Abstract
Why the sexual climax, in humans, results in a pleasurable experience remains an important biological question. Analysis of evolutionary traits in numerous Vertebrates suggests that orgasm evolved through three phylogenetic stages during the transition from external to internal fertilization and viviparity. First, orgasm is directly dependent on ejaculation in males and the expulsion of fluids from the ovarian and urethral glands (Skene’s) in females. I propose that sexual orgasm could come from the primitive reflex of discharging gametes to ensure reproduction. Thus, the understanding of orgasm should not be reduced to a penis- or a clitoris-centred paradigm. Secondly, orgasm has evolved to stimulate sexual activity because the evolutionary transition from external fertilization to internal fertilization has been accompanied in numerous species with a lessening in reproductive rates. Because sexual activity encourages reproduction, it can be argued that orgasm has evolved to increase sexual activity, particularly in viviparous species with low reproductive rates. Third, internal fertilization in the genital tract of females weakens the visibility of the putative success of fertilization. Female sexual fluids and proteins can bias fertilization in favour of preferred males. Because orgasm could promote a better choice of partner, I argue that female orgasm may have evolved as a post-copulatory selection tactic by which females can increase their control of mates.
Introduction
Why the sexual climax, in humans, leads to the experience of pleasure remains an important biological question. While the male cannot transfer gametes without experiencing an orgasm, in the human species, the female orgasm seems completely decoupled from reproduction (Cabanac Citation1971; Hrdy Citation1996; Wallen and Lloyd Citation2008). Although orgasm could result from the point that individuals with an orgasm are more successful, Wheatley and Puts (Citation2015) argued that there is actually little evidence to suggest that female orgasm can promote a better reproduction. If orgasm had a selective role, then it is difficult to understand why females show such variability in their ability to reach a climax. Analysing the evolutionary history of the sexual climax from its phylogenetic origin, we can draw some new conclusions that can shed light on the function of orgasm.
Indeed, orgasm could only be regarded as a direct selective trait if individuals with this evolutionary trait have the best reproduction. Associated with ejaculation in males, orgasm is characterized by changes in blood pressure, increased heart rate, rhythmic respiratory pattern, involuntary body movements and, in females, by spontaneous colour changes of the labia minora which engorge to twice their size, and by vaginal and anal spasms (Masters and Johnson Citation1966; Berman et al. Citation1999). The orgasm is directed by the autonomic nervous system and the spinal cord but activates numerous cortical zones through the vagus nerves mediation (Komisaruk et al. Citation2004).
Although the definition of orgasm is rather uncertain, Bancroft (Citation2005) described it as ‘a state motivated toward the experience of sexual pleasure’. In males, it is associated with ejaculation and rhythmic muscle contractions of the perineal muscles, in females, with clitoral retraction, rhythmic muscle contractions of the perineum and vagina. Orgasm also releases some neuropeptides, dopamine, oxytocin and prolactin, which cause a deep sense of well-being. Here, orgasm is defined as the culmination of sexual arousal activating the reward circuit. The increase in dopamine, oxytocin and prolactin concentration can, therefore, be considered as a signal of sexual arousal. All mammals have the physiological capacity for orgasm (Fox and Fox Citation1971) and numerous vertebrates are known to experience orgasm-like states such as primates i.e. bonobo, chimpanzee, gorilla, orangutan, proboscis monkey, macaques (Chevalier-Skolnikoff Citation1974; Allen and Lemon Citation1981; Troisi and Carosi Citation1998; Murai Citation2006; de Waal Citation2011; Grueter and Stoinski Citation2016), carnivores and rodents (Adler Citation1969; Heeb and Yahr Citation1996; Coolen et al. Citation1997; Kollack-Walker and Newman Citation1997; Tenk et al. Citation2009; Pavlicev and Wagner Citation2016), birds and reptiles (Cabanac Citation1971; Winterbottom et al. Citation1999; Ball and Balthazart Citation2011) and fishes (Petersson and Jarvi Citation2001). It has even been demonstrated that ejaculation provoked by the activation of Crz-expressing neurons is rewarding to male flies (Zer-Krispil et al. Citation2018). Thus, orgasm seems a critical component of reproductive process for many species (Balcombe Citation2009).
There are two main theories that provide an explanation for the manifestation of orgasm. It has been firstly hypothesized that orgasm would favour the persistence of bonds to ensure the best care for the offspring (Alcock Citation1987) or serve as a secondary reinforcement linking sexual behaviours and partner affiliation (Prause Citation2011; Fleischman Citation2016). However, in many species, the male provides virtually no care to the young, which reduces the interest in this hypothesis. Moreover, the strengthening of the couple’s bonds is clearly refuted by the Coolidge effect (Brown Citation1974), which leads many males, and to a lesser extent some females (Lester and Gorzalka Citation1988), to increase sexual activity by adopting a greater diversity of partners. In monkeys, females that mated with high-ranking males showed the highest frequency of orgasms (Zumpe and Michael Citation1968; Chevalier-Skolnikoff Citation1974), suggesting a role in partner preference. Finally, among the hypotheses tending to interpret orgasm as a reproductive enhancing effect, it has been assumed that female orgasm would have evolved for the selection of a partner, thus enhancing the chance of the best fertilization (Thornhill et al. Citation1995). Thus, Fox et al. (Citation1970) hypothesized that the orgasmic spasms cause contractions that may endorse sperm retention in the female genital tract, thus increasing the probability of fertilization. Fertilization of male gametes is stimulated by rhythmic contractions of the striated muscles during orgasm (Levin Citation2002; Meston et al. Citation2004; King et al. Citation2016). The allegation that female orgasms may increase conception and progeny remains controversial (Zietsch and Santtila Citation2013; Wheatley and Puts Citation2015) since female orgasms do not appear to have an active role in sperm transport during coitus (Levin Citation2011). Nonetheless, cervical excitations produce contractions of the oviduct and fallopian tubes (Komisaruk et al. Citation2004), probably via prostaglandins and are essential for sperm transport and fertilization (Adler Citation1969; Adler and Zoloth Citation1970; Wildt et al. Citation1998).
The second theory claims that female orgasm has no selective role and must be understood as a simple by-product of ontogenesis since the embryonic development of the male penis and female clitoris remains very comparable (Symons Citation1979; Gould Citation1987; Wallen and Lloyd Citation2008).
Phylogeny of the orgasm
Nevertheless, it seems quite relevant to estimate the role of orgasm by analysing how orgasm phylogenetically developed during the evolution of sexual behaviour. Analysis of evolutionary traits suggests that orgasm evolved through three phylogenetic stages during the transition from external to internal fertilization and viviparity.
First, because the orgasm is also associated with ejaculation in males and with orgasmic expulsions from ovarian and urethral glands in females, I reasoned that the sexual orgasm could originate from the ancestral reflex of gametes release in both sexes. Sex has been found to be the most common reproductive mode among eukaryotes. Originally based on meiosis, it has been hypothesized that sex could come from very archaic interactions among ‘libertine bubbles’, i.e. practising horizontal gene exchanges (see Lodé Citation2011). Eukaryotic lineages evolved from ancestors that progressively developed male and female roles (Crews Citation1982) in such a way that sexuality resulted from a series of small evolutionary changes since the primeval sexual interaction of gene transfers (Lodé Citation2011, Citation2012a, Citation2013). Subsequently, the sexual chromosomes appeared independently and repeatedly, allowing a chromosomic determination of the sexual gender. Thus, initiated by the primitive genetic interactions of the very first protoeukaryotic organisms (Lodé Citation2011), the evolution of sexuality would be caused by a succession of small events, more or less independent of each other, from genetic exchanges and recombination, from external fertilization, then from internal insemination to parental care.
Many vertebrates seem to experience numerous characteristic manifestations of an orgasm during their copulatory activity (Fox and Fox Citation1971; Gould Citation1987; Balcombe Citation2009). Females Macaca mulatta have been found to reach orgasms after artificial vaginal stimulations (Burton Citation1971). Zumpe and Michael (Citation1968) also noted pelvic movements in female (Macaca rhesus) and some mammals such as dogs, cats, guinea pigs, hamsters, gerbils, ferrets and rats are known to be stimulated by frictional means (Heeb and Yahr Citation1996; Troisi and Carosi Citation1998; Pfaus et al. Citation2016). In mammals, orgasm is accompanied by prostate discharge in males and release of the para-urethral glands [Guérin/Skene’s] in females, suggesting a similar role in gamete release in both sexes. In females, coitus causes a hormonal discharge and climax is accompanied by vaginal spasms and contractions of the uterus (Zumpe and Michael Citation1968; Fox and Fox Citation1971; Health Citation1984; Erskine et al. Citation1989; Bancroft Citation2005; Ball and Balthazart Citation2011).
In fact, most species of vertebrates have developed sensitive organs and organic receptors linked to the ‘nervous circuit of reward’ (Figure ). Many animals have nuclear habenulae, which control reward improvement mechanisms, usually associated with pleasure (Loonen and Ivanova Citation2015). Similarly, the neurochemical profiles of ventral pallidum are extremely comparable between teleostei, amphibians, reptiles, birds and mammals. Moreover, notable dopaminergic and mesolimbic neuronal circuits regulating sexual behaviour exist in early vertebrates (O’Connell and Hofmann Citation2011).
Figure 1. Simplified scheme of neural circuits of reward in the rat brain. Reward and pleasure are generated by a set of hedonic hotspots within mesocortilimbic dopamine circuit, especially from nucleus accumbens, which controls the release of dopamine from the ventral tegmental area. Beta-endorphins are released by pituitary glands. Orgasm is experienced when genitalia provokes nervous excitations towards spinal cord and brain (from Berridge and Kringelbach Citation2015 adapted).
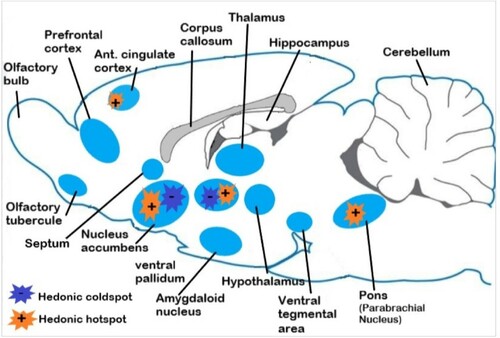
There is no reason to suppose that the intromission organs are necessary to obtain a sexual climax. In birds, for instance, the muscular contractions of the cloaca drive semen into the reproductive tract. In most vertebrates with external fertilization, basic reflex contractions accelerate gamete release, promoting the velocity of spermatozoids and expelling ova into water (Cooke et al. Citation2001). Males often build nests and compete to attract females to lay their eggs in the nests (Gross and Charnov Citation1980; Ridgway et al. Citation1989). In females, the muscles of the oviduct and those of the body wall contract causing the ova to move through the oviduct until total expulsion onto the substrate. In males, sperm is expelled onto the ova by repeated muscular contractions but survive only a few minutes, during which the fertilization occurs, may be stimulated by chemicals. A large amount of fluid is secreted by numerous glands and this reflex implies concomitant rhythmic contractions of the pelvi-perineal muscles in the two sexes.
Although stimulation of the sexual organs triggers it, orgasm is characterized by a sudden release of genital fluids. Ejaculation is the most rewarding component of male sexual behaviour in rats, Syrian hamsters and gerbils (Heeb and Yahr Citation1996; Kollack-Walker and Newman Citation1997; Tenk et al. Citation2009). The argument that the orgasm comes from the ancestral reflex of gamete release is all the more plausible because the orgasm is accompanied by ejaculation in males and by fluids from ovaries and from para-urethral glands in females. A discharge of the prostate typifies male orgasm, but in females, orgasm is associated by a release of homologous para-urethral glands, the Guérin–Skene’s glands, when stimulated from inside the vagina, evoking an identical function in both sexes (Tepper et al. Citation1984). Fluid secretion by the female glands reveals ejaculatory function equivalent to that of the male (Health Citation1984; Korda et al. Citation2010). Skene’s glands produce the same type of fluid, including enzymes and proteins, as prostate fluid with lower levels of creatinine, but they have high levels of prostate specific antigen, prostatic acid phosphatase and glucose, and it is believed that both work in the same way. Orgasm triggers many neurohormone secretions such as oxytocin, dopamine, prolactin and endorphins. Dopamine is at the heart of the mesolimbic reward system and the contribution of dopamine to the accumbens nucleus is decisive for reward associated behaviour (Yamamoto and Vernier Citation2011; Berridge and Kringelbach Citation2015; Loonen and Ivanova Citation2015). In fact, there is no objective reason why animal sexuality should be disconnected from a positive experience and no physiological element contradicts the hypothesis that stimulation of the genitals can lead to an orgasm in many vertebrates, including mammals, birds and reptiles (Cabanac Citation1971; Balcombe Citation2009; Ball and Balthazart Citation2011).
Thus, far from being limited to sexual organs, orgasm may be directly related to the strong expulsion of prostate fluids and para-urethral glands in both males and females, suggesting an ancestral role of orgasm in gamete emission.
Evolution of sexual behaviour and fertilization
Secondly, the evolutionary passage from external fertilization (ovuliparity and external fertilization) to the retention of the ova (internal fertilization) continued with egg retention and internal embryonic development (oviparity, histotrophic and hemotrophic viviparity, see Lodé Citation2012b). The evolution of viviparity in amniotes required two exaptations, the evolution of internal fertilization and a process of egg retention (see Kalinka Citation2015 for a detailed analysis). Internal fertilization in the genital track of females developed in the Late Ordovician from the retention of ova.
The evolutionary interest of the internal fertilization mode is to facilitate a terrestrial way of life while maintaining gametes in a liquid medium. On the other hand, internal fertilization requires either indirect insemination, through a spermatophore or by cloacal or vaginal coupling. The move of spermatozoids into the ejaculatory ducts results from the smooth muscle contractions associated with abundant secretions of fluids which product a pressure propelling the sperm forward. Similarly, the release of ova is assisted by the secretion of ovarian fluids. The luteinizing hormone has an important role for the release of gametes. Pituitary activation produces higher plasma concentrations of oxytocin and prolactin (Huyunh et al. Citation2013) and luteotrophic factor stimulates the vacated follicle to secrete progesterone. These hormones can have a role in inducing vaginal and uterus movements, ovulation and improving sperm and egg transport (Wildt et al. Citation1998). An increase in prolactin levels has been demonstrated during orgasm (Krüger et al. Citation2002; Haake et al. Citation2003; Huyunh et al. Citation2013). Similarly, testosterone, estradiol and progesterone are known to modulate reward processing.
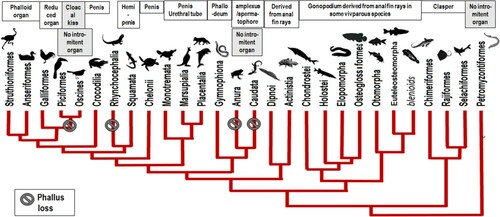
Thus, the behaviour of the male and the female helps to synchronize the maturation and release of sperm and eggs for fertilization to occur. Precisely, the evolutionary development of many intromittent organs in males (Figure ) – such as gonopodium, phallodeum, hemipenis, pseudo-penis and penis – allowing internal fertilization and directly involved in the orgasm, finds a homologue in the development of grooved tubercle in females as a rudimentary clitoral structure also implicated in the orgasm (Cibrian-Llandera et al. Citation2010) as well in fish, as in reptiles, birds or mammals. Thus, the males of Buffalo weavers (Bubalornis sp.) possess a phalloid organ that generates an orgasm-like state (Winterbottom et al. Citation1999). Such a development of specialized organic structures, therefore, supports my hypothesis of a primitive origin of orgasm linked to the basic reflex of gamete expulsion, both in males and females, thus could constitute a convergent evolution.
Figure 2. Occurrences and types of intromittent organs in males according to phylogenetic relationships.
In recent work, Pavlicev and Wagner (Citation2016) developed an analysis of induced ovulation and clitoral orgasm development. These authors present a new phylogenetic scenario in eutherian mammals based on the distance from the clitoris to the vaginal orifice, which increases with the evolution of spontaneous ovulation. This is because the longer clitoris reduces the distance to the vaginal orifice, and thereby influences the potential for stimulation. Genital organs are formed from the same undifferentiated embryonic tissues, resulting in the clitoris and penis (Wallen and Lloyd Citation2008), and leading to the conceptual dominance of a kind of a ‘penis or clitoris-centred paradigm’. By influencing the time between orgasm and ovulation, due to egg retention, the evolution of internal fertilization would have favoured the appearance of orgasm in eutherian mammals. In developing this new clitoris-centred theory, Pavlicev and Wagner (Citation2016) suggest that female orgasm may come from the reflex which, ancestrally, induces spontaneous ovulation in eutherian mammals.
Nonetheless, by emphasizing clitoral arousal, this theory is based stimuli of the sexual organs alone. These penis- or clitoris-centred theories, therefore, tend to consider that the clitoris functions as a reduced penis, suggesting that vertebrates without intromission organs could not experience a sexual climax. In females, Kraus’ corpuscles innervate both the clitoris and the vagina, so that it is disputable to differentiate clitoral orgasm from vaginal orgasm (Pfaus et al. Citation2016) although most women experience positive clitoral excitations. The penis is a specialized structure that is involved in gamete transfer and micturition but it can also become erect for other reasons. In rodents, ejaculation was found to have greater rewarding properties than intromissions (Heeb and Yahr Citation1996; Kollack-Walker and Newman Citation1997; Tenk et al. Citation2009). Moreover, even in animals that perform external fertilization, the use of a muscle reflex to expel fluids and gametes is required in both males and females (Gross and Charnov Citation1980). Internal fertilization does not depend on intromittent organs and could be achieved through a strong expulsion of sperm, which suggests that the evolutionary significance of orgasm should not be limited to a ‘penis-centred’ or a ‘clitoris-centred’ paradigm. The orgasmic response is characterized by a strong prostate flow in men and, in women, Skene glands and ovarian fluids are known to participate in the sperm nutrition process and to accelerate sperm velocity (Urbach et al. Citation2005; Santos and Taboga Citation2006; Gasparini et al. Citation2010). Antimicrobial compounds and many other secretion products from ovarian fluids and para-urethral glands promote greater receptivity of females to coitus at a higher frequency (Moalem and Reidenberg Citation2009).
Indeed, during the evolution towards internal fertilization, many species have seen their reproduction rate decrease in favour of protecting embryonic development. Over the course of evolution, many species have associated internal fertilization with a range of organic procedures that protect the intrauterine development of their offspring. But species that have developed a reproduction by internal fertilization have seen a significant decrease in their offspring. These species have then compensated for this lessening in their progeny by promoting greater ease in multiplying copulations. At the same time, the sexual climax has evolved to promote reproductive performance in low reproductive rate species because they need to have high frequency sexual intercourse. Non penetrative sexual behaviour, masturbation or same-sex sexual behaviour are common in a wide range of species, including mammals, birds, reptiles and amphibians (Balcombe Citation2009; Ball and Balthazart Citation2011). Further in humans, most homosexual men could also reach orgasm by stimulating the prostate during anal sex, demonstrating the role of fluid discharge in achieving orgasm. It is likely that the achievement of non-genital orgasms is derived from genital orgasms. The sexual climax being accompanied by a sense of pleasure provides sufficient gratification to engage in non-reproductive actions such as anal or oral sex, masturbation and homosexual behaviour (Dagg Citation1984; MacFarlane et al. Citation2010). In any case, it seems that the sexual climax has evolved by promoting the multiplication and intensity of sexual activity.
A mechanism of post-copulatory selection
Third, internal fertilization in the genital tract of females makes ovulation invisible, masking the potential success of fecundation. The assumption that orgasm promotes a better reproduction is arguable, especially given that orgasms are not necessary for fecundation to take place. Nevertheless, because it could promote better mate choice, it could be argued that female orgasm has evolved as a post-copulatory selection tactic by which females can increase their control of mates.
Mate preferences may be due to active female choice and male-male competition, and may be due to post-copulatory mate choice, with cryptic female choice and sperm competition. In most species using external fertilization, mating often depends on the female’s willingness to mate, while in many species using internal fertilization, male coercive behaviours were widely demonstrated. Spawning is an external gamete release method that easily allows post-copulatory selection to occur (Alonzo et al. Citation2016). Females of teleost fishes can control the numbers of eggs laid (Alonzo et al. Citation2016). They also present a ‘false orgasm’, demonstrating all the patterns of the spawning behaviour, without laying in the nest where the male spills out his sperm (Ridgway et al. Citation1989; Petersson and Jarvi Citation2001). Such misleading behaviours reveal that orgasm can be a signal used to manipulate males. As expected, the storage of sperm in the female spermatheca, as in salamanders, also facilitated post-copulatory sexual selection to occur (Houck and Schwenk Citation1984). It is difficult to determine the evolutionary response of males to such cryptic mate choice behaviours, but, over the course of evolution, it could be hypothesized that males should have developed contact behaviours such as amplexus or cloacal kiss as a response to these false orgasms delivering no or too few eggs to fertilize, leading to the evolutionary development of internal fertilization.
Male and female orgasms may originate from the evolution of fertilization modes but have hence followed different evolutionary pathways. It could be suspected that the evolutionary pathway from external to internal fertilization, which presupposes retention of ova in the female genital tract, may have led to a change that dissociates the signal from the reflex expulsion of gametes. Indeed, pre-existing traits are known to produce new effects without any apparent change affecting them, constituting what Gould and Vrba (Citation1982) called an exaptation. In species with internal fertilization, the disconnection between ovulation and the sexual signal may allow the female to defer her mate choice so that it results in a selective runaway. The drift of the sexual signal could, therefore, give the sexual climax a new meaning for its evolutionary maintenance, at least in spontaneous ovulators (see Pavlicev and Wagner Citation2016), since the female orgasmic signal is dissociated from the gamete release. Sexual selection can drive important evolutionary changes and could influence spermatozoid morphology (Rowe et al. Citation2015), development of baculum (Brindle and Opie Citation2016) or penis morphology for instance (Bertin and Fairbairn Citation2005; Mautz et al. Citation2013). Post-copulatory sexual selection occurs after successful copulation and insemination via two main processes: sperm competition and cryptic female choice (Olsson et al. Citation1996; Arnqvist Citation1998). Both of these processes require that a female have multiple mates.
Post-copulatory sexual selection promotes a rapid evolutionary diversification of sexual signals. In fact, phenotypic differences in males and females often resulted in sexually antagonistic coevolution (Rice Citation2000). Sexual conflicts over mating lead to an antagonistic coevolution, in which one sex develops a beneficial trait that is compensated by an opposite trait in the other sex. Models of coevolution by sexual selection can broadly be classified into those where the female preference is favoured indirectly and those where it is favoured directly (Chapman et al. Citation2003). Over the course of the evolutionary history of some groups, it could be argued that, when the males had intromission organs, the females began to be subjected to more coercion. So, the development of sexual conflict would have allowed females to dissociate the orgasmic signal associated with ovulation, thus promoting the possibility of post-copulatory sexual choice.
Ovarian fluids influence sperm behaviour and play an important role in sperm selection by females (Alonzo et al. Citation2016). In many species, ovarian fluids released with eggs provide gamete-recognition proteins (Vacquier Citation1998; Swanson and Vacquier Citation2002). Recent evidences show that fluids and proteins can bias fertilization in favour of preferred males in species with external fertilization (Urbach et al. Citation2005; Rosengrave et al. Citation2008; Gasparini et al. Citation2010; Alonzo et al. Citation2016) so that these fluids allow opportunities for cryptic female choice. In mammals, such as in otters, badgers or rodents, the probability of superfoetation makes possible to multiply pairings (Yamaguchi et al. Citation2006; Annavi et al. Citation2014), hence allowing the female to use orgasm as a selective choice of the partner. Furthermore, in all mammalian species, spermatozoa are stopped in the caudal isthmus before ovulation to form an oviductal sperm reservoir. The fact that ovarian fluids can influence the sperm velocity of unrelated males (Rosengrave et al. Citation2008; Gasparini and Pilastro Citation2011; Fitzpatrick and Evans Citation2014) supports the thesis that such post-copulatory mechanisms are capable of biasing fertilization by favouring unrelated males. The release of fluids involved in sexual orgasm could, therefore, reduce the cost of mating with relatives (see Fitzpatrick and Evans Citation2014). It could be inferred that, in the course of evolution, orgasm evolved to increase sexual activity and favour partner selection, as species increasingly exhibited a lower reproductive rate. Cervical stimulation is vital to successful sperm transport and fertilization (Adler Citation1969). The intensity of the sexual orgasm suggests to the partners that this sexual activity, and especially the copulation, probably ended in potential fertilization, providing the protagonists with sufficient diversion that attracts the male’s attention to wait for a putative favourable outcome.
Linking orgasm and reproduction, the female orgasm has been assumed to be dependent on factors related to male traits indicating paternal investment (Sherlock et al. Citation2016). Since orgasm is completely optional in many species, including humans, its role in choosing a better mating and avoiding inbreeding has yet to be determined. Nonetheless, in the absence of visible competition between males, it would potentially allow the female to assess the quality of the partner. It is likely that genetic elements indicating the quality of the partner could be sought in the unrelated and in the difference or complementarity of their MHC/HLA system (Bruford and Jordan Citation1998; Eklund Citation1998; Penn Citation2002). Indeed, ovarian fluids are capable to bias fertilizations for unrelated males in some fishes (Swanson and Vacquier Citation2002; Gasparini and Pilastro Citation2011) and in hens Gallus gallus, phenotypic indices help females to discriminate among unrelated males, but post-mating cryptic choice strategies are mainly based on MHC dissimilarity (Gillingham et al. Citation2009; Løvlie et al. Citation2013). In duck, Anas platyrhynchos, female sperm selection acts as a mechanism of inbreeding avoidance (Denk et al. Citation2005) and Setchell et al. (Citation2011) pointed out that the olfactory profile is related to MHC dissimilarity and pedigree relatedness in mandrill, Mandrillus sphinx, providing a potential mechanism for selective mate choice. Dissimilarities in MHC are also involved in sexual choice in numerous animals, from tunicates to salmonids for instance (Scofield et al. Citation1982; Skarstein et al. Citation2005).
One could, therefore, consider that the expression of a false orgasm in females is used as a diversion, suggesting that copulation is going well. Many male primates exhibit significant sexual coercion (Goetz et al. Citation2007; Knott et al. Citation2010). Although these are common and known strategies in many primates, post-copulatory cryptic choice and confusion of paternity are poorly documented in humans (Knott et al. Citation2010). Such diversionary behaviour could be important strategies to reduce sexual coercion. It is even possible that sperm-sensitive receptors may be involved in the oviduct or uterus and that sperm recognition proteins may allow sperm selection (Swanson and Vacquier Citation2002; Berlin et al. Citation2008).
Numerous primate females show their receptivity with phenotypic cues as excessive sexual swellings (Deschner et al. Citation2004) in contrast with concealed ovulation in humans (Schoroder Citation1993) or in langurs (Heistermann et al. Citation2001). Moderate swellings were also found in some mustelids with multiple mating, such as mink (Neovison vison, Mustela lutreola), martens (Martes foina) (Lodé Citation1991), ferret or polecat (Mustela putorius) (Lodé Citation2001) and in at least one bird species (Prunella collaris) (Nakamura Citation1998). In polecat, for instance, the strong congestion of the vulva is associated with the follicular maturation, so that ovulation is generally induced by long copulation, but spontaneous ovulation may also occur. In fact, in primates, excessive swelling is a morphological signal that indicates ovulation in females according to the graduated signal hypothesis (Nunn Citation1999). This morphological signal it is also supposed to be used to confuse belief in male paternity (Hrdy Citation1979; Pagel Citation1994). Similarly, a prolonged period of sexual receptivity, like in humans, may have evolved as a female strategy to confuse fatherhood (Heistermann et al. Citation2001). Clearly, females can manipulate male behaviour to bias paternity. In humans, women can even simulate and express fake orgasms in order to pretend to be interested in a male partner (Ellsworth and Bailey Citation2013).
The question of the function of orgasm opens up many unresolved issues, both about the role of the different fluids involved and about the diversity of sexual behaviour. Prolactin, luteinizing hormone, and oxytocin that accompany the orgasm have originally evolved in the context of viviparity and could participate in mate choice. The transition from internal fertilization to viviparity could hence be the most remarkable step in the evolution of the orgasm. Moreover, significant associations between sexual characters, intromittent organs and lineage-specific diversification rates showed that viviparity as a new reproductive life-history trait has boosted evolutionary diversification (Helmstetter et al. Citation2016).
Conclusions
Although the analysis of natural history suggests that orgasm comes from the discharge mechanisms of gametes, its current evolutionary significance in vertebrates would rather derive from post-copulatory selective mechanisms. Orgasm has its origin in the evolution of fertilization patterns, but the orgasmic signal entails a positive experience, which emphasizes the success of the relationship. The orgasm could find its current evolutionary significance in the preference of an unrelated sexual partner, in species using internal fertilization. In any case, orgasm has naturally a behavioural reinforcement effect of reproductive activities.
Acknowledgements
Thanks are due to two anonymous reviewers who improved the manuscript and to Dominique Le Jacques and Marie-Loup Lélias for their assistance in the study.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Adler NT. 1969. The effect of males’ copulatory behaviour on successful pregnancy of the female rat. J Comp Phys. 69:613.
- Adler NT, Zoloth SR. 1970. Copulatory behaviour can inhibit pregnancy in female rats? Science. 168:1480–1482. doi: https://doi.org/10.1126/science.168.3938.1480
- Alcock J. 1987. Ardent adaptation. Nat Hist. 96:4.
- Allen ML, Lemon WB. 1981. Orgasm in female primates. Am J Primatol. 1:15–34. doi: https://doi.org/10.1002/ajp.1350010104
- Alonzo SH, Stiver KA, Marsh-Rollo SE. 2016. Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat Commun. 7:2452. doi: https://doi.org/10.1038/ncomms12452
- Annavi G, Newman C, Dugdale HL, Buesching CD, Sin YW, Burke T, Macdonald DW. 2014. Neighbouring-group composition and within-group relatedness drive extra–group paternity rate in the European badger Meles meles. J Evol Biol. 27:2191–2203. doi: https://doi.org/10.1111/jeb.12473
- Arnqvist G. 1998. Comparative evidence for the evolution of genitalia by sexual selection. Nature. 393:784–786. doi: https://doi.org/10.1038/31689
- Balcombe J. 2009. Animal pleasure and its moral significance. App Anim Behav Sci. 118:212. doi: https://doi.org/10.1016/j.applanim.2009.02.012
- Ball GF, Balthazart J. 2011. Sexual arousal, is it for mammals only? Horm Behav. 59:645–655. doi: https://doi.org/10.1016/j.yhbeh.2010.11.001
- Bancroft J. 2005. The endocrinology of sexual arousal. J End. 186:411–427. doi: https://doi.org/10.1677/joe.1.06233
- Berlin S, Qu L, Ellegren H. 2008. Adaptive evolution of gamete-recognition proteins in birds. J Mol Evol. 67:488–496. doi: https://doi.org/10.1007/s00239-008-9165-6
- Berman JR, Berman L, Goldstein I. 1999. Female sexual dysfunction, incidence, pathophysiology, evaluation, and treatment options. Urology. 54:385–391. doi: https://doi.org/10.1016/S0090-4295(99)00230-7
- Berridge KC, Kringelbach ML. 2015. Pleasure systems in the brain. Neuron. 86:646–664. 101016/jneuron201502018
- Bertin A, Fairbairn DJ. 2005. One tool, many uses: precopulatory sexual selection on genital morphology in Aquarius remigis. J Evol Biol. 18:949–961. doi: https://doi.org/10.1111/j.1420-9101.2005.00913.x
- Brindle M, Opie C. 2016. Postcopulatory sexual selection influences baculum evolution in primates and carnivores. Proc R Soc B Biol Sci. 283:20161736. 101098/rspb20161736
- Brown RE. 1974. Sexual arousal, the Coolidge effect and dominance in the rat Rattus norvegicus. Anim Behav. 22:634–637. doi: https://doi.org/10.1016/S0003-3472(74)80009-6
- Bruford MW, Jordan WC. 1998. New perspectives on mate choice and the MHC. Heredity. 81:127–133. doi: https://doi.org/10.1046/j.1365-2540.1998.00428.x
- Burton F. 1971. Sexual climax in female Macaca mulatta. Proc 3rd Int Congr Primat Zurich. 3:180–191.
- Cabanac M. 1971. Physiological role of pleasure. Science. 173:1103–1107. doi: https://doi.org/10.1126/science.173.4002.1103
- Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol Evol. 18:41–47. doi: https://doi.org/10.1016/S0169-5347(02)00004-6
- Chevalier-Skolnikoff S. 1974. Male–female, female–female, and male–male sexual behavior in the stumptail monkey, with special attention to the female orgasm. Archiv Sex Behav. 3:95. 101007/BF01540994
- Cibrian-Llandera Tl, Tecamachaltzi-Silvaran M, Triana-Del DR, Pfaus JG, Manzo J, Coria-Avila GA. 2010. Clitoral stimulation modulates appetitive sexual behavior and facilitates reproduction in rats. Physiol Behav. 100:148–153. doi: https://doi.org/10.1016/j.physbeh.2010.02.015
- Cooke SJ, McKinley RS, Philipp DP. 2001. Physical activity and behavior of a centrarchid fish. Micropterus Salmoides Lacépède, during spawning. Ecol Freshwater Fish. 10:227–237. 101034/j1600–06332001100405x
- Coolen LM, Olivier B, Peters HJ, Veening JG. 1997. Demonstration of ejaculation-induced neural activity in the male rat brain using 5-HT1A agonist 8-OH-DPAT. Physiol Behav. 62:881–891. doi: https://doi.org/10.1016/S0031-9384(97)00258-8
- Crews D. 1982. On the origin of sexual behaviour. Psychoneuroendocrinology. 7:259–270. doi: https://doi.org/10.1016/0306-4530(82)90030-0
- Dagg AI. 1984. Homosexual behaviour and female-male mounting in mammals – a first survey. Mammal Rev. 14:155–185. doi: https://doi.org/10.1111/j.1365-2907.1984.tb00344.x
- Denk AG, Holzmann A, Peters A, Vermeirssen ELM, Kempenaers B. 2005. Paternity in mallards: effects of sperm quality and female sperm selection for inbreeding avoidance. Behav Ecol. 16:825. doi: https://doi.org/10.1093/beheco/ari065
- Deschner T, Heistermann M, Hodges K, Boesch C. 2004. Female sexual swelling size, timing of ovulation, and male behavior in wild West African chimpanzees. Horm Behav. 46:204–215. doi: https://doi.org/10.1016/j.yhbeh.2004.03.013
- de Waal F. 2011. Le singe en nous, Fayard/Pluriel, Paris.
- Eklund AC. 1998. Use of MHC for mate choice in wild house mice Mus domesticus. Genetica. 104:245–248. doi: https://doi.org/10.1023/A:1026417522110
- Ellsworth RM, Bailey DH. 2013. Human female orgasm as evolved signal: a test of two hypotheses. Arch Sex Behav. 42:1545–1554. doi: https://doi.org/10.1007/s10508-013-0152-7
- Erskine MS, Kornberg E, Cherry JA. 1989. Paced copulation in rats: effects of intromission frequency and duration on luteal activation and estrous length. Physiol Behav. 45:33–39. doi: https://doi.org/10.1016/0031-9384(89)90163-7
- Fitzpatrick JL, Evans JP. 2014. Post-copulatory inbreeding avoidance in guppies. J Evol Biol. 27:2585–2594. Epub 2014 Dec 4. doi: https://doi.org/10.1111/jeb.12545
- Fleischman DS. 2016. An evolutionary behaviorist perspective on orgasm. Socioaffect Neurosci Psychol. 6:32130. doi: https://doi.org/10.3402/snp.v6.32130
- Fox CA, Fox BA. 1971. A comparative study of coital physiology, with special reference to the sexual climax. J Rep Fert. 24:319–336. doi: https://doi.org/10.1530/jrf.0.0240319
- Fox CA, Wolff HS, Baker JA. 1970. Measurement of intra-vaginal and intra-uterine pressures during human coitus by radio-telemetry. J Rep Fert. 22:243–251. doi: https://doi.org/10.1530/jrf.0.0220243
- Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc Biol Sci. 278:2495–2501.doi: https://doi.org/10.1098/rspb.2010.2369
- Gasparini C, Simmons LW, Beveridge M, Evans JP. 2010. Sperm swimming velocity predicts competitive fertilization success in the green swordtail Xiphophorus helleri. PLoS One. 5:e12146. doi: https://doi.org/10.1371/journal.pone.0012146
- Gillingham MAF, Richardson DS, Løvlie H, Moynihan A, Worley K, Pizzari T. 2009. Cryptic preference for MHC-dissimilar females in male red junglefowl, Gallus Gallus. Proc R Soc B. 276:1083–1092. doi: https://doi.org/10.1098/rspb.2008.1549
- Goetz AT, Shackelford TK, Platek SM, Starratt VG, McKibbin WF. 2007. Sperm competition in humans: implications for male sexual psychology, physiology, anatomy, and behaviour. Ann Rev Sex Res. 18(1).
- Gould SJ. 1987. Freudian slip. Nat Hist. 96:14–21.
- Gould SJ, Vrba ES. 1982. Exaptation – a missing term in the science of form. Paleobiol. 8:4–15. doi: https://doi.org/10.1017/S0094837300004310
- Gross MR, Charnov EL. 1980. Alternative male life histories in bluegill sunfish. Proc Natl Acad Sci. 77:6937–6940. doi: https://doi.org/10.1073/pnas.77.11.6937
- Grueter CC, Stoinski TS. 2016. Homosexual behavior in female mountain gorillas: reflection of dominance, affiliation, reconciliation or arousal? PLoS ONE. 11(5):e0154185. doi:https://doi.org/10.1371/journal.pone.0154185.
- Haake P, Schedlowski M, Exton MS, Giepen C, Hartmann U, Osterheider M, Flesch M, Janssen OE, Leygraf N, Krüger THC. 2003. Acute neuroendocrine response to sexual stimulation in sexual offenders. Can J Psychol. 48:265–271.
- Health D. 1984. An investigation into the origins of a copious vaginal discharge during intercourse, ‘enough to wet the bed’ that ‘is not urine’. J Sex Res. 20:194–210. doi: https://doi.org/10.1080/00224498409551217
- Heeb MM, Yahr P. 1996. . c-Fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience. 72:1049–1071. [PubMed: 8735229]. doi: https://doi.org/10.1016/0306-4522(95)00602-8
- Heistermann M, Ziegler T, van Schaik CP, Launhardt K, Winkler P, Hodges JK. 2001. Loss of oestrus, concealed ovulation and paternity confusion in free-ranging Hanuman langurs. Proc Biol Sci R Soc B. 268:2445–2451. doi: https://doi.org/10.1098/rspb.2001.1833
- Helmstetter AJ, Papadopulos AS, Igea J, Van Dooren TJ, Leroi AM, Savolainen V. 2016. Viviparity stimulates diversification in an order of fish. Nat Commun. 7:11271. doi:https://doi.org/10.1038/ncomms11271.
- Houck LD, Schwenk H. 1984. The potential for long-term sperm competition in a Plethodontid salamander. Herpetologica. 40:410–415. wwwjstororg/stable/3892093.
- Hrdy SB. 1979. Infanticide among animals, a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol. 1:13–40. doi: https://doi.org/10.1016/0162-3095(79)90004-9
- Hrdy SB. 1996. The evolution of female orgasms: logic please but no atavism. Anim Behav. 52:851–852. doi: https://doi.org/10.1006/anbe.1996.0230
- Huyunh HK, Willemsen ATM, Holstege G. 2013. Female orgasm but not male ejaculation activates the pituitary A PET neuro-imaging study. NeuroImage. 76:178–182. doi: https://doi.org/10.1016/j.neuroimage.2013.03.012
- Kalinka AT. 2015. How did viviparity originate and evolve? Of conflict, co-option, and cryptic choice. BioEssays. 37:721–731. doi: https://doi.org/10.1002/bies.201400200
- King R, Dempsey M, Valentine KA. 2016. Measuring sperm backflow following female orgasm: a new method. Socioaffect Neurosci Psychol. 1927. 103402/snpv631927.
- Knott CD, Thompson ME, Stumpf RM, McIntyre MH. 2010. Female reproductive strategies in orangutans, evidence for female choice and counterstrategies to infanticide in a species with frequent sexual coercion. Proc Roy Soc B: Biol Sci. 277:105–113. doi: https://doi.org/10.1098/rspb.2009.1552
- Kollack-Walker S, Newman SW. 1997. Mating-induced expression of c-fos in the male Syrian hamster brain: role of experience, pheromones, and ejaculations. J Neurobiol. 1997(32):481–501. [PubMed: 9110260]. doi: https://doi.org/10.1002/(SICI)1097-4695(199705)32:5<481::AID-NEU4>3.0.CO;2-1
- Komisaruk BR, Whipple B, Crawford A, Grimes S, Liu WC, Kalnin A, Mosier C. 2004. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 1024:77–88. doi: https://doi.org/10.1016/j.brainres.2004.07.029
- Korda JB, Goldstein SW, Sommer F. 2010. Sexual medicine history: the history of female ejaculation. J Sex Med. 7:1965–1975. doi: https://doi.org/10.1111/j.1743-6109.2010.01720.x
- Krüger TH, Haake P, Hartmann U, Schedlowski M, Exton MS. 2002. Orgasm-induced prolactin secretion: feedback control of sexual drive? Neurosci Biobehav Rev. 26:31–44. PMID, 11835982. doi: https://doi.org/10.1016/S0149-7634(01)00036-7
- Lester GL, Gorzalka BB. 1988. Effect of novel and familiar mating partners on the duration of sexual receptivity in the female hamster. Behav Neural Biol. 49:398–405. PMID 3408-3449 doi: https://doi.org/10.1016/S0163-1047(88)90418-9
- Levin R. 2002. The physiology of sexual arousal in the human female, a recreational and pro-creational synthesis. Arch Sex Behav. 31:405–411. doi: https://doi.org/10.1023/A:1019836007416
- Levin RJ. 2011. Can the controversy about the putative role of the human female orgasm in sperm transport be settled with our current physiological knowledge of coitus? J Sex Med. 8:1566–1578. doi: https://doi.org/10.1111/j.1743-6109.2010.02162.x
- Lodé T. 1991. Conspecific recognition and mating in stone marten Martes foina. Acta Theriol. 36:275–283. doi: https://doi.org/10.4098/AT.arch.91-28
- Lodé T. 2001. Mating system and genetic variance in a polygynous mustelid, the European polecat. Genes Gen Syst. 76:221–227. doi: https://doi.org/10.1266/ggs.76.221
- Lodé T. 2011. Sex is not a solution for reproduction: the libertine bubble theory. BioEssays. 33:419–422. doi: https://doi.org/10.1002/bies.201000125
- Lodé T. 2012a. Have sex or not? Lessons from bacteria. Sex Dev. 6:325–328. doi: https://doi.org/10.1159/000342879
- Lodé T. 2012b. Oviparity or viviparity? That is the question. Reprod Biol. 12:259–264. doi: https://doi.org/10.1016/j.repbio.2012.09.001
- Lodé T. 2013. Adaptive significance and long-term survival of asexual lineages. Evol Biol. 40:450–460. doi: https://doi.org/10.1007/s11692-012-9219-y
- Loonen AJM, Ivanova SA. 2015. Circuits regulating pleasure and happiness: the evolution of reward-seeking and misery-fleeing behavioral mechanisms in vertebrates. Front Neurosci. 9:394. doi: https://doi.org/10.3389/fnins.2015.00394
- Løvlie H, Gillingham MA, Worley K, Pizzari T, Richardson DS. 2013. Cryptic female choice favours sperm from major histocompatibility complex-dissimilar males. Proc R Soc B Biol Sci. 280:1296. doi: https://doi.org/10.1098/rspb.2013.1296
- MacFarlane GR, Blomberg SP, Vasey PL. 2010. Homosexual behaviour in birds: frequency of expression is related to parental care disparity between the sexes. Anim Behav. 80:375–390. doi: https://doi.org/10.1016/j.anbehav.2010.05.009
- Masters WH, Johnson VE. 1966. Human sexual response. Boston: Little Brown.
- Mautz BS, Wong BB, Peters RA, Jennions MD. 2013. Penis size interacts with body shape and height to influence male attractiveness. Proc Natl Acad Sci. 110:6925–6930. doi: https://doi.org/10.1073/pnas.1219361110
- Meston CM, Levin RJ, Sipski ML, Hull EM, Heiman JR. 2004. Women’s orgasm. Annu Rev Sex Res. 15:173–257.
- Moalem S, Reidenberg JS. 2009. Does female ejaculation serve an antimicrobial purpose? Med Hyp. 73:1069–1071. doi: https://doi.org/10.1016/j.mehy.2009.07.024
- Murai T. 2006. Mating behaviors of the proboscis monkey (Nasalis larvatus). Am J Primatol. 68:832–837. PMID: 16847976. doi: https://doi.org/10.1002/ajp.20266
- Nakamura M. 1998. Multiple mating and cooperative breeding in polygynandrous alpine accentors. I. Competition among females. Anim Behav. 55:259–275. doi: https://doi.org/10.1006/anbe.1997.0725
- Nunn CL. 1999. The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav. 58:229–246. doi: https://doi.org/10.1006/anbe.1999.1159
- O’Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Compar Neurol. 519:3599–3639. doi: https://doi.org/10.1002/cne.22735
- Olsson M, Shine R, Madsen T, Gullberg A, Tegelstrom H. 1996. Sperm selection by females. Nature. 383:585. doi: https://doi.org/10.1038/383585a0
- Pagel M. 1994. The evolution of conspicuous oestrous advertisement in old world monkeys. Anim Behav. 47:1333–1341. doi: https://doi.org/10.1006/anbe.1994.1181
- Pavlicev M, Wagner G. 2016. The evolutionary origin of female orgasm. J Exp Zool B Mol Dev Evol. 326B:326–337. doi: https://doi.org/10.1002/jez.b.22690
- Penn PJ. 2002. The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology. 108:1–21. doi: https://doi.org/10.1046/j.1439-0310.2002.00768.x
- Petersson E, Jarvi T. 2001. ‘False orgasm’ in female brown trout: trick or treat? Anim Behav. 61:497–501. doi: https://doi.org/10.1006/anbe.2000.1585
- Pfaus JG, Scardochio T, Parada M, Gerson C, Quintana GR, Coria-Avila GA. 2016. Do rats have orgasms? Socioaffect Neurosci Psychol. 6:3402. 103402/snpv631883.
- Prause N. 2011. The human female orgasm: critical evaluations of proposed psychological sequelae. Sex Relat Ther. 26:315–328. doi: https://doi.org/10.1080/14681994.2011.651452
- Rice WR. 2000. Dangerous liaisons. PNAS. 97:12953–12955. doi: https://doi.org/10.1073/pnas.97.24.12953
- Ridgway MS, Goff GP, Keenleyside HA. 1989. Courtship and spawning behavior in smallmouth bass (Micropterus dolomieui). Am Midl Nat. 122:209–213. doi: https://doi.org/10.2307/2425905
- Rosengrave P, Taylor H, Montgomerie R, Metcalf V, McBride K, Gemmell N. 2008. Chemical composition of seminal and ovarian fluids of Chinook salmon Oncorhynchus tshawytscha and their effects on sperm motility traits. Comp Bioch Phys A: Mol Int Phys. 152:123–129. doi: https://doi.org/10.1016/j.cbpa.2008.09.009
- Rowe M, Albrecht T, Cramer ER, Johnsen A, Laskemoen T, Weir TJ, Lifjeld JT. 2015. Postcopulatory sexual selection is associated with accelerated evolution of sperm morphology. Evolution. 69:1044–1052. Epub2015 Mar 21 doi: https://doi.org/10.1111/evo.12620
- Santos FCA, Taboga SR. 2006. Female prostate: a review about the biological repercussions of this gland in humans and rodents. Anim Rep. 3:3–18.
- Schoroder I. 1993. Concealed ovulation and clandestine copulation, a female contribution to human evolution. Ethol Sociobiol. 14:381–389. doi: https://doi.org/10.1016/0162-3095(93)90026-E
- Scofield VL, Schlumpberger JM, West LA, Weissman IL. 1982. Protochordate allorecognition is controlled by a MHC-like gene system. Nature. 295:499–502. 101038/295499a0
- Setchell JM, Vaglio S, Abbott KM, Moggi-Cecchi J, Boscaro F, Pieraccini G, Knapp LA. 2011. Odour signals major histocompatibility complex genotype in an old world monkey. Proc Roy Soc B: Biol Sci. 278:274–280. doi: https://doi.org/10.1098/rspb.2010.0571
- Sherlock JM, Sidari MJ, Harris EA, Barlow FK, Zietsch BP. 2016. Testing the mate-choice hypothesis of the female orgasm: disentangling traits and behaviours. Socioaffect Neurosci Psychol. 6:31562. doi: https://doi.org/10.3402/snp.v6.31562
- Skarstein F, Folstad I, Liljedal S, Grahn M. 2005. MHC and fertilization success in the Arctic charr Salvelinus alpinus. Behav Ecol Sociobiol. 57:374–380. doi: https://doi.org/10.1007/s00265-004-0860-z
- Swanson WJ, Vacquier VD. 2002. The rapid evolution of reproductive proteins. Nat Rev Gen. 3:137–144. doi: https://doi.org/10.1038/nrg733
- Symons D. 1979. The evolution of human sexuality. Oxford: Oxford University Press.
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. 2009. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 55:93–97. doi: https://doi.org/10.1016/j.yhbeh.2008.08.012
- Tepper SL, Jagirdar J, Heath D, Geller SA. 1984. Homology between the female paraurethral Skene’s glands and the prostate immunohistochemical demonstration. Arch Pathol Lab Med. 108:423–425.
- Thornhill R, Gangestad SX, Comer R. 1995. Human female orgasm and mate fluctuating asymmetry. Anim Behav. 50:1601–1615. doi: https://doi.org/10.1016/0003-3472(95)80014-X
- Troisi A, Carosi M. 1998. Female orgasm rate increases with male dominance in Japanese macaques. Anim Behav. 56:1261–1266. doi: https://doi.org/10.1006/anbe.1998.0898
- Urbach D, Folstad I, Rudolfsen G. 2005. Effects of ovarian fluid on sperm velocity in Arctic charr Salvelinus alpinus. Behav Ecol Sociobiol. 57:438–444. doi: https://doi.org/10.1007/s00265-004-0876-4
- Vacquier VD. 1998. Evolution of gamete recognition proteins. Science. 281(281):1995–1998. doi: https://doi.org/10.1126/science.281.5385.1995
- Wallen K, Lloyd E. 2008. Clitoral variability compared with penile variability supports non-adaptation of female orgasm. Evol Dev. 10:1e2.
- Wheatley JR, Puts DA. 2015. Evolutionary science of female orgasm. In: The evolution of sexuality, 123–148. Springer. Retrieved from http//linkspringercom/chapter/101007/978–3–319–09384–0-7
- Wildt L, Kissler S, Licht P, Becker B. 1998. Sperm transport in the human female genital tract and its modulation by oxytocin as assessed by hysterosalpingoscintigraphy, hysterotonography, electrohysterography and Doppler sonography. Hum Reprod Update. 4:655–666. doi: https://doi.org/10.1093/humupd/4.5.655
- Winterbottom M, Burke T, Birkhead TR. 1999. A stimulatory phalloid organ in a weaver bird. Nature. 399:28. doi: https://doi.org/10.1038/19884
- Yamaguchi N, Dugdale HL, Macdonald DW. 2006. Female receptivity, embryonic diapause, and superfetation in the European badger Meles meles, implications for the reproductive tactics of males and females. Quat Rev Biol. 81:33–48. doi: https://doi.org/10.1086/503923
- Yamamoto K, Vernier P. 2011. The evolution of dopamine systems in chordates. Front Neuroanat. 5:21. doi: https://doi.org/10.3389/fnana.2011.00021
- Zer-Krispil S, Zak H, Shao L, Ben-Shaanan S, Tordjman L, Bentzur A, Shmueli A, Shohat-Ophir G. 2018. Ejaculation induced by the activation of Crz neurons is rewarding to Drosophila males. Curr Biol. 28:1445–1452.e3. doi: https://doi.org/10.1016/j.cub.2018.03.039
- Zietsch BP, Santtila P. 2013. No direct relationship between human female orgasm rate and number of offspring. Anim Behav. 86:253–255. doi: https://doi.org/10.1016/j.anbehav.2013.05.011
- Zumpe D, Michael RP. 1968. The clutching reaction and orgasm in the female rhesus monkey (Macaca mulatta). J End. 40:117. doi: https://doi.org/10.1677/joe.0.0400117
