in South Africa coupled with the dissemination of the National Strategic Framework to reduce AMR [Citation71–73]. However, further studies are needed to substantiate any perceptions.
Since URTIs were the most frequent diagnosis (22.8%), this may explain why the penicillins were the most prescribed antimicrobials (). Other studies have also shown that the penicillins (β-lactams) are among the most prescribed and dispensed class of antibiotics in ambulatory care given the high prevalence rates of URTIs [Citation2,Citation65,Citation74–77]. β-lactams have continued to be the mainstay of antimicrobial therapy due to their wide spectrum of activity against both gram positive and negative organisms.
Isoniazide and a combination of rifampicin, isoniazid, pyrazinamide and ethambutol (Rifafour®) were also among the most prescribed antimicrobials in the surveyed CHCs across South Africa. This high rate may reflect the relatively high prevalence of TB in South Africa with eight countries currently accounting for two thirds of the global total prevalence of TB. South Africa currently contributes 3.6% of global cases, similar to other countries including Bangladesh (3.6%) and Nigeria (4.4%) [Citation78]. We have seen high rates of TB in other PPS studies in South Africa [Citation30]; although lower in others [Citation29]. These findings may reflect the different nature of patients treated in the different healthcare settings in South Africa. However, these findings make it mandatory to assess the profile of patients attending ambulatory healthcare centers in Africa and wider when seeking to compare antimicrobial utilization patterns including their rates across LMICs. This is because high rates of TB are typically not seen in a number of other LMICs, or high-income countries, making comparisons regarding antimicrobial prescribing difficult without such knowledge. This suggestion is further endorsed by a study conducted among PHCs in Botswana where high rates of prescribing of metronidazole were documented, which was due to a high burden of sexually transmitted and gynecological infections [Citation2]. This is different to ambulatory care prescribing seen in many other countries where high rates of URTIs are seen [Citation10,Citation75].
It was also encouraging to see a high rate of prescribing of antibiotics in the Access group with no prescribing of Reserve antibiotics (). These rates are considerably higher compared with the findings of Hsia et al. (2018) where the utilization of antibiotics in the Access category only accounted for 33.3% of total utilization among the hospitals in South Africa taking part in the global PPS study [Citation42]. However, this needs further evaluation especially given high prevalence rates for URTIs seen in our study, which are typically viral in origin.
It was also encouraging to see low use of injections in the surveyed CHCs (1.9%; ). This compares to appreciably higher rates of injectable administration in hospitals in South Africa (64.3%) and wider across Africa (63.1% to over 80% of administered antimicrobials) [Citation29,Citation33,Citation36,Citation56]. Alongside this, it was encouraging to see high rates of adherence to the South African EML-STG when antimicrobials were prescribed (93.4%). This differs from recent studies where there have been concerns with guideline implementation in ambulatory care in South Africa [Citation20,Citation79] and among other African countries [Citation52,Citation54]. Compliance is important to enhance the quality of prescribing as well as improve stock control systems to limit the potential for shortages of antimicrobials and the concerns this cause if no suitable alternatives have been discussed beforehand [Citation17,Citation80–82].
There were reports regarding difficulties in obtaining microbiological results among the surveyed CHCs resulting in none of the files reviewed having culture results within them. This may be attributed to limited services currently at CHCs in South Africa, distance to such facilities and a delay in feedback of the results. Engler et al. (2021) in their study highlighted the challenges pertaining to the collection and reporting of culture and sensitivity across public healthcare facilities in South Africa [Citation83]. These challenges are enhanced in CHCs, which have to wait for any results from the nearest referral hospital making it scarce to find such results in patient’s files in CHCs. However, in the case of patients with TB, they contrast with the findings of McCarthy et al (2018) where empiric treatment accounted for only 15% of patients initially treated for TB in primary healthcare settings [Citation25]. We are not sure of the reasons behind this appreciable difference. This urgently needs to be addressed including whether CHCs are dealing with follow-up TB patients or patients presenting to health care facilities for the first time. Generally though, limited diagnostic and surveillance data are currently the major shortcomings among public healthcare facilities in South Africa, which need addressing going forward to improve future antimicrobial prescribing and reduce AMR [Citation83]. This also includes greater documentation generally in patients’ notes of the rationale behind the antimicrobials prescribed including any diagnostic tests used.
In addition, ambulatory care facilities across South Africa do face many challenges especially among both PHC and CHC facilities where antimicrobial stewardship activities and ASPs are not fully implemented. This is important when initiating or reviewing potential treatment approaches for patients with infectious diseases attending CHCs to improve future prescribing. This especially given the concerns that can exist when inappropriately treating patients with infectious diseases in ambulatory care across Africa [Citation20,Citation53,Citation54,Citation70]. We will continue to monitor the situation to improve future antimicrobial prescribing.
Another identified concern was that not all prescriptions were written by their INN name (), which adds to costs given the low prices for multi-sourced medicines that have been obtained in countries versus originator prices [Citation84–86]. The Medicines and Related Substances Control Amendment Act No. 90 of 1997 ensures that the South African government can undertake a variety of activities to provide a supply of affordable medicines. Further to this, multi-sourced medicines (generics) are the first choice in the public sector whilst those patients in medical aid schemes, as medical consumers, have the option whether or not to choose a generic medicine [Citation87]. However, medicine prices became a critical issue from mid-2000 across South Africa. Consequently, generic medicines have been recommended for wider use, including the private sector. We are aware of concerns with the quality of generics in some African countries [Citation88]; however, this should not apply to South Africa with its comprehensive procurement and regulatory practices coupled with patients obtaining their antibiotics directly from CHCs with no co-payment [Citation17]. We will also be following this up in future studies.
Finally, of particular relevance to South Africa and other LMICs, is the potential contribution of nurses in the ambulatory care setting to improve future antibiotic utilization and reduce AMR [Citation3,Citation19,Citation20,Citation89]. In our study, 36.2% of the antimicrobials prescribed were by nurses. Consequently, there is a need to ensure their full knowledge regarding appropriate prescribing of antimicrobials against recognized guidance contained in the South African EML-STG and audit subsequent prescribing habits [Citation20]. This is similar to the situation for other HCPs across Africa and to other LMICs to ensure appropriate knowledge regarding antibiotics, AMR and ASPs and to promote high adherence to national treatment guidelines [Citation10,Citation90,Citation91]. As a result, enhancing appropriate prescribing of antimicrobials. These are projects for the future.
The results of this study will be disseminated among the CHCs taking part and others within the public healthcare system in South Africa. Hopefully, this will result in the implementation or progression of antimicrobial stewardship teams in line with Government guidance to maintain surveillance of current antimicrobial use by training all health care workers [Citation71,Citation83]. As a result, improving future antimicrobial prescribing, which includes greater collection of microbiological data to inform future empiric prescribing.
We are aware there are limitations with this study. These include the fact that we only included two CHCs from each province. In addition, the retrospective nature of the study precluded direct contact with prescribers to ascertain the rationale behind their prescribing of antimicrobials. This included the prescribing of antimicrobials for patients with URTIs, which are essentially viral in origin. No dosage data was collected, which could have added to other potential quality indicators for the study. In addition, the length of time taken for data collection, as well as the days when the surveys were undertaken in each hospital, may have influenced admission patterns. However, despite these limitations, we believe the findings are robust providing future guidance.
5. Conclusions
The prevalence of antimicrobial prescribing among CHCs in South Africa was 21.5% and URTIs were the most frequently diagnosed condition. This resulted in amoxicillin being the most prescribed antimicrobial. It was encouraging to see high adherence to the South African EML-STG when antimicrobials were prescribed, with the majority of antimicrobials in the WHO Access list. However, there were concerns with the extent of antimicrobial prescribing for patients with URTIs, which are essentially viral in origin, coupled with a lack of sensitivity testing. Alongside this, not all antimicrobials were prescribed by INN. The establishment of pertinent ASPs among CHCs should help improve antimicrobial prescribing in the future. This could include more routine testing of patients to identify resistant organisms to help improve future empiric prescribing.
Conflicts of interest and funding
The study was funded by the ENAABLERS grant and as part of a Newton Scholarship awarded to Natalie Schellack, when at SMU, jointly funded by the South African Medical Research Council and UK Medical Research Council.
Disclosure of any financial/other conflicts of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author Contributions
Conceptualization, NS, BG, DK, MB and JCM; methodology, PS, NS, DK and JCM; data curation and analysis, PS, NS, DK, and AK; writing—original PS and BBG.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors gratefully acknowledge Ms Petra Gaylard, a consultation statistician, for the statistical analysis of the data. We thank all the Departments of Health in the provinces, and the hospital management of the participating hospitals for allowing us to collect data from there. We also thank the MPharm interns and the support staff at SMU for their assistance with data collection.
Data Availability Statement
Additional data is available on reasonable request.
References
- Godman B, Haque M, McKimm J, et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin. 2020;36(2):301–327.
- Mashalla Y, Setlhare V, Massele A, et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: findings and implications. Int J Clin Pract. 2017;71(12):e13042.
- Rezal RS, Hassali MA, Alrasheedy AA, et al. Prescribing patterns for upper respiratory tract infections: a prescription-review of primary care practice in Kedah, Malaysia, and the implications. Expert Rev Anti Infect Ther. 2015;13(12):1547–1556.
- Teixeira Rodrigues A, Roque F, Falcao A, et al. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013;41(3):203–212.
- Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during influenza seasons. JAMA Network Open. 2018;1(2):e180243.
- Wei X, Zhang Z, Hicks JP, et al. Long-term outcomes of an educational intervention to reduce antibiotic prescribing for childhood upper respiratory tract infections in rural China: follow-up of a cluster-randomised controlled trial. PLoS Med. 2019;16(2):e1002733.
- Alshaikh FS, Godman B, Sindi ON et al. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and meta-analysis. PLoS One. 2022;17(8):e0272375
- Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–e70.
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6(1):47.
- Godman B, Egwuenu A, Haque M, et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life. 2021;11(6).
- Hofer U. The cost of antimicrobial resistance. Nat Rev Microbiol. 2019;17(1):3.
- Godman B, Egwuenu A, Wesangula E et al. Tackling antimicrobial resistance across sub-Saharan Africa; current challenges and implications for the future. Expert Opinion on Drug Safety. 2022
- Iwu CD, Patrick SM. An insight into the implementation of the global action plan on antimicrobial resistance in the WHO African region: a roadmap for action. Int J Antimicrob Agents. 2021;58(4):106411.
- Department of Health Republic of South Africa. SOUTH AFRICAN ANTIMICROBIAL RESISTANCE NATIONAL STRATEGY FRAMEWORK; A ONE HEALTH APPROACH - 2017–2024. 2017. Available at URL: https://www.knowledgehub.org.za/system/files/elibdownloads/2020-03/AMR%20National%20Action%20Plan%202018%20-%202024.pdf
- Auta A, Hadi MA, Oga E, et al. Global access to antibiotics without prescription in community pharmacies: a systematic review and meta-analysis. J Infect. 2019;78(1):8–18.
- National department of Health, Republic of South Africa - republic of South Africa. Implementation Plan for Antimicrobial Resistance National Strategy Framework 2014-2019. [cited 18 June 2022]. Available at URL: http://www.health.gov.za/index.php/antimicrobial-resistance
- Meyer JC, Schellack N, Stokes J, et al. Ongoing initiatives to improve the quality and efficiency of medicine use within the public healthcare system in South Africa; a preliminary study. Front Pharmacol. 2017;8:751.
- Reublic of South Africa Statistics Department. Mid-year population estimates 2021. [Accessed 18 June 2022]. Available at URL: http://www.statssa.gov.za/publications/P0302/P03022021.pdf.
- McKenzie A, Schneider H, Schaay N, et al. PRIMARY HEALTH CARE SYSTEMS (PRIMASYS)- Case study from South Africa. 2017. [cited 17 June 2022]. Available at URL: https://www.who.int/alliance-hpsr/projects/alliancehpsr_southafricaprimasys.pdf?ua=1.
- Matsitse TB, Helberg E, Meyer JC, et al. Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: findings and implications. Expert Rev Anti Infect Ther. 2017;15(10):963–972.
- Stott BA, Moosa S. Exploring the sorting of patients in community health centres across Gauteng Province, South Africa. BMC Fam Pract. 2019;20(1):5.
- Manderson L. Prescribing, care and resistance: antibiotic use in urban South Africa. Humanit Soc Sci Commun. 2020;7(1):77.
- Torres N, Chibi B Antibiotic use and resistance in South Africa: the need for better data. 2019. [cited 17 June 2022]. Available at URL: http://www.hsrc.ac.za/en/review/hsrc-review-june-2019/antibiotic-use-and-resistance-in-sa.
- Ncube NB, Solanki GC, Kredo T, et al. Antibiotic prescription patterns of South African general medical practitioners for treatment of acute bronchitis. S Afr Med J. 2017;107(2):119–122.
- McCarthy K, Fielding K, Churchyard GJ, et al. Empiric tuberculosis treatment in South African primary health care facilities - for whom, where, when and why: implications for the development of tuberculosis diagnostic tests. PLoS One. 2018;13(1):e0191608.
- Schellack N, Benjamin D, Brink A, et al. A situational analysis of current antimicrobial governance, regulation, and utilization in South Africa. Int J Infect Dis. 2017;64:100–106.
- Kruger D, Dlamini NN, Meyer JC, et al. Development of a web-based application to improve data collection of antimicrobial utilization in the public health care system in South Africa. Hosp Pract. 2021;49(3):184–193.
- Finlayson H, Versporten A, Whitelaw A, Goossens H, et al. The global point prevalence survey of antimicrobial consumption and resistance (global-pps): results of antimicrobial prescribing in a south African tertiary hospital. [cited 8 June 2022]. Available at URL: http://www.global-pps.com/wp-content/uploads/ECCMID-2016_South-Africa.pdf. Available at URL.
- Skosana PP, Schellack N, Godman B, et al. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev Anti Infect Ther. 2021;19(10):1353–1366.
- Dlamini NN, Meyer JC, Kruger D, et al. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: a pilot study and implications. Hosp Pract. 2019;47(2):88–95.
- Skosana PP, Schellack N, Godman B, et al. A national, multicentre, web-based point prevalence survey of antimicrobial use and quality indices among hospitalised paediatric patients across South Africa. J Glob Antimicrob Resist. 2021;29:542–550.
- Massele A, Tiroyakgosi C, Matome M, et al. Research activities to improve the utilization of antibiotics in Africa. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):1–4.
- Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–e29.
- ECDC. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals 2011–2012. [cited 8 June 2022]. Available at URL: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf.
- Plachouras D, Karki T, Hansen S, et al. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro surveillance. 2018; 23(46)
- Anand Paramadhas BD, Tiroyakgosi C, Mpinda-Joseph P, et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev Anti Infect Ther. 2019;17(7):535–546.
- Gwebu PC, Meyer JC, Schellack N, et al. A web-based point prevalence survey of antimicrobial use and quality indicators at Raleigh Fitkin Memorial Hospital in the Kingdom of Eswatini and the implications. Hosp Pract. 2022;1–8.
- National Department of Health (NDoH). Regulations relating to categories of hospitals. Government Notices. Pretoria, South Africa. [cited 10 June 2022]. Available at URL: http://www.health.gov.za/index.php/2014-03-17-09-09-38/legislation/joomla-split-menu/category/84-2012r?download=138:regulations-relating-to-categories-of-hospitals-r185-20122
- WHO. WHO Collaborating Centre for Drug Statistics Methodology. ATC/ DDD Index. [cited 10 June 2022]. Available at URL: https://www.whocc.no/
- Sharland M, Pulcini C, Harbarth S, et al. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect Dis. 2018;18(1):18–20.
- Sharland M, Gandra S, Huttner B, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use-the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. 2019;19(12):1278–1280.
- Hsia Y, Lee BR, Versporten A, et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Health. 2019;7(7):e861–e71.
- Hsia Y, Sharland M, Jackson C, et al. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: an analysis of sales data from 70 middle-income and high-income countries. Lancet Infect Dis. 2019;19(1):67–75.
- Klein EY, Milkowska-Shibata M, Tseng KK, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000-15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1):107–115.
- Saleem Z, Hassali MA, Godman B, et al. Sale of WHO AWaRe groups antibiotics without a prescription in Pakistan: a simulated client study. J Pharm Policy Pract. 2020;13(1):26.
- Afriyie DK, Sefah IA, Sneddon J, et al. Antimicrobial point prevalence surveys in two Ghanaian hospitals: opportunities for antimicrobial stewardship. JAC Antimicrob Resist. 2020;2(1):dlaa001.
- Mwita JC, Ogunleye OO, Olalekan A, et al. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: a Narrative Review and the Implications. Int J Gen Med. 2021;14:515–530.
- Zhang HL, Bodinayake C, Wijayaratne GB, et al. Point-prevalence survey of outpatient antibiotic prescription at a tertiary medical center in Sri Lanka: opportunities to improve prescribing practices for respiratory illnesses. BMC Infect Dis. 2021;21(1):97.
- Kurdi A, Hasan AJ, Baker KI, et al. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan regional government of Northern Iraq: the need for urgent action. Expert Rev Anti Infect Ther. 2021;19(6):805–814.
- Versporten A, Bielicki J, Drapier N, et al. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: developing hospital-quality indicators of antibiotic prescribing for children. J Antimicrob Chemother. 2016;71(4):1106–1117.
- Saleem Z, Hassali MA, Versporten A, et al. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: findings and implications. Expert Rev Anti Infect Ther. 2019;17(4):285–293.
- Olaru ID, Meierkord A, Godman B, et al. Assessment of antimicrobial use and prescribing practices among pediatric inpatients in Zimbabwe. J Chemother. 2020;32(8):456–459.
- Campbell S, Meyer JC, Godman B. Why compliance to national prescribing guidelines is important especially across sub-Saharan Africa and suggestions for the future. J Biomed Pharm Sci. 2021;4: 316.
- Sefah IA, Essah DO, Kurdi A, et al. Assessment of adherence to pneumonia guidelines and its determinants in an ambulatory care clinic in Ghana: findings and implications for the future. JAC Antimicrob Resist. 2021;3(2):dlab080.
- Momanyi L, Opanga S, Nyamu D, et al. Antibiotic prescribing patterns at a leading referral hospital in Kenya: a point prevalence survey. J Res Pharm Pract. 2019;8(3):149–154.
- Okoth C, Opanga S, Okalebo F, et al. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract. 2018;46(3):128–136.
- Mustafa ZU, Salman M, Yasir M, et al. Antibiotic consumption among hospitalized neonates and children in Punjab province, Pakistan. Expert Rev Anti Infect Ther. 2022;20(6):931–939.
- Fink G, D’Acremont V, Leslie HH, et al. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: a cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect Dis. 2020;20(2):179–187.
- Nepal A, Hendrie D, Robinson S, et al. Analysis of patterns of antibiotic prescribing in public health facilities in Nepal. J Infect Developing Countries. 2020;14(1):18–27.
- Ahiabu MA, Tersbol BP, Biritwum R, et al. A retrospective audit of antibiotic prescriptions in primary health-care facilities in Eastern Region, Ghana. Health Policy Plan. 2016;31(2):250–258.
- Chem ED, Anong DN, Akoachere JKT. Prescribing patterns and associated factors of antibiotic prescription in primary health care facilities of Kumbo East and Kumbo West Health Districts, North West Cameroon. PloS one. 2018;13(3):e0193353.
- Kasabi GS, Subramanian T, Allam RR, et al. Prescription practices & use of essential medicines in the primary health care system, Shimoga district, Karnataka, India. Indian J Med Res. 2015;142(2):216–219.
- Raza UA, Khursheed T, Irfan M, et al. Prescription patterns of general practitioners in peshawar, Pakistan. Pak J Med Sci. 2014;30(3):462–465.
- Greer RC, Intralawan D, Mukaka M, et al. Retrospective review of the management of acute infections and the indications for antibiotic prescription in primary care in northern Thailand. BMJ open. 2018;8(7):e022250.
- Ab Rahman N, Teng CL, Sivasampu S. Antibiotic prescribing in public and private practice: a cross-sectional study in primary care clinics in Malaysia. BMC Infect Dis. 2016;16(1):208.
- Sulis G, Adam P, Nafade V, et al. Antibiotic prescription practices in primary care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2020;17(6):e1003139.
- Ofori-Asenso R, Agyeman AA. A review of injection and antibiotic use at primary health care (public and private) centers in Africa. J Pharm Bioallied Sci. 2015;7(3):175–180.
- Hassali MA, Kamil TK, Md Yusof FA, et al. General practitioners’ knowledge, attitude and prescribing of antibiotics for upper respiratory tract infections in Selangor, Malaysia: findings and implications. Expert Rev Anti Infect Ther. 2015;13(4):511–520.
- Soleymani F, Godman B, Yarimanesh P, et al. Prescribing patterns of physicians working in both the direct and indirect treatment sectors in Iran; findings and implications. Jphs. 2019;10:407–413.
- Tiroyakgosi C, Matome M, Summers E, et al. Ongoing initiatives to improve the use of antibiotics in Botswana: university of Botswana symposium meeting report. Expert Review of Anti-infective Therapy. 2018;16(5):381–384.
- Engler D, Meyer JC, Schellack N, et al. Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: are we there yet? J Chemother. 2021;33(1):21–31.
- Brink AJ, Messina AP, Feldman C, et al. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016;16(9):1017–1025.
- National Department of Health (NDoH). Antimicrobial resistance. National Strategy Framework 2014-2024. Pretoria, South Africa. [cited 10 June 2022]. Available at URL: http://www.health.gov.za/index.php/antimicrobial-resistance.
- Shamsuddin S, Akkawi ME, Zaidi ST, et al. Antimicrobial drug use in primary healthcare clinics: a retrospective evaluation. Int J Infect Dis. 2016;52:16–22.
- Godman B, Haque M, McKimm J et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: findings and implications for the future. Curr Med Res Opin. 2020;36(2):301–27.
- Pereko DD, Lubbe MS, Essack SY. Surveillance of antibiotic use in the private sector in Namibia using sales and claims data. J Infect Developing Countries. 2016;10(11):1243–1249.
- Kilipamwambu A, Bwire GM, Myemba DT, et al. WHO/INRUD core prescribing indicators and antibiotic utilization patterns among primary health care facilities in Ilala district, Tanzania. JAC-Antimicrob Resist. 2021;3(2). DOI:10.1093/jacamr/dlab049
- WHO. Global tuberculosis reports. 2020. Available at URL: https://www.who.int/teams/global-tuberculosis-programme/tb-reports.
- Kredo T, Cooper S, Abrams AL, et al. ‘Building on shaky ground’-challenges to and solutions for primary care guideline implementation in four provinces in South Africa: a qualitative study. BMJ open. 2020;10(5):e031468.
- Ofori-Asenso R, Brhlikova P, Pollock AM. Prescribing indicators at primary health care centers within the WHO African region: a systematic analysis (1995-2015). BMC Public Health. 2016;16:724.
- Niaz Q, Godman B, Massele A, et al. Validity of World Health Organisation prescribing indicators in Namibia’s primary healthcare: findings and implications. Int J Qual Health Care. 2019;31(5):338–345.
- Chigome AK, Matlala M, Godman B, et al. Availability and use of therapeutic interchange policies in managing antimicrobial shortages among south African public sector hospitals; findings and implications. Antibiotics. 2019;9(1). DOI:10.3390/antibiotics9010004
- Engler D, Meyer JC, Schellack N, et al. Antimicrobial stewardship activities in public healthcare facilities in South Africa: a baseline for future direction. Antibiotics. 2021;10(8):996.
- Godman B, Fadare J, Kwon HY, et al. Evidence-based public policy making for medicines across countries: findings and implications for the future. J Comp Eff Res. 2021;10(12):1019–1052.
- Godman B, Massele A, Fadare J, et al. Generic drugs – essential for the sustainability of healthcare systems with numerous strategies to enhance their use. Pharmaceutical Sciences And Biomedical Analysis Journal. 2021;4(1):126.
- Kaiser AH, Hehman L, Forsberg BC, et al. Availability, prices and affordability of essential medicines for treatment of diabetes and hypertension in private pharmacies in Zambia. PloS one. 2019;14(12):e0226169.
- South African Government. Medicines and Related Substances Control Amendment Act 1997. [cited 8 June 2022]. https://www.gov.za/sites/default/files/gcis_document/201409/a90-97.pdf.
- Fadare JO, Adeoti AO, Desalu OO, et al. The prescribing of generic medicines in Nigeria: knowledge, perceptions and attitudes of physicians. Expert Rev Pharmacoecon Outcomes Res. 2016;16(5):639–650.
- Brink AJ, Cotton M, Feldman C, et al. Updated recommendations for the management of upper respiratory tract infections in South Africa. S Afr Med J. 2015;105(5):344–352.
- Hoxha I, Malaj A, Kraja B, et al. Are pharmacists’ good knowledge and awareness on antibiotics taken for granted? The situation in Albania and future implications across countries. J Glob Antimicrob Resist. 2018;13:240–245.
- Kalungia AC, Mwambula H, Munkombwe D, et al. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: implications for future policy and practice. J Chemother. 2019;31(7–8):378–387.
