?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Many diseases are associated with oxidative stress caused by reactive oxygen species and free radicals generated in living cells. This study deals with finding naturally occurring antioxidants of plant origin. The study investigated the phytochemistry, antioxidant and free radical scavenging potential of ethanolic leaf extract of Adhatoda vasica (ELEAV) using different antioxidant models. The phytochemical analysis of ELEAV revealed the presence of alkaloids, flavonoids, terpenoids, saponins, phenols and steroids. ELEAV also showed an antioxidant capacity, with inhibition of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical by 69.23%. ELEAV had potent inhibitory effects on scavenging nitric oxide, superoxide and hydroxyl radicals, by 65.24%, 61.54% and 61.24%, respectively. Moreover, ELEAV displayed a concentration-dependent reducing power activity and potent inhibitory effects on ferric ion-induced lipid peroxidation (68.26%) in bovine brain extract. These findings confirm the biological efficacy of A. vasica as a potential source of natural antioxidants.
Introduction
Cellular metabolism results in the production of biologically significant by-products, called reactive oxygen species (ROS), including nitric oxide, superoxide, hydroxyl, hydrogen peroxide and singlet oxygen radicals (Kirkinezos & Moraes Citation2001). These ROS, produced by a number of exogenous sources, are potentially damaging transient chemical species and are considered to be important factors in causing decreased cell fluidity and permeability, cell dysfunction and mutations, and in the onset of diseases such as ageing, arteriosclerosis, cancer, cardiovascular diseases, diabetes and strokes (Alma et al. Citation2003). ROS are produced in the intracellular system, including cytoplasmic molecules, cytoplasmic proteins, membrane enzymes, peroxisomes, and mitochondrial and microsomic electron transport systems (Martinez Citation1995; Gulcin Citation2006). The administration of antioxidants can significantly control the severity of chronic diseases, providing a close relationship between free radical scavenging activity and the involvement of endocrinological responses (Wiseman & Halliwell Citation1996).
The importance of dietary antioxidant components in the prevention of chronic disease and improvement of health quality has attracted huge research attention in the past few decades (Alvarado et al. Citation2006; Skotti et al. Citation2014). These compounds are able to remove oxygen free radicals formed in cells and thus protect the human body from diseases and also retard rancidity from the lipid peroxidation of foods (Ishino et al. Citation2010). Nowadays, a variety of synthetic antioxidants is commonly used in the food industry for food safety purposes. However, the use of these compounds has been restricted by the regulatory authorities owing to their long-term toxicity and carcinogenicity (Gulcin et al. Citation2010). Hence, interest has greatly increased in finding naturally occurring antioxidants to replace synthetic antioxidants for use in food and for medical purposes.
Medicinal plants are important sources of natural products which differ widely in terms of their structures, biological properties and mechanisms of action. Various phytochemical components, especially polyphenols such as tannins, flavonoids, alkaloids, phenyl propanoids and phenolic acids, are known to have antioxidant and free radical scavenging efficacy. Polyphenols have various biological effects, which are mainly attributed to their antioxidant activities in scavenging free radicals, inhibition of lipid peroxidation and metal chelation. Polyphenols share the same general chemical pattern, with one or more phenolic groups which react as hydrogen donors and thus neutralize free radicals (Amari et al. Citation2014). Polyphenols are natural biologically active components found in every part of the plant, including the leaves, flowers, shoot, stem and root, which work as a defence mechanism against diseases or, more accurately, protect the plant from disease (Amari et al. Citation2014).
Adhatoda vasica is a perennial plant, well known for its efficacy in the Ayurveda system of medicine, with several medicinal properties (Manjunath Citation1948; Maurya & Singh Citation2010; Kaur et al. Citation2012). The plant grows throughout India, even at the higher altitudes of the Himalayan region, and is also found in Myanmar, Sri Lanka, Burma and Malaysia. Adhatoda vasica has been used in the Indian indigenous system of medicine for thousands of years, especially in the treatment of respiratory disorders including coughs, colds, asthma and bronchitis (Kaur et al. Citation2012). The leaves of A. vasica have been found to contain several alkaloids including vasicinone, vasicinol, adhatodine and peganine, as well as steroids and flavonoids such as astragalin, kaempferol, apigenin and quercetin (Maurya & Singh Citation2010). Moreover, A. vasica possesses several biological activities including anti-inflammatory, antispasmodic, antibleeding, antidiabetic and antijaundice effects (Maurya & Singh Citation2010). Other species of the genus Adhatoda, such as A. zeylanica, have also been found to display several biological activities, including antioxidant, antidiabetic and antibacterial actions (Iiango et al. Citation2009).
To the authors’ knowledge, there is no systematic report available in the literature on the antioxidant efficacy of the ethanolic leaf extract of Adhatoda vasica (ELEAV). Therefore, the aim of this research is to determine the potential efficacy of ELEAV in various antioxidant and radical scavenging models, as well as its inhibitory effect on lipid peroxidation, and to carry out phytochemical analysis.
Materials and methods
Chemicals and instruments
The chemicals and reagents used in this study, including 1,1-diphenyl-2-picrylhydrazyl (DPPH), sodium nitroprusside (SNP), Griess reagent, trichloroacetic acid (TCA), bovine brain extract, nitroblue tetrazolium (NBT), ferric chloride, potassium ferricyanide and gallic acid, as well as standard antioxidant compounds ascorbic acid and butylated hydroxyanisole (BHA), were purchased from Sigma-Aldrich (St Louis, MO, USA) and were of analytical grade. Spectrophotometric measurements were taken using a 96-well microplate enzyme-linked immunosorbent assay (ELISA) reader (Infinite M200; Tecan, Mannedorf, Switzerland).
Plant material and extraction
Dried leaf powder of A. vasica was a gift from Jeevan Herbal Products (Sagar, MP, India). The dried leaf powder (100 g) was extracted by drenching in ethanol in a conical flask for 7 days at room temperature. The solvent was filtered, followed by distillation under reduced pressure using a rotary evaporator (Eyela, China) until the solvent was completely dry. Finally, the extract, with a yield of 6.9 mg/100 g, was preserved in a sealed vial at 4°C until tested and analysed.
Preliminary phytochemical screening
A qualitative phytochemical screening of ELEAV to detect the presence of essential phytoconstituents, such as alkaloids, tannins, saponins, flavonoids, anthraquinone glycoside, steroids, terpenes, glycosides, proteins, amino acids, reducing sugars and phenol, was carried out using standard biochemical procedures as described previously (Edeoga et al. Citation2005; Patra et al. Citation2009).
Determination of DPPH radical scavenging activity
The antioxidant activity of ELEAV, based on the scavenging of stable DPPH free radical, was determined as described previously (Bajpai et al. Citation2013). Different concentrations of ELEAV (25–150 µg/ml) were added to 0.004% methanolic solution of DPPH in a 1:1 ratio in a 96-well microplate. The mixture was incubated at 37°C in the dark for 30 min with shaking (150 rpm). Absorbance was recorded at 517 nm using the Tecan ELISA reader against a blank sample. All the tests were run in triplicate. Ascorbic acid was used as the reference compound in the concentration range of 25–150 µg/ml. The per cent inhibition activity was calculated using the following formula:
where Acontrol is the absorbance of the control reaction and Atest represents the absorbance of a test reaction.
Determination of nitric oxide radical scavenging activity
SNP automatically generates nitric oxide, in aqueous solution at physiological pH, which intermingles with oxygen to generate nitrite ions that can be anticipated by Griess reagent [1% sulfanilamide, 2% phosphoric acid and 0.1% naphthyl ethylene diamine dihydrochloride (NED)]. Scavengers of free radicals result in the reduced production of nitric oxide radicals. In this assay, a solution of SNP (10 mM) in phosphate-buffered saline (PBS, pH 7.4) was mixed with different concentrations of ELEAV (20–100 µg/ml). The mixture was incubated at 37°C for 60 min in the light. Half the quantity of the aliquots was taken and mixed with an equal quantity of Griess reagent, and the mixture was incubated at 25°C for 30 min in the dark. The absorbance of the pink chromophore generated during diazotization of nitrite ions with sulfanilamide and subsequent coupling with NED was read at 546 nm against a blank (Bajpai et al. Citation2013). All the tests were performed in triplicate. Ascorbic acid was used as the standard reference compound in the concentration range of 20–100 µg/ml. The per cent inhibition activity was calculated by the same formula as used for determination of DPPH radical scavenging activity (see above).
Determination of superoxide radical scavenging activity
Superoxide radical scavenging activity of ELEAV was measured by the reduction of NBT according to the previously reported method (Bajpai et al. Citation2013). The non-enzymatic phenazine methosulfate–nicotinamide adenine dinucleotide (PMS/NADH) system generates superoxide radicals, which reduce NBT to a purple formazan. In this assay, the reaction mixture (150 µl) contained phosphate buffer (0.2 M, pH 7.4), NADH (73 µM), NBT (50 µM), PMS (15 µM) and various concentrations (50–250 µg/ml) of ELEAV and standard compound. After incubation for 60 min at room temperature, the absorbance of the reaction mixture was measured at 560 nm against an appropriate blank to determine the quantity of formazan generated. All tests were performed three times. Ascorbic acid was used as a standard. The per cent inhibition activity was calculated by the same formula as used for determination of DPPH radical scavenging activity (see above).
Determination of hydroxyl radical scavenging activity
To determine the hydroxyl radical scavenging activity of ELEAV, the previously described method was adopted (Bajpai et al. Citation2013). The assay is based on quantification of the degradation product of 2-deoxy-2-ribose sugar by condensation with 2-thiobarbituric acid (TBA). In this assay, hydroxyl radicals were generated by Fenton's reaction using the Fe3+–ascorbate–EDTA–H2O2 system. The reaction mixture in a total volume of 240 µl contained 2-deoxy-2-ribose (3 mM), KH2PO4-KOH buffer (20 mM, pH 7.4), FeCl3 (0.1 mM), EDTA (0.1 mM), H2O2 (2 mM), ascorbic acid (0.1 mM) and various concentrations (100–500 µg/ml) of ELEAV or standard compound. After incubation for 45 min at 37°C, 40 µl of 2.8% TCA and 40 µl of TBA (0.5% in 0.0255 M NaOH solution containing 0.02% BHA) were added in the reaction mixture, and the mixture was incubated at 95°C for 15 min to develop the pink colour. After cooling, the absorbance was measured at 532 nm against an appropriate blank solution. All tests were performed three times. BHA was used as the reference standard. The per cent inhibition activity was calculated by the same formula as used for determination of DPPH radical scavenging activity (see above).
Determination of lipid peroxidation inhibitory effect
A previously reported method was adopted to determine the Fe3+/ascorbic acid-dependent non-enzymatic lipid peroxidation activity of ELEAV in bovine brain extract (Bajpai et al. Citation2013). The reaction mixture, in the absence and presence of ELEAV (50–250 µg/ml) or reference compound, containing 50 µl of bovine brain phospholipids (5 mg/ml), 1 mM FeCl3 and 1 mM ascorbic acid in 20 mM phosphate buffer with a final volume of 330 µl, was incubated at 37°C for 1 h. The hydroxyl radicals generated in the reaction initiated the lipid peroxidation, resulting in malondialdehyde (MDA) production that was measured by the TBA reaction. All tests in this assay were performed three times. BHA was used as the reference compound. The per cent inhibition activity was calculated by the same formula as used for determination of DPPH radical scavenging activity (see above).
Determination of reducing power activity
The ferric ion (Fe3+) reducing power of ELEAV was determined by the previously reported method (Bajpai et al. Citation2013). Aliquots (50 µl) of different concentrations of ELEAV (5–25 µg/ml) and/or the positive control, ascorbic acid, were mixed with 50 µl phosphate buffer (0.2 M, pH 6.6) and 50 µl potassium ferricyanide (1% w/v in H2O), followed by incubation at 50°C for 20 min in the dark. After incubation, 50 µl of TCA (10% w/v in H2O) was added to terminate the reaction and the mixture was subjected to centrifugation at 3000 rpm for 10 min. For the final reaction mixture, the supernatant (50 µl) was mixed with 50 µl distilled water and 10 µl FeCl3 solution (0.1% w/v in H2O). The reaction mixture was incubated for 10 min at room temperature and the absorbance was measured at 700 nm against an appropriate blank solution. A higher absorbance of the reaction mixture indicated greater reducing power ability. All tests were run in triplicate. Ascorbic acid was used as the standard drug.
Statistical analysis
The data are expressed as the mean ± SD of three independent experiments. Data were analysed using one-way analysis of variance (ANOVA) and the Student's t-test. Values were considered to be statistically significant at p < 0.05.
Results and discussion
Phytochemical analysis
Several recent reports have confirmed that phytochemicals including alkaloids, glycosides, terpenoids, saponins, phenols and steroids have enormous antioxidant and free radical scavenging activities (Farhan et al. Citation2012; Amari et al. Citation2014). Plant extracts rich in polyphenols and essential phytoconstituents have been shown to display potent antioxidant and free radical scavenging activities in various antioxidant models (Farhan et al. Citation2012; Amari et al. Citation2014). As shown in , ELEAV contains various phytochemicals or polyphenols (such as flavonoids, tannins, phenolics, steroids, terpenoids and alkaloids) which are known to have potent antioxidant or free radical scavenging activities. In general, the antioxidant compounds of essential oils are terpenoids, which are phenolic in nature, and it would seem rational that their antioxidant mode of action could be related to that of other compounds. Although several different methods have been developed to evaluate the antioxidant activity of biological samples, it is relatively difficult to measure each antioxidant component separately. Therefore, this study explored a new therapeutic agent of plant origin, namely ELEAV, and attempted to confirm its biological efficacy in various in vitro antioxidant models, including its lipid peroxidation inhibitory efficacy.
Table 1. Phytochemical analysis of ethanolic leaf extract of Adhatoda vasica (ELEAV).
Scavenging activity of DPPH radical
The DPPH radical is a stable free radical, which has been widely used as a sensitive and rapid tool to estimate the free radical scavenging activity of both hydrophilic and lipophilic antioxidants (Archana et al. Citation2005). Antioxidants neutralize the free radicals on interaction with DPPH by transferring electrons or hydrogen atoms to DPPH (Archana et al. Citation2005). This method determines the antiradical power of an antioxidant by measuring the decrease in the absorbance of DPPH, resulting in a colour change from purple to yellow, through the donation of hydrogen to form a stable DPPH molecule (Matthaus Citation2002). DPPH shows strong absorbance in the radical form at a wavelength of 517 nm, which disappears after the acceptance of an electron or a hydrogen radical from an antioxidant compound to become a stable diamagnetic molecule (Matthaus Citation2002).
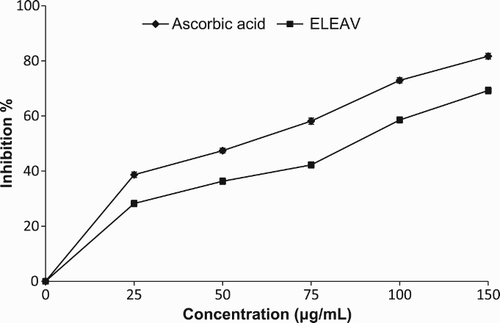
shows a significant decrease in the concentration of DPPH radicals due to the scavenging ability of ELEAV and the standard compound, ascorbic acid. The scavenging effects on the DPPH radical of ELEAV and the standard at the concentration of 100 µg/ml were 58.54% and 72.91%, respectively. However, 150 µg/ml of ELEAV inhibited 69.23% of DPPH radicals. The results were concentration dependent and statistically significant (p < 0.05). Similar findings on the DPPH radical scavenging activities of various plant extracts have been observed previously (Farhan et al. Citation2012; Amari et al. Citation2014).
Scavenging activity of nitric oxide radical
As a cell signalling molecule, nitric oxide has been associated with a variety of physiological processes in the human body. It transmits signals from vascular endothelial cells to vascular smooth muscle cells, resulting in vasodilatation (Aliev et al. Citation2009). It plays an important role in vital physiological functions in the respiratory, immune, neuromuscular and other systems. This molecule also regulates the release of neurotransmitters, and is involved in neuronal excitability, learning and memory processes, as well as inflammatory bowel syndrome, sepsis, septic shock, cephalalgia, dementia, multiple sclerosis and stroke (Aliev et al. Citation2009). In addition, it has been shown that nitric oxide modulates neurotoxin-induced cellular damage and is involved in neuronal cell death in Parkinson's disease and other neurodegenerative disorders such as Alzheimer's disease (Aliev et al. Citation2009). A number of related reports suggest that nitric oxide may modulate iron-catalysed oxidation reactions such as the superoxide-anion driven Fenton's reaction, resulting in the production of strong oxidants such as the hydroxyl radical and organometallic complexes (Dadashpour et al. Citation2011).
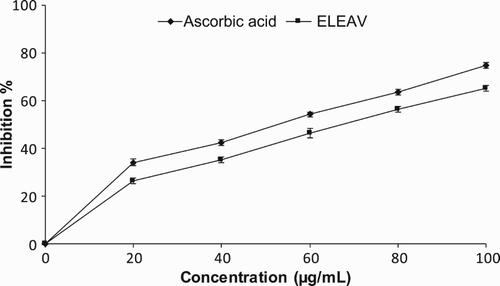
The mechanisms by which nitric oxide may inhibit lipid peroxidation have not been studied in depth; however, one possible mechanism relates to its ability to terminate the propagation of lipid peroxidation reactions. shows the scavenging effects of ELEAV and the standard on the nitric oxide radicals at the concentration of 80 µg/ml, which were 56.23% and 63.57%, respectively, whereas ELEAV at 100 µg/ml inhibited 65.24% of nitric oxide radicals. The results were statistically significant (p < 0.05) and concentration dependent. The nitric oxide radical scavenging activities of various plant-based products and extracts have been reported previously (Farhan et al. Citation2012; Amari et al. Citation2014).
Scavenging activity of superoxide radical
Superoxide radicals, which are highly toxic ROS, are generated in the body by various biological and metabolic reactions. Although the relatively weak superoxide oxidants have only limited chemical reactivity, they are potential precursors of highly reactive species including hydrogen peroxide, hydroxyl radicals and singlet oxygen, causing lipid peroxidation (Kanatt et al. Citation2007). Superoxide radical scavenging capacity is the first line of defence against oxidative stress in humans. The superoxide anion is an oxygen-centred radical with selective reactivity (Kanatt et al. Citation2007). It has been reported that the antioxidant properties of some plant products are effective mainly via scavenging of superoxide anion radicals (Dadashpour et al. Citation2011).
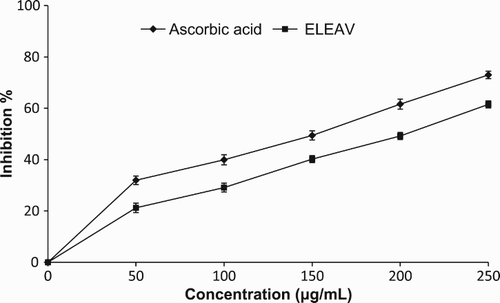
In this assay, superoxide anions derived from dissolved oxygen by the PMS/NADH system reduce NBT. Superoxide radical reduces the yellow dye (NBT2+) to produce blue formazan, which is measured spectrophotometrically at 560 nm. Antioxidants inhibit the formation of blue NBT. The decrease in absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion radical in the reaction mixture. shows the concentration-dependent inhibition of superoxide radical generation by all concentrations of ELEAV and the standard compound tested. At the concentration of 250 µg/ml, the superoxide radical inhibitory effects of ELEAV and ascorbic acid were 61.54% and 73.01%, respectively. Various plant-based products and extracts have previously been shown to exert superoxide scavenging efficacy (Hazra et al. Citation2010).
Scavenging activity of hydroxyl radical
The hydroxyl radical has been implicated as a highly damaging ROS in free radical pathology, capable of damaging almost every molecule in living cells (Halliwell Citation1991; Uttara et al. Citation2009). The best characterized biological damage caused by the hydroxyl radical is its capacity to stimulate lipid peroxidation, which occurs when the hydroxyl radical is generated close to membranes and attacks the fatty acid side-chains of the membrane phospholipids (Halliwell Citation1991; Uttara et al. Citation2009). This radical can also be formed from superoxide anion and hydrogen peroxide, in the presence of metal ions (Cu2+ and Fe2+). The hydroxyl radical has the capacity to join nucleotides in DNA and cause strand breakage, which contributes to carcinogenicity, mutagenicity and cytotoxicity (Valko et al. Citation2007). The hydroxyl radical scavenging capacity of a drug is directly related to its antioxidant activity. The highly reactive hydroxyl radicals can cause oxidative damage to DNA, lipids and proteins (Valko et al. Citation2007). The effect of ELEAV on the inhibition of free radical-mediated deoxyribose damage was assessed by means of the iron (II)-dependent DNA damage assay. Fenton's reaction generates hydroxyl radicals which degrade DNA deoxyribose sugar, using Fe2+ salts as an important catalytic component (Valko et al. Citation2007). Oxygen radicals may attack DNA either at the sugar or at the base, giving rise to a large number of products. ELEAV was capable of reducing DNA damage at all the concentrations used.
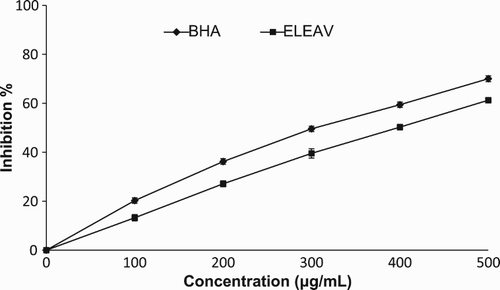
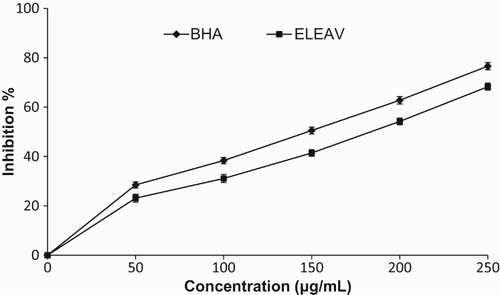
In this assay, ELEAV was analysed for hydroxyl radical scavenging activity in order to examine its antioxidant properties. As shown in , ELEAV displayed the potential for hydroxyl radical scavenging activity in a concentration-dependent manner. At 500 µg/ml, the hydroxyl radical scavenging activities of ELEAV and BHA were 61.24% and 70.02%, respectively. The ability of ELEAV to quench hydroxyl radicals seems to be directly related to the prevention of propagation of the process of lipid peroxidation and it seems to be a good scavenger of ROS, thus reducing the rate of the chain reaction. BHA was used as a reference standard. The hydroxyl radical scavenging activity of various plant extracts has been reported previously (Hazra et al. Citation2010).
Lipid peroxidation inhibition
Lipid peroxidation affects the colour, flavour, texture and nutritional value of food or food products, and is considered a major cause of food deterioration (Balu et al. Citation2005). During the process of lipid peroxidation, free radicals steal electrons from the lipids in cell membranes, resulting in a loss of membrane fluidity, an increase in membrane permeability and a decrease in physiological performance, thus endangering cell viability (Balu et al. Citation2005). The chemical structure of iron, and its capacity to drive one-electron reactions, makes iron a key factor in the formation of free radicals. In biological systems, lipid peroxidation generates many aldehyde products, among which MDA is considered to be the most important derivative (Barrera et al. Citation2008). Lipid peroxidation begins by a free radical chain reaction mechanism. It mostly affects polyunsaturated fatty acids, and is a major cause of cell membrane disruption and cell damage (Barrera et al. Citation2008). This process is initiated by hydroxyl and superoxide radicals, leading to the formation of peroxy radicals that eventually propagate the chain reaction in lipids. Therefore, antioxidants capable of scavenging peroxy radicals could prevent lipid peroxidation.
This assay measured the great potential of ELEAV to inhibit lipid peroxidation in bovine brain extract, induced by the Fe3+/ascorbate system. The inhibitory effect of ELEAV on Fe3+-induced lipid peroxidation in bovine brain homogenates is presented in . At the concentration of 250 µg/ml, ELEAV and BHA showed 68.26% and 76.59% inhibition of lipid peroxides, respectively. ELEAV protected considerably against lipid peroxidation induced by Fe3+, by reducing lipid peroxidation in a concentration-dependent manner, and the results were statistically significant (). Other plant extracts have also shown protective effects against Fe3+-induced lipid peroxidation (Geetha & Vasudevan Citation2004). The inhibition of Fe3+-induced lipid peroxidation by ELEAV may be due to Fe3+ chelation and its hydroxyl radical scavenging abilities (Balu et al. Citation2005; Barrera et al. Citation2008). This capacity could be important because Fe3+ can stimulate free radical formation; thus, when complexes are formed between ELEAV and Fe3+, lipid peroxidation could be prevented or reduced (Balu et al. Citation2005; Barrera et al. Citation2008). ELEAV was able to scavenge the hydroxyl radical produced during Fe3+-catalysed decomposition of hydrogen peroxide (Fenton's reaction); the highly reactive hydroxyl radical initiates a process of membrane lipid peroxidation that could lead to alterations in cell structure and function (Barrera et al. Citation2008).
Reducing power ability
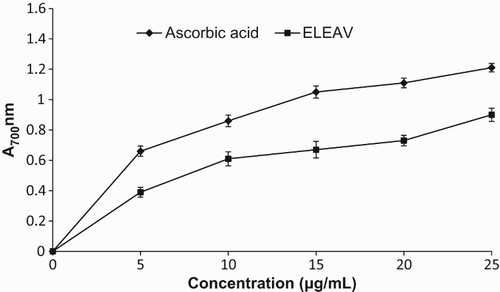
Several studies have shown that the electron donation capacity reflects the reducing power of biologically active compounds in relation to their antioxidant activity (Gulcin et al. Citation2010). Antioxidants are reducing agents, and inactivation of oxidants by reductants can be described as a reduction–oxidation (redox) reaction, in which one reaction species is reduced at the expense of the oxidation of the other (Gulcin et al. Citation2010). The reduction of Fe3+ is often used as an indicator of electron donating ability, which is an important mechanism of phenolic antioxidant action. In the reducing power assay, the presence of antioxidants in the sample would result in the reduction of Fe3+ to Fe2+ by the donation of an electron. The amount of Fe2+ complex can be then be monitored by measuring the formation of Perl's Prussian blue ferric ferrocyanide (Fe4[Fe(CN)6]3) at 700 nm. Increasing absorbance at 700 nm indicates an increase in reductive ability. shows the reducing power of ELEAV as a function of its concentration. It was found in this assay that the reducing power of ELEAV and ascorbic acid increased with the increase in their concentrations. At the concentration of 25 µg/ml, the reducing power efficacies of ELEAV and ascorbic acid were 0.90 and 1.21, respectively. Similarly, various plant extracts containing different phytochemicals have shown antioxidant activity through their reductive capacity in an Fe3+–Fe2+ system (Lizcano et al. Citation2012). The results obtained in this study indicate that the marked reducing power of ELEAV may be attributable to its antioxidant activity.
Figure 6. Reducing power activity of ethanolic leaf extract of Adhatoda vasica (ELEAV) and standard compound, ascorbic acid.
The antioxidant activity of plants comes mainly from the active compounds or essential phytoconstituents that they contain (Wojdylo et al. Citation2007). Although the activity of synthetic antioxidants is often reported to be higher than that of natural antioxidants, their higher toxicity and carcinogenicity prohibit their use at certain levels (Gulcin et al. Citation2010). At certain concentrations, various herbal products and/or extracts rich in polyphenols noticeably slow down the formation of conjugated diolefin. In this regard, there has been increasing interest in polyphenolic compounds in the food industry because of their inhibitory effects on lipid peroxidation and on the formation of off-flavours and other objectionable compounds, which could lead to improvements in the quality and nutritional value of fresh and processed foods (Wojdylo et al. Citation2007).
Based on the findings of this study, it can be concluded that ELEAV, containing alkaloids, glycosides, terpenoids, saponins, phenols and steroids as essential phytochemicals, exhibited significant antioxidant and free radical scavenging activities. ELEAV also exerted potent antioxidant activity through its reducing power ability. Moreover, ELEAV had a potent inhibitory effect on ferric ion-induced lipid peroxidation. The antioxidant activity of herbal extracts is of potential interest to those in the food industry who are looking for compounds with significant biological potential to be used as alternatives to chemical and conventional food preservation systems. Hence, it is claimed that ELEAV could be a source of natural antioxidants for use in the food industry against oxidative deterioration, as well as a useful therapeutic agent in the prevention of oxidative stress-related degenerative diseases. Further studies are planned to isolate individual biologically active constituents from ELEAV and establish their precise antioxidant mechanisms of action in vivo.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aliev G, Palacios HH, Lipsitt AE, Fischbach K, Lamb BT, Obrenovich ME, Morales L, Gasimov E, Bragin V. 2009. Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotox Res. 16:293–305. doi: https://doi.org/10.1007/s12640-009-9066-5
- Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T. 2003. Screening, chemical composition and antioxidant and antimicrobial activities of the essential oils from Origanum syriacum L. growing in Turkey. Biol Pharm Bull. 26:1725–1729. doi: https://doi.org/10.1248/bpb.26.1725
- Alvarado C, Álvarez P, Puerto M, Gausserès N, Jiménez L, De la Fuente M. 2006. Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition. 22:767–777. doi: https://doi.org/10.1016/j.nut.2006.05.007
- Amari NO, Bouzouina M, Berkani A, Lotmani B. 2014. Phytochemical screening and antioxidant capacity of the aerial parts of Thymelaea hirsuta L. Asian Pac J Trop Dis. 4:104–109. doi: https://doi.org/10.1016/S2222-1808(14)60324-8
- Archana B, Dasgupta N, De B. 2005. In vitro study of antioxidant activity of Syzygium cumini fruit. Food Chem. 90:727–733. doi: https://doi.org/10.1016/j.foodchem.2004.04.033
- Bajpai VK, Sharma A, Kim SH, Baek KH. 2013. Phenolic content and antioxidant capacity of essential oil obtained from sawdust of Chamaecyparis obtusa by microwave-assisted hydrodistillation. Food Technol Biotechnol. 51:360–369.
- Balu M, Sangeetha P, Haripriya D, Panneerselvam C. 2005. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci Lett. 383:295–300. doi: https://doi.org/10.1016/j.neulet.2005.04.042
- Barrera G, Pizzimenti S, Dianzani MU. 2008. Lipid peroxidation: control of cell proliferation, cell differentiation and cell death. Mol Aspects Med. 29:1–8. doi: https://doi.org/10.1016/j.mam.2007.09.012
- Dadashpour M, Rasooli I, Sefidkon F, Rezaei MB, Alipour D, Astaneh S. 2011. Lipid peroxidation inhibition, superoxide anion and nitric oxide radical scavenging properties of Thymus daenensis and Anethum graveolens essential oils. J Med Plants. 10:109–120.
- Edeoga HO, Okwa DE, Mbaebie BO. 2005. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 4:685–688. doi: https://doi.org/10.5897/AJB2005.000-3127
- Farhan H, Malli F, Rammal H, Hijazi A, Bassal A, Ajouz N, Badran B. 2012. Phytochemical screening and antioxidant activity of Lebanese Eryngium creticum L. Asian Pac J Trop Biomed. 2:S1217–S1220. doi: https://doi.org/10.1016/S2221-1691(12)60388-8
- Geetha RK, Vasudevan DM. 2004. Inhibition of lipid peroxidation by botanical extracts of Ocimum sanctum: in vivo and in vitro studies. Life Sci. 76:21–28. doi: https://doi.org/10.1016/j.lfs.2004.05.036
- Gulcin I. 2006. Antioxidant and antiradical activities of L-Carnitine. Life Sci. 78:803–811. doi: https://doi.org/10.1016/j.lfs.2005.05.103
- Gulcin I, Elias R, Gepdiremen A, Chea A, Topal F. 2010. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzy Inhib Med Chem. 25:44–53. doi: https://doi.org/10.3109/14756360902932792
- Halliwell B. 1991. Reactive oxygen species in living systems: source, biochemistry and role in human disease. Am J Med. 91:S14–S22. doi: https://doi.org/10.1016/0002-9343(91)90279-7
- Hazra B, Sarkar R, Biswas S, Mandal N. 2010. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Comp Alt Med. 10:1–15. doi: https://doi.org/10.1186/1472-6882-10-20
- Iiango K, Chitra V, Kanimozhi P, Balaji G. 2009. Antidiabetic, antioxidant and antibacterial activities of leaf extracts of Adhatoda zeylanica Medic (Acanthaceae). J Pharm Sci Res. 1:67–73.
- Ishino K, Wakita C, Shibata T, Toyokuni S, Machida S, Matsuda S, Matsuda T, Uchida K. 2010. Lipid peroxidation generates body odor component trans-2-nonenal covalently bound to protein in vivo. J Biol Chem. 285:15302–15313. doi: https://doi.org/10.1074/jbc.M109.068023
- Kanatt KR, Chander R, Sharma A. 2007. Antioxidant potential of mint (Mentha spicata L.) in radiation processed lamb meat. Food Chem. 100:451–458. doi: https://doi.org/10.1016/j.foodchem.2005.09.066
- Kaur I, Chauhan PK, Jaryal M, Saxena S, Kanisha. 2012. Antioxidant and antimicrobial activity of leaf extract of Adhatoda vasica against the bacteria isolated from the sputum samples of asthmatic patients. Int J Drug Res Tech. 2:273–278.
- Kirkinezos IG, Moraes CT. 2001. Reactive oxygen species and mitochondrial diseases. Cell Dev Biol. 12:449–457. doi: https://doi.org/10.1006/scdb.2001.0282
- Lizcano LJ, Viloria-Bernal M, Vicente R, Berrueta LA, Gallo B, Martinez-Canamero M, Ruiz-Larrea MB, Ruiz-Sanz JI. 2012. Lipid oxidation inhibitory effects and phenolic composition of aqueous extracts from medicinal plants of Colombian Amazonia. Int J Mol Sci. 13:5454–5467. doi: https://doi.org/10.3390/ijms13055454
- Manjunath BL. 1948. The wealth of India, A Dictionary of Indian raw materials and industrial products. CSIR Delhi, 2:31–32.
- Martinez CM. 1995. Review: oxygen free radicals and human disease. Biochimie. 77:147–161. doi: https://doi.org/10.1016/0300-9084(96)88119-3
- Matthaus B. 2002. Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem. 50:3444–3452. doi: https://doi.org/10.1021/jf011440s
- Maurya S, Singh D. 2010. Quantitative analysis of total phenolic content in Adhatoda vasica Nees extracts. Int J Pharm Tech Res. 2:2403–2406.
- Patra JK, TPanigrahi K, Rath SK, Dhal NK, Thatoi HN. 2009. Phytochemical screening and antimicrobial assessment of leaf extracts of Excoecaria agallocha L.: a mangal species of Bhitarkanika, Orissa, India. Adv Nat Appl Sci. 3:241–246.
- Skotti E, Anastasaki E, Kanellou G, Polissiou M, Tarantilis PA. 2014. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind Crops Prod. 53:46–54. doi: https://doi.org/10.1016/j.indcrop.2013.12.013
- Uttara B, Singh AV, Zamboni P, Mahajan RT. 2009. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 7:65–74. doi: https://doi.org/10.2174/157015909787602823
- Valko M, Leibfritz D, Moncola J, Cronin MTD, Mazura M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 39:44–84. doi: https://doi.org/10.1016/j.biocel.2006.07.001
- Wiseman H, Halliwell B. 1996. Damage to DNA by reactive oxygen and nitrogen species: role of inflammatory disease and progression to cancer. Biochem J. 313:17–29.
- Wojdylo A, Oszmianski J, Czemerys R. 2007. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 105:940–949. doi: https://doi.org/10.1016/j.foodchem.2007.04.038