Abstract
Nanotechnology involves understanding and manipulating materials normally in the size range of 1 to 100 nm, where particles show completely novel physico-chemical properties from their bulk counterpart. In the last two decades a number of precisely engineered nanomaterials have been developed for diagnostic and therapeutic applications. For diagnostic applications, nanoparticles allow detection at the molecular scale. Some fluorescent nanohybrids can serve the dual purpose of detection and destruction of pathogenic microbes. The specificity and sensitivity of magnetic resonance imaging can be greatly improved by using nanoparticles as contrast enhancement material. As therapeutic agent, they allow targeted delivery and sustained release of drug molecules and they also have huge potential in tissue engineering. Given the vast range of application of nanomaterials in the biomedical domain, this review focuses on the major nanoparticle based diagnostic and therapeutic modalities.
Introduction
With the arrival of micro and nano technologies, fabrication of nanoscale structures and devices with unique phenomena of supramolecular assembly have become recurrent and this has increased their potential for novel intersection in a vast range of fields such as engineering, physics, chemistry, biology, computer science and more. In the last decade a plethora of research has been done on the applications of nanotechnology in biomedical research, involving visualization, manipulation and fabrication of materials on the scale of 100 nm down to 1 nm. Nanoscale materials are increasingly making a major impact on human health, and as they are used progressively more in diagnostic and therapeutic applications they have the potential to revolutionize medicine.
Generally, three groups of nanomaterials are of intense interest: zero-dimensional materials, so-called quantum dots, with variations in shape and diameter, one-dimensional materials, including nanorods and nanowires, and two-dimensional materials, including nanobelts, nanodisks, films, and nanosheets. Herein we will focus on nanoparticles (NPs) that are being used widely in different biomedical applications.
NPs have become popular in biomedical applications due to their ultra small size, extremely high surface area to volume ratio, tunable optical emission, enhanced mechanical properties, and superparamagnetic behavior; in addition they need to be biocompatible and synthesized by simple methods. Nanoscale devices smaller than 50 nm can easily enter most cells, while those smaller than 20 nm can move out of the blood vessels as they circulate through the body. Due to their small size, nanoscale devices can readily interact with biomolecules both extracellularly and intracellularly. By gaining access to most areas of the body, they have the potential to detect disease, monitor cells within the living system and deliver treatment in previously unachievable ways (Patra et al. Citation2008). Though applications and products dealing with drug delivery systems dominate the nanomedicine market, based on their unique features NPs can be used in areas such as imaging (Park et al.Citation2010), detecting biomolecules (Liu et al. Citation2004), and tissue engineering (Harrison & Atala Citation2007).
NPs with dual functionality can be used for diagnostic and therapeutic purposes (e.g. Fe2O3-Pt NPs) (Gao et al.Citation2008). NPs can be used as carriers for different drugs. They can be used to reduce toxicity of a therapeutic drug (Vlerken & Amiji Citation2006), or for pH-triggered drug release (Lim et al. Citation2011). NPs are currently being used for hyperthermia treatment (Maier-Hauff et al. Citation2011), and photodynamic therapy (Robertson et al. Citation2009). For example, AuroShell is a nanoproduct that kills cells at the target tumor site by emitting heat upon absorption of near-infrared (NIR) light (Leung et al. Citation2013).
This article will review the biomedical application of NPs in some prominent diagnostic and therapeutic fields.
Nanoparticle based biosensors
Many kinds of NPs, such as metal, oxide, semiconductor and carbon NPs (CNPs), have been used for constructing biosensors. These NPs play different roles in different sensing systems. The majority of the biosensing applications based on NPs that have been developed so far are based on gold NPs (GNPs), due to their unique optical properties and ease of use with different biomarkers in aqueous solution (Baptista et al. Citation2008; Sperling et al. Citation2008; Boisselier& Astruc Citation2009). An interesting optical property of GNPs is that their optical absorption and scattering peaks can be tuned by varying their size (at around 20 nm range) and shape; in particular these peaks can be put in the NIR optical window (800–1300 nm). Moreover, they are capable of transferring electrons efficiently between different electro-active species and electrode materials. In addition, the light-scattering properties and extremely large enhancement ability of the local electromagnetic field enables GNPs to be used as signal amplification tags in diverse biosensors (Lia et al. Citation2010). Three main types of GNP based biosensors are used: optical, electrochemical and piezo-electric biosensors. GNPs can act as optical biosensors as these NPs can augment the change of refractive index and enhance electron transfer. Based on these features DNA sensors with GNPs were developed and these were 1000 times more sensitive than those without GNPs (He et al.Citation2000). Electron transfer from redox proteins, particularly redox enzymes, to electrodes has been a subject of extensive research for making bioelectrocatalysts. It was seen that electron transfer rate was 5000 per second in the presence of GNPs (compared with the rate at which molecular oxygen, which is a cosubstrate for the enzyme, accepts electrons), while it was 700 per second without GNPs (Xiaoet al. Citation2003). Spadavecchia et al. (Citation2013) demonstrated that conjugation of gold nanostructures with thiolated DNA produces an additional plasmonic band, well separated from the localized plasmon resonance band of GNPs. By identifying the appearance of this additional plasmonic band upon hybridization, DNA as low as 200 pM can be detected. In electrochemical biosensors changes in electrical states are the basis for detection. Here GNPs, which have unique catalytic properties, are used as an immobilization platform. GNP based glucose biosensors have a detection limit of 0.18 μM (Andreescu & Luck Citation2008). A NADH sensor based on GNPs shows 780 mV potential decreases without any electron transfer mediators (Jena & Raj Citation2006). Piezo-electric biosensors are principally based on changes in mass. Improved sensitivity was obtained using GNP functionalities like biomolecular immobilization and amplification of mass change. An example of such a biosensor is a DNA sensor using GNPs as amplification tags with detection limit of (Liu et al. Citation2004).
Silver NPs (AgNPs) are of enormous current research attention in the field of novel biosensor development because of their catalytic properties (Sun & Xia Citation2002; Radet al. Citation2011). Yanxia et al. (Citation2008) developed a H2O2 biosensor based on the direct electrochemistry of hemoglobin (Hb) in Hb–Ag sol on a glassy carbon (GC) electrode. A novel H2O2 biosensor based on the direct electrochemistry and electrocatalysis of myoglobin (Mb) immobilized on AgNP doped carbon nanotube (CNT) film with hybrid sol–gel techniques has been reported by Liu and Hu (Citation2009).
Magnetic NPs (MNPs) are also leading to more sensitive, rapid and cost effective biosensors because most biological samples exhibit negligible magnetic susceptibility, and hence the background against which measurements are made is extremely low. MNP based biosensors are being used to detect nucleic acids, enzymes, other proteins, drugs, pathogens and tumor cells with excellent sensitivity (Haun et al. Citation2011). MNPs have been successfully applied in diagnostic magnetic resonance (DMR). The core principle behind DMR is the use of MNP as proximity sensors that modulate the transverse relaxation time of neighboring water molecules. This signal can be quantified using magnetic resonance imagers or nuclear magnetic resonance (NMR) relaxometers, including miniaturized NMR detector chips that are capable of performing highly sensitive measurements on microliter sample volumes and in a multiplexed format.
Claussen et al. (Citation2012) made a hybrid biosensor, where glucose oxidase is immobilized on a 3D matrix consisting of multilayered graphene petal nanosheets and Pt NPs. This biosensor has exceptional glucose sensitivity.
As CNT has high electrocatalytic activity, a huge number of studies directed at using CNT in electrochemical biosensing are going on. In addition to having small size, nanoscale sensors made from CNTs exhibit higher signal-to-noise ratios in comparison to microelectrodes. A glucose biosensor based on multiwalled CNTs and zinc oxide composite was developed by Palanisamy et al. (Citation2012) and this biosensor can be used for detecting glucose in urine sample with high reproducibility and repeatability.
Nanoparticles as imaging and contrast enhancer agents
Preliminary reports have demonstrated the potential of metal and MNPs as color contrast agents for several techniques such as confocal microscopy, confocal endoscopy, and atomic force microscopy. In tissue and cell studies the addition of GNPs to collagen has been shown to reduce auto fluorescence, an effect attributed to the catalytic effect of the NPs on NADH to NAD conversion (Ivan et al. Citation2007). This alteration of GNP mediated cellular auto fluorescence might be helpful in using GNPs as a novel class of contrast agents for fluorescence based detection of cancer tissues. Using the surface-enhanced Raman scattering (SERS) effect, the sensitivity of reporter dye molecules, directly attached to the surface of GNPs through Au-S or Au-N interactions, can be greatly enhanced and this hybrid may be used for more sensitive cancer cell imaging (Christiansen et al. Citation2007; Luet al. Citation2010; Jiang et al. Citation2011). Optical coherence tomography (OCT) uses the scattering function of gold nanoshells for in vivo imaging (Agrawal et al. Citation2006; Adler et al. Citation2008; Skrabalak et al. Citation2008).
NPs labeled with fluorescent dyes such as fluorescein isothiocyanate (FITC) and rhodamine B isothiocyanate (RITC) have wide application in bioimaging (Santra et al.Citation2006; Park et al. Citation2010), but they have the problem of photobleaching. Semiconductor quantum dots (QDs) such as ZnSe, ZnS and CdS, together with carbon dots, which have excellent photoluminescent properties, are also being used for imaging purposes (Hoshino et al. Citation2004; Jaiswal et al.Citation2004; Li & Zhu Citation2013). QD based fluorescence biosensors in particular have become very popular over the past two decades due to their high quantum yield, narrow and tunable emission spectrum and good photostability. Fluorescent NPs (both QDs and fluorescent dye tagged NPs) are being used extensively in both in vitro and in vivo imaging. They have become especially popular for cancer cell imaging (Sage Citation2004; Xing et al. Citation2006; Li et al. Citation2010).
Our research group has developed a ‘glow nanohybrid’ where a highly fluorescent CNP is chemically grafted on surface functionalized ZnO nanorods (Mitra &Chandraet al. Citation2012). This nanohybrid serves a dual purpose – it can kill bacteria as ZnO has well-known antibacterial property and simultaneously it can detect bacteria because of the presence of highly fluorescent CNPs. For making this conjugate, first ZnO nanorods were functionalized with amine groups followed by attachment of CNPs, activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The prepared CNP grafted ZnO nanorods preserved good dispersity along with uniformity in size distribution. This CNP grafted ZnO nanorod could successfully inhibit Staphylococcus aureus and Escherichia coli growth. The unique chemical grafting made the nanohybrid fluorescent, emitting bright green light under UV, and it can effectively detect bacteria under a fluorescent microscope.
In this context the role of MNPs as contrast enhancing agents is very significant. Magnetic iron oxide NPs have been extensively used as magnetic resonance imaging (MRI) contrast agents due to their ability to shorten T2 relaxation times in the liver, spleen, and bone marrow (Na et al. Citation2009). More recently, uniform ferrite NPs have been successfully employed as new T2 MRI contrast agents with improved relaxation properties. Iron oxide NPs functionalized with targeting agents have been used for targeted imaging via the site-specific accumulation of NPs at the targets of interest. Lai et al. (Citation2012) have developed FITC-incorporated silica-coated FePt NPs that exhibited not only significant T1 and T2 magnetic resonance (MR) contrast abilities but also a fluorescent property. When gadolinium, confined into nanoporous silicon particle, is used as MRI contrast agent, it gives improved T1 relaxivity and ultimately enhanced image contrast is obtained (Ananta et al. Citation2010). A target-specific MRI contrast agent for tumor cells expressing a high affinity folate receptor using folic acid and polyamidoamine (PAMAM) dendrimer was synthesized by Swanson et al. (Citation2008). Surface modified dendrimer was functionalized for targeting with folic acid (FA) and the primary amines of the dendrimer of remaining terminal were conjugated with the bifunctional DOTA-NCS (2-(4-isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic) chelator that forms stable complexes with gadolinium (Gd III). The targeted dendrimer contrast NPs can infiltrate tumors and are retained in tumor cells up to 48 hours post-injection. These dendrimer NPs have huge potential for targeted cancer imaging due to the prolonged clearance time, compared with the current clinically approved gadodiamide contrast agent. Jin et al. (Citation2010) have devised an iron oxide and gold coupled core-shell NP, which not only offers contrast for electron microscopy and MRI, but also enables a new imaging mode called magnetomotive photoacoustic imaging, with remarkable contrast enhancement.
MnO NPs have also been recently explored as a new T1 MRI contrast agent (Baek et al. Citation2010). But the contrast signal of MnO based NPs is weak for a long time in in vivo MRI tracking, and hence further improvement in this regard is needed (Kim et al. Citation2011).
Nanomaterials in drug delivery
Most research in the field of nanomedicine is on the application of nanomaterials in drug delivery systems (DDS). Application of NPs in drug delivery has the following goals: specific drug targeting and delivery, maintaining biocompatibility during application and therapeutic processes, and faster production (Jong & Born Citation2008). The release mechanism of drugs, the diffusion coefficient and the biodegradation rate are the main factors which govern the drug release rate. Here, drugs may be bound to the NPs either within the production process of NPs or by adsorption of drugs to NPs. Many of the pharmacological properties of conventional (‘free’) drugs can be improved through the use of DDS. DDS are designed to alter the pharmacokinetics (PK) and biodistribution (BD) of their associated drugs, or to function as drug reservoirs.
Packaging of chemotherapeutics within nanosized formulations like liposomes has been of considerable interest in the past few years (Crommelin & Storm Citation2003; Metselaar& Storm Citation2005; Minko et al. Citation2006) and liposomes are considered to be one of the most successful DDS (Kaneda Citation2000); a number of liposome-based systems have been approved by the US Food and Drug Administration (FDA) for disease treatment in the clinic. Although due to their small size liposomes can enter the arterioles and epithelial fenestration, drug solubility is an important concern for liposome based DDS. Hydrophilic drugs can be readily entrapped with a high degree of latency within the aqueous interior of liposomes, but neutral hydrophobic drugs or those with intermediate solubilities tend to be rapidly released in the presence of plasma proteins or cell membranes. Fortunately, excellent retention of drugs that are hydrophobic weak bases (such as doxorubicin and vincristine) has been achieved through ‘remote loading’ techniques that rely on pH or chemical gradients across the liposome bilayer to accumulate and retain the drug (Cullis et al. Citation1997). Doxil was the first liposomal drug formulation approved by the FDA for the treatment of AIDS associated with Kaposi's sarcoma (Northfelt et al. Citation1998). Other liposomal drugs used in clinical practice today include AmBisome (amphotericin B liposomes), DaunoXome (daunorubicin liposomes), DepoCyt (cytarabine liposomes), and Visudyne (verteporfin liposomes). Attachment of therapeutic molecules to inert polymers decreases drug clearance by the kidneys and by immune recognition. In general, when a drug is associated with a carrier, the drug clearance decreases (the half-life increases), the volume of distribution decreases, and the area under the time–concentration curve increases (Allen & Cullis Citation2004). Paclitaxel encapsulated in vitamin E TPGS-emulsified poly(D, L-Lactic-c-glycolic acid) (PLGA) NPs showed enhanced cytotoxicity for tumor cells in vitro, and it maintained a sustainable therapeutic potential in an animal model system (Win & Feng Citation2006). Poly-4-vinylpyridine N-oxide (PVNO) polymer coated micro quartz particles were shown to have efficient distribution without showing any macrophage related toxicity and genotoxicity (Schins et al. Citation2002; Albrecht et al. Citation2005). Polyethylene glycol (PEG) coated magnetic NPs of 40–50 nm size are reported to be well taken up by endocytosis (Gupta & Curtis Citation2004).
The blood–brain barrier (BBB) significantly hinders the passage of systemically delivered therapeutics and the brain extracellular matrix limits the distribution and longevity of locally delivered agents. So the central nervous system (CNS) poses a unique challenge for drug delivery. Polymeric NPs represent a promising solution to these problems. Encapsulation of paclitaxel in cityl alcohol/polysorbate NPs has been reported to enhance drug uptake in rat brain (Koziara et al. Citation2006). Poly(butylcyanoacrylate) (PBCA) NPs were the first polymer-based NP carrier used to deliver dalargin (a compound with opioid activity) to the CNS (Kreuter et al. Citation1995). Systemically-administered poly(lactic acid) (PLA) NPs, loaded with breviscapine (a flavonoid), have been shown to penetrate the BBB (Liu et al. Citation2008). Geldenhuys et al. (Citation2011) have demonstrated that surface modification of paclitaxel-loaded PLGA NPs with glutathione may also improve BBB bypass. Surface functionalization with PEG-PLA NPs with lectins like wheat gram protein, which specifically bind to the sialic acid present in large amounts in nasal cavities, have shown to increase brain uptake enormously (Gao et al. Citation2006). MnO NP is also found to translocate to the brain through olfactory route (Elder et al. Citation2006).
Conjugation of specific proteins like antibodies to NP surface has great potential for more specific and immunologically directed targeting (Nobs et al. Citation2004; Prinzen et al.Citation2007). Peptide coated QDs less than 10 nm in size are being targeted specifically to the lung and tumor vasculature (Akerman et al. Citation2002).
Mesoporous silica NP has also become a multifunctional delivery platform that has been shown at both in vitro and in vivo levels to be capable of delivering chemotherapeutic agents to a variety of cancer cell types (Lee et al. Citation2009; Meng et al. Citation2010; He et al. Citation2011). The major advantage of using a mesoporous silica based carrier is that its internal ordered pore structures may be tailored to host differently sized drug molecules and its release profile can be predetermined. Mesoporous silica NP based drug carriers have been shown to be able to incorporate a high dosage of drugs in the internal pore system owing to their large internal surface areas and pore volumes as well as their tunable pore size. This carrier allows effective and protective packaging of hydrophobic and charged anticancer drugs for controlled and on demand delivery.
Silica coated iron oxide NPs, covalently functionalized with light-responsive azobenzene derivatives, have been recently reported as novel target delivery systems (Turcheniuk et al. Citation2013). This composite has unique magnetic targeting and stimuli-responsive release properties. The cargo molecules inside the pores cannot diffuse out because of the blocking by high density azobenzene chains. But visible light irradiation causes trans/cis isomerization of azobenzene chains and it helps in release of guest molecules out of the pores. Phosphonic acid has strong affinity for iron oxide NP through the formation of bidendate Fe-O-P bonds. This NP can further be functionalized with rhodamine B isothiocyanate, propargyl folate and paclitaxel (Das et al. Citation2011). These trifunctional NPs could selectively target and induce apoptosis to folate-receptor overexpressing cancer cells with higher efficacy in comparison with the free drug. Iron oxide NPs can also be used for delivery of chemotherapeutics such as gemcitabine (Lee et al. Citation2013).
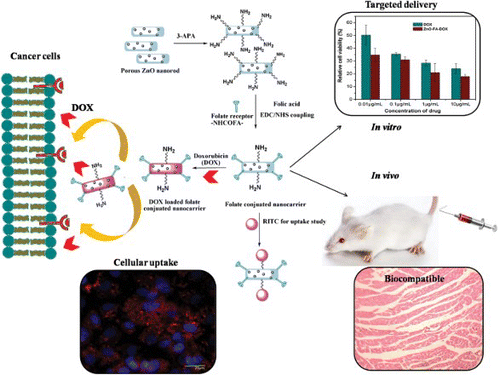
Our research group has developed surface functionalized porous ZnO nanorods for cancer cell specific targeted drug delivery (TDD) (Mitra & Bano et al. Citation2012). Here porous ZnO nanorods were prepared by a simple route and had very high surface area of with uniformly distributed pores of 15 nm. These were then functionalized with 3-aminophosphonic acid followed by folic acid to yield folate conjugated porous ZnO nanorods (ZnO-FA). Its extremely high surface area and uniformly distributed pores on its surface made the nanocarrier suitable for high drug loading (88%) of the anticancer drug doxorubicin (DOX). A pH triggered drug release was observed with minimum release in pathophysical conditions. In vitro efficacy of DOX loaded ZnO-FA (ZnO-FA-DOX) was evaluated against breast cancer cells MDA-MB-231 (). This nano-hybrid was much more effective than DOX alone or ZnO-FA. Moreover, the targeted scaffold was functionalized with a pendant –NH2 group and was covalently bonded with the fluorescent dye RITC for cellular uptake and imaging studies in MDA-MB-231 cells (). In vivo acute and intravenous toxicological evaluation in murine model system complemented the biocompatibility of ZnO-FA-DOX in TDD. So this porous ZnO based nanocarrier has great potential in TDD.
Figure 1. Surface functionalized mesoporous ZnO nanorod mediated cancer cell specific targeted delivery. Porous ZnO nanorods were functionalized with 3-aminophosphonic acid followed by folic acid. The nanocarrier was then loaded with anticancer drug doxorubicin. Efficacy of doxorubicin loaded Zno nanorods was shown in breast cancer cells MDAMB-231. Its biocompatibility was evaluated in a murine model system.
Nanoparticles in tissue engineering, hyperthermia and photodynamic therapy
Engineered NPs which are capable of translocation through the bloodstream and can reach specific targets are now the subject of large numbers of studies in the field of biomedicine, because they are useful in diagnostics and drug delivery to specific targets; also such NPs are now also being developed for treating diseases. This makes them attractive even at the clinical level.
Nanotechnology in tissue engineering
Bone is a natural composite of hierarchically arranged collagen fibrils, hydroxyapatite (HAp) and proteoglycans. Hence, various tissue engineering strategies have adopted composite scaffold approaches to closely mimic the bone in structure and composition. NPs including calcium triphosphate and HAp have been fabricated into porous 3D scaffolds for bone repair or regeneration purposes (Khan et al.Citation2006). This method allows bone composition to be mimicked; also the incorporation of nanoceramics enhances the mechanical strength of bones. Researchers are now evaluating the use of polymer or ceramic matrices containing single multi-walled CNTs to improve the mechanical properties of the artificial bony structure (Harrison & Atala Citation2007). CNTs offer excellent properties, such as high tensile strength, high flexibility, and low density that can be exploited to develop more successful orthopedic implant materials.
Yamashita et al. (Citation2009) investigated the effects of rough and smooth surfaces of ceria and yttria stabilized zirconium dioxide, which is promising as a dental implant material. Tan et al. (Citation2011) created a heparin/chitosan NP immobilized decellularized bovine jugular vein scaffold to increase the loading capacity and allow for controlled release of vascular endothelial growth factor (VEGF). This was successfully used to reconstruct dog pulmonary and right ventricle with potential regeneration capability.
Hyperthermia
NP mediated hyperthermia treatment (killing of tumor cells by generating slightly increased temperature) is principally based on delivery of MNP through a magnetically governed processes to the target area (Corchero & Villaverde Citation2009). These NPs can be activated either by using radiofrequency (RF) pulses or infrared light to release heat and kill the target tissue. Super paramagnetic iron oxide NP is currently being used in hyperthermia applications in vivo (Maier-Hauff et al.Citation2011). The surface plasmon resonance of metal NPs is another property which is used for irreversible thermal ablation of tumors (Chen et al. Citation2010). Optically activated gold nanoshells are reported to reduce tumor size in both mouse and human xenograft models of triple-negative breast cancer, without a concomitant increase in the percentage of cancer stem cells (Atkinson et al. Citation2010). Although hyperthermia treatment using NPs has certain disadvantages as most NPs have a high specific absorption rate, research is currently going on in this particular field to increase efficiency.
Photodynamic therapy
Photodynamic therapy is used to destroy tumor cells and is based on concentration of singlet oxygen (1O2) as part of ROS production in localized area (Takahashi et al. Citation2002; Oberdanner et al. Citation2005). Here photosensitizers are activated with appropriate excitation wavelength to produce ROS, thereby killing target cells. Some NPs, which can produce ROS upon excitation (Lee & Kopelman Citation2011), are now being used in photodynamic therapy. Conventionally UV light is used to excite photosensitizers. As such types of excitement cannot penetrate deep inside the tissue, a new generation of photodynamic therapy has been developed using NIR wavelengths as excitation energy. The penetrating capability of NIR is much deeper than conventional UV excitation. Another advancement introduced in this area is the introduction of a newer class of NPs, namely upconverting NPs (UCNPs), which on efficient encapsulation of photosensitizer molecules can perform better. For example, Guo et al. (Citation2010) developed mesoporous silica coated onto sodium yttrium fluoride upconversion nanocrystals to form a core-shell structure and then loaded with the photosensitizer zinc (II)-phthalocyanine (ZnPc) into the porous silica. On irradiation with 980 nm, NPs emitted visible light that activated ZnPc molecules in the silica shell through resonance energy transfer showing a strong photodynamic cell destruction effect on MB49 cells. Another novel class of multifunctional NPs (MFNPs) based on UCNPs with combined optical and magnetic properties was shown to be useful in multimodality imaging and therapy (Cheng et al.Citation2011, Citation2012).
Outlook
The unique and superior properties of NPs have shown strong potential for rapid development of nanotechnology based biomedical applications. A large number of nanoscience researchers are trying to exploit the exciting, novel properties of nanomaterials to use them in the field of biomedical sciences worldwide. There are already some NP based products are available in the market and many are in the pipeline for commercialization. This review article focused on some of the major biomedical applications (diagnostics, drug delivery and photodynamic and hyperthermia mediated therapy) of nanomaterials.
NPs have already revolutionized the field of biosensing. As far as the field of therapeutics is concerned, the currently approved NP systems have obviously improved the therapeutic index of drugs. Sometimes some surface functionalized NPs, e.g. glycan functionalized diamond NPs, can themselves be used as an antibacterial agent (Barraset al. Citation2013). But next generation NPs will have targeting ligands such as antibodies, peptides, or aptamers tagged to them and they will not only improve the efficacy of the drug but also reduce their side effects. Many multifunctional NPs have been developed which are capable of targeting, imaging and therapy. It is expected that in the future many more optimally designed NPs will enter the clinic. But until now there has not been sufficient and comprehensive data on the long-term biotoxicity profile of most NPs. The bio-distribution and release kinetics of the NPs from biological systems should also be investigated in detail. Collaborative, multidisciplinary research endeavors are still needed for more successful application of nanomaterials in biomedical science.
References
- Adler DC, Huang SW, Huber R, Fujimoto JG. 2008. Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography. Opt Express. 16:4376–4393. doi: 10.1364/OE.16.004376
- Agrawal A, Huang S, Wei Haw Lin A, Lee MH, Barton JK, Drezek RA, Pfefer TJ. 2006. Quantitative evaluation of optical coherence tomography signal enhancement with gold nanoshells. J Biomed Opt. 11:041121. doi: 10.1117/1.2339071
- Akerman ME, Chan WC, Laakkonen P, Bhatia SN, Ruoslahti E. 2002. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 99:12617–12621. doi: 10.1073/pnas.152463399
- Albrecht C, Knaapen AM, Becker A, Höhr D, Haberzett P, van Schooten FJ, Borm PJA, Schins RPF. 2005. The crucial role of particle surface reactivity in respirable quartz-induced reactive oxygen/nitrogen species formation and APE/Ref-1 induction in rat lung. Respir Res. 6:129. doi: 10.1186/1465-9921-6-129
- Allen TM, Cullis PR. 2004. Drug delivery systems: entering the mainstream. Science. 303:1818–1822. doi: 10.1126/science.1095833
- Ananta JS, Godin B, Sethi R, Moriggi L, Liu X, Serda RE, Krishnamurthy R, Muthupillai R, Bolskar RD, Helm L, et al. 2010. Geometrical confinement of gadolinium-based contrast agents in nanoporous particles enhances T1 contrast. Nat Nanotechnol. 5:815–821. doi: 10.1038/nnano.2010.203
- Andreescu S, Luck LA. 2008. Studies of the binding and signaling of surface-immobilized periplasmic glucose receptors on gold nanoparticles: a glucose biosensor application. Anal Biochem. 375:282–290. doi: 10.1016/j.ab.2007.12.035
- Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, Woodward WA, Krishnan S, Chang JC, Rosen JM. 2010. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med. 2:55–79. doi: 10.1126/scitranslmed.3001447
- Baek MJ, Park JY, Xu W, Kattel K, Kim HG, Lee EJ, Patel AK, Lee JJ, Chang Y, Kim TJ, Bae JE, Chae KS, Lee GH. 2010. Water-soluble MnO nanocolloid for a molecular T1 MR imaging: a facile one-pot synthesis, in vivo T1 MR images, and account for relaxivities. ACS Appl Mater Interfaces. 2:2949–2955. doi: 10.1021/am100641z
- Baptista P, Pereira E, Eaton P, Doria G, Miranda A, Gomes I, Quaresma P, Franco R. 2008. Gold nanoparticles for the development of clinical diagnosis methods. Anal Bioanal Chem. 391:943–950. doi: 10.1007/s00216-007-1768-z
- Barras A, Martin FA, Bande O, Baumann JS, Ghigo JM, Boukherroub R, Beloin C, Siriwardena A, Szunerits S. 2013. Glycan-functionalized diamond nanoparticles as potent E. coli anti-adhesive. Nanoscale. 5:2307–2316. doi: 10.1039/c3nr33826f
- Boisselier E, Astruc D. 2009. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 38:1759–1782. doi: 10.1039/b806051g
- Chen YS, Frey W, Kim S, Homan K, Kruizinga P, Sokolov K, Emelianov S. 2010. Enhanced thermal stability of silica-coated gold nanorods for photoacoustic imaging and image-guided therapy. Opt Express. 18:8867–8878. doi: 10.1364/OE.18.008867
- Cheng L, Yang K, Li Y, Chen J, Wang C, Shao M, Lee ST, Liu Z. 2011. Facile preparation of multifunctional upconversion nanoprobes for multimodal imaging and dual-targeted photothermal therapy. Angew Chem Int Ed Engl. 50:7385–790.
- Cheng L, Yang K, Li Y, Zeng X, Shao M, Lee ST, Liu Z. 2012. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials. 33:2215–2222. doi: 10.1016/j.biomaterials.2011.11.069
- Christiansen SH, Becker M, Fahlbusch S, Michler J, Sivakov V, Andrä G, Geiger R. 2007. Signal enhancement in nano-Raman spectroscopy by gold caps on silicon nanowires obtained by vapour-liquid-solid growth. Nanotechnology. 18:035503. doi: 10.1088/0957-4484/18/3/035503
- Claussen JC, Kumar A, Jaroch DB, Khawaja MH, Hibbard AB, Porterfield DM, Fisher TS. 2012. Nanostructuring platinum nanoparticles on multilayered graphene petal nanosheets for electrochemical biosensing. Adv Funct Mater. 22: 3399–3405. doi: 10.1002/adfm.201200551
- Corchero JL, Villaverde A. 2009. Biomedical applications of distally controlled magnetic nanoparticles. Trends Biotechnol. 27:468–476. doi: 10.1016/j.tibtech.2009.04.003
- Crommelin DJ, Storm G. 2003. Liposomes: from the bench to the bed. J Liposome Res. 13:33–36. doi: 10.1081/LPR-120017488
- Cullis PR, Hope MJ, Bally MB, Madden TD, Mayer LD, Fenske DB. 1997. Influence of pH gradients on the transbilayer transport of drugs, lipids, peptides and metal ions into large unilamellar vesicles. Biochim Biophy. Acta (Review on Biomembranes). 1331:187–211.
- Das M, Bandyopadhyay D, Mishra D, Datir S, Dhak P, Jain S, Maiti TK, Basak A, Pramanik P. 2011. “Clickable”, trifunctional magnetite nanoparticles and their chemoselective biofunctionalization. Bioconjugate Chem. 22:1181–1193. doi: 10.1021/bc2000484
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdörster G. 2006. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 114:1172–1178. doi: 10.1289/ehp.9030
- Gao J, Liang G, Cheung JS, Pan Y, Kuang Y, Zhao F, Zhang B, Zhang X, Wu EX, Xu B. 2008. Multifunctional yolk–shell nanoparticles: a potential mri contrast and anticancer agent. J Am Chem Soc. 130:11828–11833. doi: 10.1021/ja803920b
- Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, Fu S. 2006. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 27:3482–3490. doi: 10.1016/j.biomaterials.2006.01.038
- Geldenhuys W, Mbimba T, Bui T, Harrison K, Sutariya V. 2011. Brain-targeted delivery of paclitaxel using glutathione-coated nanoparticles for brain cancers. J Drug Target. 19:837–845. doi: 10.3109/1061186X.2011.589435
- Guo HC, Qian HS, Idris NM, Zhang Y. 2010. Singlet oxygen-induced apoptosis of cancer cells using upconversion fluorescent nanoparticles as a carrier of photosensitizer. Nanomed-Nanotechnol. 6:486–495. doi: 10.1016/j.nano.2009.11.004
- Gupta AK, Curtis ASG. 2004. Surface modified supermagnetic nanoparticles for drug delivery: interaction studies with human firbroblasts in culture. J Mater Sci: Mat in Med. 15:493–496.
- Harrison BS, Atala A. 2007. Carbon nanotube applications for tissue engineering. Biomaterials. 28:344–353. doi: 10.1016/j.biomaterials.2006.07.044
- Haun JB, Yoon TJ, Lee H, Weissleder R. 2011. Molecular detection of biomarkers and cells using magnetic nanoparticles and diagnostic magnetic resonance. Methods Mol Biol. 726:33–49. doi: 10.1007/978-1-61779-052-2_3
- He L, Musick MD, Nicewarner SR, Salinas FG, Benkovic SJ, Natan MJ, Keating CD. 2000. Colloidal Au-enhanced surface plasmon resonance for ultrasensitive detection of DNA hybridization. J Am Chem Soc. 122:9071–9077. doi: 10.1021/ja001215b
- He Q, Zhang Z, Gao F, Li Y, Shi J. 2011. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: effects of particle size and PEGylation. Small. 7: 271–280. doi: 10.1002/smll.201001459
- Hoshino A, Hanaki K, Suzuki K, Yamamoto K. 2004. Applications of T-lymphoma labeled with fluorescent quantum dots to cell tracing markers in mouse body. Biochem Biophys Res Commun. 314:46–53. doi: 10.1016/j.bbrc.2003.11.185
- Ivan ES, Huang X, Fima M, Joseph OH, Randall K, Mostafa ES. 2007. Effect of plasmonic gold nanoparticles on benign and malignant cellular autofluorescence: a novel probe for fluorescence based detection of cancer technology in cancer research & treatment. Tech Canc Res Treat. 6:403–412.
- Jaiswal JK, Goldman ER, Mattoussi H, Simon SM. 2004. Use of quantum dots for live cell imaging. Nat Methods. 1:73–78. doi: 10.1038/nmeth1004-73
- Jena BK, Raj CR. 2006. Electrochemical biosensor based on integrated assembly of dehydrogenase enzymes and gold nanoparticles. Anal Chem. 15:6332–6339. doi: 10.1021/ac052143f
- Jiang L, Qian J, Cai F, He S. 2011. Raman reporter-coated gold nanorods and their applications in multimodal optical imaging of cancer cells. Anal Bioanal Chem. 400:2793–2800. doi: 10.1007/s00216-011-4894-6
- Jin Y, Jia C, Huang SW, O'Donnell M, Gao X. 2010. Multifunctional nanoparticles as coupled contrast agents. Nat Commun. 1:41. doi: 10.1038/ncomms1042
- Jong WHD, Borm PJA. 2008. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 3:133–149. doi: 10.2147/IJN.S596
- Kaneda Y. 2000. Virosomes: evolution of the liposome as a targeted drug delivery system. Adv Drug Delivery Rev. 43:197–205. doi: 10.1016/S0169-409X(00)00069-7
- Khan Y, El-Amin SF, Laurencin CT. 2006. In vitro and in vivo evaluation of a novel polymer-ceramic composite scaffold for bone tissue engineering. Conference Proceedings of the IEEE Engineering in Medicine and Biology Society. New York, USA. 1:529–530.
- Kim T, Momin E, Choi J, Yuan K, Zaidi H, Kim J, Park M, Lee N, McMahon MT, Quinones-Hinojosa A, et al. 2011. Mesoporous silica-coated hollow manganese oxide nanoparticles as positive T(1) contrast agents for labeling and MRI tracking of adipose-derived mesenchymal stem cells. J Am Chem Soc. 133:2955–2961. doi: 10.1021/ja1084095
- Koziara JM, Lockman PR, Allen DD, Mumper RJ. 2006. The blood-brain barrier and brain drug delivery. J Nanosci Nanotechnol. 6:2712–2735. doi: 10.1166/jnn.2006.441
- Kreuter J, Alyautdin RN, Kharkevich DA, Ivanov AA. 1995. Passage of peptides through the bloodbrain barrier with colloidal polymer particles (nanoparticles). Brain Res. 674:171–174. doi: 10.1016/0006-8993(95)00023-J
- Lai SM, Tsai TY, Hsu CY, Tsai JL, Liao MY, Lai PS. 2012. Bifunctional silica-coated superparamagnetic fept nanoparticles for fluorescence/MR dual imaging. J Nano Mat. 2012:1–7. doi: 10.1155/2012/631584
- Lee CH, Cheng SH, Wang YJ, Chen YC, Chen NT, Souris J, Chen CT, Mou CY, Yang CS, Lo LW. 2009. Near-Infrared mesoporous silica nanoparticles for optical imaging: characterization and in vivo biodistribution. Adv Funct Mater. 19:215–222. doi: 10.1002/adfm.200800753
- Lee GY, Qian WP, Wang L, Wang YA, Staley CA, Satpathy M, Nie S, Mao H, Yang L. 2013. Theranostic nanoparticles with controlled release of gemcitabine for targeted therapy and MRI of pancreatic cancer. ACS Nano. 26:2078–89. doi: 10.1021/nn3043463
- Lee YE, Kopelman R. 2011. Polymeric nanoparticles for photodynamic therapy. Methods Mol Biol. 726:151–178. doi: 10.1007/978-1-61779-052-2_11
- Leung JP, Wu S, Chou KC, Signorell R. 2013. Investigation of sub-100 nm gold nanoparticles for laser-induced thermotherapy of cancer. Nanomaterials. 3:86–106. doi: 10.3390/nano3010086
- Li J, Zhu JJ. 2013. Quantum dots for fluorescent biosensing and bio-imaging applications. Analyst. 138:2506–2515. doi: 10.1039/c3an36705c
- Li Z, Wang Y, Wang J, Tang Z, Pounds JG, Lin Y. 2010. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem. 82:7008–7014. doi: 10.1021/ac101405a
- Lia Y, Schluesenerb HJ, Xua S. 2010. Gold nanoparticle-based biosensors. Gold Bull. 43:29–41. doi: 10.1007/BF03214964
- Lim EK, Huh YM, Yang J, Lee K, Suh JS, Haam S. 2011. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv Mater. 23:2436–2442. doi: 10.1002/adma.201100351
- Liu CY, Hu JM. 2009. Hydrogen peroxide biosensor based on the direct electrochemistry of myoglobin immobilized on silver nanoparticles doped carbon nanotubes film. Biosens Bioelectron. 24:2149–2154. doi: 10.1016/j.bios.2008.11.007
- Liu M, Li H, Luo G, Liu Q, Wang Y. 2008. Pharmacokinetics and biodistribution of surface modification polymeric nanoparticles. Arch Pharm Res. 31:547–554. doi: 10.1007/s12272-001-1191-8
- Liu T, Tang J, Jiang L. 2004. The enhancement effect of gold nanoparticles as a surface modifier on DNA sensor sensitivity. Biochem Biophys Res Commun. 313:3–7. doi: 10.1016/j.bbrc.2003.11.098
- Lu W, Singh AK, Khan SA, Senapati D, Yu H, Ray PC. 2010. Gold nano-popcorn-based targeted diagnosis, nanotherapy treatment, and in situ monitoring of photothermal therapy response of prostate cancer cells using surface. J Am Chem Soc. 132:18103–18114. doi: 10.1021/ja104924b
- Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, Thiesen H, Budach V, Jordan A. 2011. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 103:317–324. doi: 10.1007/s11060-010-0389-0
- Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. 2010. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 4:4539–4550. doi: 10.1021/nn100690m
- Metselaar JM, Storm G. 2005. Liposomes in the treatment of inflammatory disorders. Expert Opin Drug Deliv. 2:465–476. doi: 10.1517/17425247.2.3.465
- Minko T, Pakunlu RI, Wang Y, Khandare JJ, Saad M. 2006. New generation of liposomal drugs for cancer. Anticancer Agents Med Chem. 6:537–552. doi: 10.2174/187152006778699095
- Mitra S, Bano S, Patra P, Chandra S, Debnath N, Das S, Banerjee R, Kundu SC, Pramanik P, Goswami A. 2012. Porous ZnO nanorod for targeted delivery of doxorubicin: in vitro and in vivo response for therapeutic applications. J Mat Chem. 22:24145–24154. doi: 10.1039/c2jm35013k
- Mitra S, Chandra S, Laha D, Patra P, Debnath N, Pramanik A, Pramanik P, Goswami A. 2012. Unique chemical grafting of carbon nanoparticle on fabricated ZnO nanorod: antibacterial and bioimaging property. Mater Res Bull. 47: 586–594. doi: 10.1016/j.materresbull.2011.12.036
- Na BHB, Song IC, Hyeon T. 2009. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 21:2133–2148. doi: 10.1002/adma.200802366
- Nobs L, Buchegger F, Gurny R, Allémann E. 2004. Poly(lactic acid) nanoparticles labeled with biologically active Neutravidin for active targeting. Eur J Pharm Biopharm. 58: 483–490. doi: 10.1016/j.ejpb.2004.04.006
- Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan C, Du Mond LD, Mamelok RD, Henry DH. 1998. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi's sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 16:2445–2451.
- Oberdanner CB, Plaetzer K, Kiesslich T, Krammer B. 2005. Photodynamic treatment with fractionated light decreases production of reactive oxygen species and cytotoxicity in vitro via regeneration of glutathione. Photochem Photobiol. 81:609–613. doi: 10.1562/2004-08-23-RN-284.1
- Palanisamy S, Cheemalapati S, Chen SM. 2012. Enzymatic glucose biosensor based on multiwalled carbon nanotubes-zinc oxide composite. Int J Electrochem Sci. 7:8394–8407.
- Park KS, Tae J, Choi B, Kim YS, Moon C, Kim SH, Lee HS, Kim J, Kim J, Park J, et al. 2010. Characterization, in vitro cytotoxicity assessment, and in vivo visualization of multimodal, RITC-labeled, silica-coated magnetic nanoparticles for labeling human cord blood–derived mesenchymal stem cells. Nanomed: Nanotechnol Biol Med. 6:263–276.
- Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P. 2008. Application of gold nanoparticles for targeted therapy in cancer. J Biomed Nanotechnol. 4:1–34.
- Prinzen L, Miserus RJHM, Dirksen A, Hackeng TM, Deckers N, Bitsch NJ, Remco TA, Douma K, Heemskerk JW, Eline Kooi M, et al. 2007. Optical and magnetic resonance imaging of cell death and platelet activation using Annexin A5-functionalized quantum dots. Nano Lett. 7:93–100. doi: 10.1021/nl062226r
- Rad AS, Mirabi A, Binaian E, Tayebi H. 2011. Review on glucose and hydrogen peroxide biosensor based on modified electrode included silver nanoparticles. Int J Electrochem Sci. 6:3671–3683.
- Robertson CA, Evans DH, Abrahamse H. 2009. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J Photochem Photobiol, B. 96:1–8. doi: 10.1016/j.jphotobiol.2009.04.001
- Sage L. 2004. Finding cancer cells with quantum dots. Anal Chem. 76:453A. doi: 10.1021/ac0347718
- Santra S, Liesenfeld B, Bertolino C, Dutta D, Cao Z, Tan W, Moudgil BM, Mericle RA. 2006. Fluorescence lifetime measurements to determine the core-shell nanostructure of FITC-doped silica nanoparticles: an optical approach to evaluate nanoparticle photostability. J Lumin. 117: 75–82. doi: 10.1016/j.jlumin.2005.04.008
- Schins RP, Duffin R, Höhr D, Knaapen AM, Shi T, Weishaupt C, Stone V, Donaldson K, Borm PJ. 2002. Surface modification of quartz inhibits toxicity, particle uptake, and oxidative DNA damage in human lung epithelial cells. Chem Res Toxicol. 9:1166–1173. doi: 10.1021/tx025558u
- Skrabalak SE, Chen J, Sun Y, Lu X, Au L, Cobley CM, Xia Y. 2008. Gold nanocages: synthesis, properties, and applications. Acc Chem Res. 41:1587–1595. doi: 10.1021/ar800018v
- Spadavecchia J, Barras A, Lyskawa J, Woisel P, Laure W, Pradier CM, Boukherroub R, Szunerits S. 2013. Approach for plasmonic based DNA sensing: amplification of the wavelength shift and simultaneous detection of the plasmon modes of gold nanostructures. Anal. Chem. 85: 3288–3296. doi: 10.1021/ac3036316
- Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. 2008. Biological applications of gold nanoparticles. Chem Soc Rev. 37:1896–1908. doi: 10.1039/b712170a
- Sun YG, Xia YN. 2002. Shape-controlled synthesis of gold and silver nanoparticles. Science. 298:2176–2179. doi: 10.1126/science.1077229
- Swanson SD, Kukowska - Latallo JF, Patri AK, Chen C, Ge S, Cao Z, Kotlyar A, East AT, Baker JR. 2008. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int J Nanomedicine. 3: 201–210.
- Takahashi M, Nagao T, Imazeki Y, Matsuzaki K, Minamitani H. 2002. Roles of reactive oxygen species in monocyte activation induced by photochemical reactions during photodynamic therapy. Front Med Biol Eng. 11: 279–294. doi: 10.1163/156855701321138932
- Tan Q, Tang H, Hu J, Hu Y, Zhou X, Tao Y, Wu Z. 2011. Controlled release of chitosan/heparin nanoparticledelivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int J Nanomedicine. 6:929–942. doi: 10.2147/IJN.S18753
- Turcheniuk K, Tarasevych AV, Kukhar VP, Boukherroub R, Szunerits S. 2013. Recent advances in surface chemistry strategies for the fabrication of functional iron oxide based magnetic nanoparticles. Nanoscale. 5:10729–10752. doi: 10.1039/c3nr04131j
- Vlerken LE, Amiji MM. 2006. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Deliv. 3:205–216. doi: 10.1517/17425247.3.2.205
- Win KY, Feng SS. 2006. In vitro and in vivo studies on vitamin E TPGS-emulsified poly(D,L-lactic-co-glycolic acid) nanoparticles for paclitaxel formulation. Biomaterials. 27: 2285–2291. doi: 10.1016/j.biomaterials.2005.11.008
- Xiao Y, Patolsky F, Katz E, Hainfeld JF, Willner I. 2003. “Plugging into Enzymes”: nanowiring of redox enzymes by a gold nanoparticle. Science. 299:1877–1881. doi: 10.1126/science.1080664
- Xing Y, Smith AM, Agrawal A, Ruan G, Nie S. 2006. Molecular profiling of single cancer cells and clinical tissue specimens with semiconductor quantum dots. Int J Nanomedicine. 1:473–481. doi: 10.2147/nano.2006.1.4.473
- Yamashita D, Machigashira M, Miyamoto M, Takeuchi H, Noguchi K, Izumi Y, Ban S. 2009. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent Mater J. 28:461–470. doi: 10.4012/dmj.28.461
- Yanxia X, Chengguo H, Shengshui H. 2008. Hydrogen peroxide biosensor based on direct electrochemistry of hemoglobin in Hb–Ag sol films. Sensor Actuat B-Chem. 130:816–822. doi: 10.1016/j.snb.2007.10.048