Abstract
Objective
To investigate the difference in the characteristics between patients with emphysematous pancreatitis (EP) who survived and those who died.
Methods
PubMed search was performed to gather EP cases from March 1959 to February 2019. Forty-two articles with 58 EP cases were identified and met the study’s inclusion criteria. The elderly were defined as individuals aged >65 years. Data on patients’ demographics, clinical symptoms, laboratory results, treatments, outcomes, and mortality were collected and analyzed by chi-square test and Student’s t-test. p-Value <.05 (2-tailed) was set as the significance level.
Results
Forty-seven men and eleven women aged 61.3 ± 15.9 (mean ± standard deviation) years were included. The elderly accounted for 43.1% (n = 25) of cases. There were 20 mortality cases, and 38 cases survived, with an overall mortality rate of 34.5%. Sex, underlying diseases, etiologies, and laboratory results were not significantly related to mortality. Older age was significantly related to mortality (p = .001). The shock was more commonly seen in the mortality group (100%) than in the survival group (21%) (p < .001). In contrast, fever was less frequent in the mortality group than in the survival group (25 vs. 71%, p = .002).
Conclusions
EP patients have a high mortality rate (34.5%). Older age, afebrile status, and presence of shock are associated with high mortality. To improve the survival of this aggressive group, a further prospective investigation involving a larger sample size is necessary.
Introduction
Acute pancreatitis (AP) is a commonly seen emergency in daily clinical practice. It has a wide range of severity degrees. The majority of AP patients experienced a benign course, but approximately 5–10% of AP patients developed acute necrotizing pancreatitis (ANP), with morbidity and mortality risk increased in patients with organ failure and infectious complicationsCitation1.
Gallstones and alcohol consumption are the main and important causes of APCitation2. Other important causes include hypertriglyceridemia, post-endoscopic retrograde cholangiopancreatography (ERCP), medications, genetic causes, and pancreatic duct injuryCitation3–7. Some rare causes of AP include biliary sludge and microlithiasis, hypercalcemia, infections, biliary obstruction, biliary cysts, and idiopathic factorsCitation8–11.
Infected pancreatic necrosis (IPN) may occur in as high as 40% of patients with ANPCitation12. The mortality rate of IPN was reported to be 9.8%-47% in combination with early severe pancreatitis, despite advanced modern intensive careCitation13–16. Emphysematous pancreatitis (EP), characterized by gas appearing within or around the pancreatic necrosis, is an uncommon variant of ANP. Gas-forming bacteria may reach the pancreatic bed by the bloodstream, lymphatic channel, fistula from the nearby bowel, translocation from the transverse colon, or reflux via opening of the ampulla of VaterCitation17. Although the presence of air itself does not definitely represent infection, EP was previously regarded as a fulminant type of IPN. Several case reports mentioned the potentially high mortality of EP despite aggressive surgical interventions, and there were also cases successfully treated with antibiotics aloneCitation18–22. We aimed to identify the risk factors of mortality in EP by retrospectively collecting EP cases through a PubMed database search, and to investigate the difference in characteristics between EP patients who survived and those who died.
Materials and methods
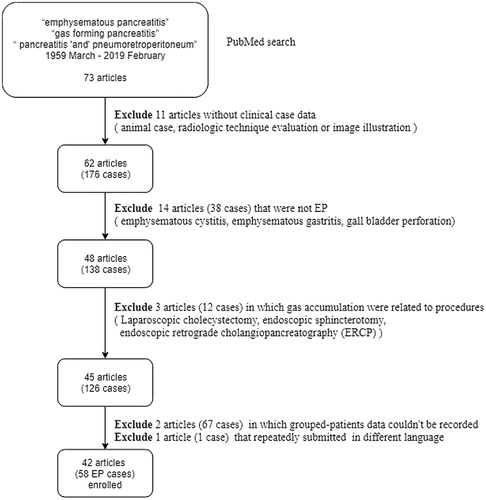
A retrospective cohort study was designed to investigate the risk factors of mortality in patients with EP. By searching the PubMed database using the keywords “emphysematous pancreatitis”, “gas-forming pancreatitis”, and “pancreatitis, pneumo-retroperitoneum”, 73 articles were found primarily from March 1959 to February 2019.
According to the inclusion and exclusion criteria shown in , 42 articles with 58 EP cases were included. We divided the patients into the following groups: survival group (n = 38) and mortality group (n = 20). Data of patients, including age, sex, history of comorbidities, etiologies, symptoms (abdominal pain, vomiting, etc.), clinical findings, laboratory results, culture test results from the pancreatic fluid, treatment procedures, outcomes, and mortality or survival, were recorded. Fever was defined as body temperature of ≥38 °C, and shock as presenting systolic blood pressure (SBP) <90 mmHg or diastolic blood pressure (DBP) <60 mmHg, which requires inotropic agent administration or intensive care. The elderly were defined as patients aged >65 years. For patients with missing data (n = 4) on lipase level, the mean lipase level was used in the analysis.
Comparative analyses of the characteristics were performed between the survival and mortality groups. Relative risk factors for mortality were also investigated. Categorical variables were analyzed by using the chi-square test, whereas continuous variables were analyzed by using Student’s t-test. The significance level was set at p-value <.05 (2-tailed). Data were analyzed using IBM SPSS 20 (IBM Corporation, Armonk, NY) statistical software.
Results
General description of the selected cases
We included a total of 58 cases, with 13 cases from India, 12 cases from Spain, 6 cases from the United States of America, 5 cases from Canada, 4 cases from Germany, 3 cases from England, 3 cases from Japan, 2 cases from Korea, and 10 individual cases from Australia, France, Italy, Netherlands, Iceland, Luxembourg, Portugal, Romania, Israel, and Singapore. Of them, one case each occurred in 1959, 1993, 1995, 2001, 2005–2008, 2012, 2015, 2016, and 2018–2019; two cases in 1996 and 2003–2004; three cases in 1986, 2010, and 2014; four cases in 2011; six cases in 2017; nine cases in 2009; and 11 cases in 2013.
The general characteristics of the included cases are shown in . The numbers of treatment with drainage in a specific year were as follows: three in 2010, two in 2013, and one in 1995, 2003, 2011, 2014, 2015, and 2017. The numbers of treatment with surgical interventions were as follows: four in 2017, three in 1986, 2009, and 2013, two in 1996, 2003, and 2004, and one in 1959, 1995, 2001, 2005, 2006, 2008, 2010, 2011, 2012, and 2014).
Table 1. General data of 58 emphysematous pancreatitis cases.
Characteristics of the survival and mortality groups
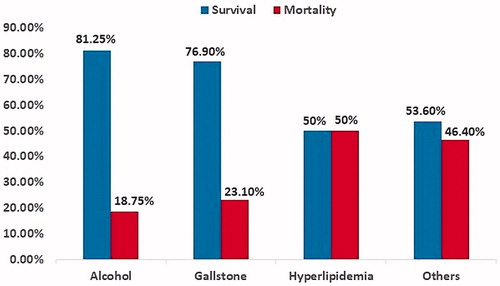
There were 20 and 38 cases in the mortality and survival groups. The comparisons between the survival and mortality groups are shown in . EP is commonly seen in male patients. Older age was significantly related to mortality (p = .001), with age of 70.1 ± 12.2 (mean ± standard deviation (SD)) years in the mortality group and 56.6 ± 15.7 years in the survival group. The shock was significantly more common in the mortality group (100%) than in the survival group (21%) (p < .001). In contrast, fever was significantly less frequent in the mortality group than in the survival group (25 vs. 71%, p = .002). Length of stay (LOS) was significantly shorter in the mortality group (12.3 ± 22.1 days) than in the survival group (30.8 ± 25.1 days) (p = .006). Sex, co-morbidities (DM), etiologies (), laboratory tests [white blood cell (WBC) count, amylase, lipase], and methods of treatment (drainage, surgery) were not statistically significant between the survival and mortality groups.
Table 2. Comparison of survival and mortality cases of emphysematous pancreatitis.
Bacteriology statistics
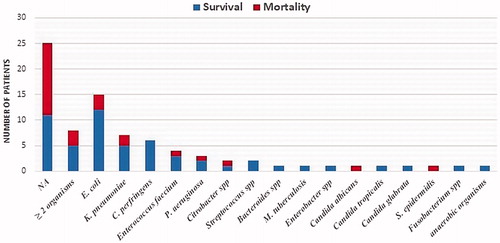
Positive results of the pancreatic fluid culture test were found in 33 of the 58 cases (56.9%), with 27 cases (71.1%) from the survival group and 6 cases (30%) from the mortality group. Culture results were not available in 25 cases, and multi-bacterial growth was found in 8 cases. The identified pathogens in the pancreatic fluid are shown in . The five most common micro-organisms were Escherichia coli, Klebsiella pneumoniae, Clostridium perfringens, Enterococcus faecium, and Pseudomonas aeruginosa.
Discussion
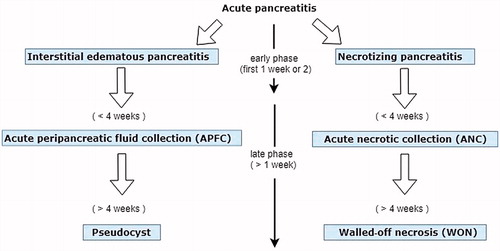
AP can be classified as IEP and NP. The revised Atlanta classification in 2012 modernized the terminology of its natural course, severity, and systemic and local complications, including pancreatic fluid accumulationCitation1. The dynamic process of disease progression and local complication is shown in . The early phase (usually within the first 1–2 weeks) is manifested by systemic inflammatory response syndrome (SIRS) and compensatory anti-inflammatory response syndrome (CARS) predisposing the patient to transient (<48 h) or persistent organ failure and risk of infection. In the late phase, ongoing systemic inflammation and persistent/transient organ failure last for weeks to months, with progression of local complication and attack of subsequent infection. Local complications include acute pancreatic fluid collection (APFC), pseudocyst, acute necrotic collection (ANC), and walled-off necrosis (WON). ANP develops in approximately 5–10% of patients with AP, which was correlated with an increased risk of infection, eventful outcome, and high mortalityCitation1.
IPN is the most serious complication of ANP, and with concomitant organ failure, it carries a higher risk for mortality than sterile necrosis (35.2 vs. 19.8%)Citation23. EP, although rare and not specially sorted, was categorized as an IPN owing to the presence of gas bubbles, usually representing a pathognomonic sign of infection of the necrosisCitation1. The most common cause of gas inside or around the pancreas is bacterial infection, although there are other causes such as colonic fistula, enterocutaneous fistula, perforated duodenal ulcer, duodenal diverticulum, or air reflux from duodenal instrumentationCitation17. Infectious pathogens usually reach the pancreas through the bloodstream, lymphatic channel, fistula, translocation from transverse colon, or reflux via opening of the ampulla of VaterCitation17,Citation24. EP was traditionally considered to be a fulminant type of IPN and related to poor outcome, and surgical treatment was believed to be standard for this entityCitation25. Given the step-up approach and minimal invasive strategies used in IPN treatment, evolution of EP treatment had become more applicable in the last 10 years. The actual incidence and mortality of EP were not well known owing to the limited researches focused on EP, with most of them being single striking case reports and few being case series. In 57 patients who underwent necrosectomy for IPN, EP was found in 19.3% (11/57) of cases, with mortality rate of 36.4% (4/11)Citation25. In that study, surgical intervention was applied to all patients with EP. Local complications were higher in the EP group than in the non-emphysematous-IPN group (p = .049), but there was no significant difference in mortality between the two groups (p = .739), owing to prompt recognition of EP, intensive care strategy, and timely surgical interventionCitation25. Successful conservative treatment was demonstrated in another study that analyzed nine initially stable EP cases out of 242 pancreatitis patients from January 2007 to December 2009Citation19. Fine needle aspiration for culture and antibiotics were adopted for all nine patients. Two patients required additional percutaneous catheter drainage. Surgical necrosectomy was performed in only one patient with worsening organ failure. Only one patient requiring surgery died, with a mortality rate of 11% (1/9)Citation19. In a retrospective study conducted between April 2003 and March 2011, EP accounted for 17% (56/327) of necrotizing pancreatitis casesCitation26. The prevalence of organ failure in EP was 66.1% (37/56). Medical treatment had a good response in selected stable patients (20/56), and surgical necrosectomy was preserved for the other 36 patients with persistent decompensated organ failure. The overall mortality rate was 10.7% (6/56). Morbidity was higher in the surgical treatment group, but no significant difference in mortality was found between the medical (0%) and surgical treatment groups (16.6%, 6/36) (p = .06)Citation26.
Epidemiology
Advancing age and number of chronic comorbid conditions were reported to be strong predictors of early death among patients with first-time acute pancreatitisCitation27. Severe pancreatitis more frequently resulted in death in the oldest patients (≥80 years) (11.97%, p = .00) than in the patients aged 65–79 years (4.59%, p = .10) and younger patients (<65 years) (2.31%)Citation28. Among the rare studies on EP involving a larger series, age was not focused on and the number of elderly patients was small; thus, the influence of age on mortality was difficult to investigateCitation18,Citation19,Citation25,Citation26. Our study enrolled nearly-equivalent elderly and non-elderly patients (43.1 vs. 56.9%) and revealed the relationship between age and mortality in EP (p = .001), with age of 70.1 ± 12.2 years (mean ± SD) in the mortality group and 56.6 ± 15.7-years-old in the survival group. A similar result was also noted in another study, with age of 66.9 ± 13.7 years in EP patients who died and 57.1 ± 16.9 years in those who survived (p = .019)Citation29. However, age was not an independent predictor of severity or mortality in the multivariable analysisCitation18,Citation19. Male predominance was consistent with other researches, but no statistical significance was found between sex and mortalityCitation19,Citation25,Citation26.
Comorbidities
In our study, no comorbid disease was correlated with mortality in EP, as shown in and . There was no case with preexisting end-stage renal disease (ESRD) in this cohort. In an analysis of Scottish healthcare database enrolling 2053 cases of AP, only 7 patients (0.3%) were previously on dialysis. Preexisting dialysis did not predict the progression of AP severityCitation30. In contrast, the incidence of AP in ESRD patients on dialysis was reported at 5.17 per 1000 person-years, with an overall mortality of 8.1%. The risk factors for mortality after an AP attack in patients on dialysis were male sex, advanced age, AP severity, and presence of diabetes mellitus or liver diseaseCitation31. The presence of uremia was seldom mentioned in the literature of EP and IPN. Thus, the relationship between uremia and mortality of EP could not be determined.
The prevalence of DM history in EP patients was 24.1% in our study, which was a little higher than that in AP patients reported previously (17.7–19.4%)Citation30,Citation32. However, no correlation was found in having DM with increased mortality in EP, similar to the previous report on AP. However, the mortality of AP patients with diabetes did not significantly differ or was even lower than that of AP patients without diabetesCitation30,Citation32,Citation33.
Etiologies
In our study, EP in most patients was due to idiopathic etiologies (46.6%), especially in the mortality group (65%). Among the others with known etiology, alcohol consumption (25.9%) and gallstone (20.7%) were still the leading two reasons. Hyperlipidemia, although also a frequent etiology of AP or IPN, was rarely found in our EP patients, specifically in only two EP patients (3.4%), with one patient who survived and the other who died. There is no significant statistical difference between etiology and mortality in EP patients.
Symptoms
Epigastralgia and vomiting
All EP patients in our study had epigastralgia. Vomiting was present in 48.3% of EP patients (28/58), with both survival and mortality groups having comparable rates (50 vs. 45%), without a significant difference (p = .787).
Fever
In a study of 75 patients with AP between 1997 and 1998, Bohidar et al. demonstrated that fever was observed in 60% of all patients. Fever was frequently observed in those with organ failure or with higher Balthazar CT severity scores (83% in those scoring >7 vs. 52% in those scoring < 7). However, only 18% (8/45) of the patients with fever was caused by IPN. The proportion of AP patients with fever that was not caused by infection of the pancreas was 82%, that by pancreatic inflammation per se was 22%, and that by non-pancreatic infection was 60%. The mortality rate, although not statistically significant, was higher in patients who developed fever than in those who did not (7/45 vs. 1/30, p = .09). In 8 patients with IPN, all had fever and 3 patients (37.5%) diedCitation34. In our study, 55.2% of all EP patients presented with fever, which was much lower compared to that (100%) in IPN patients with fever previously reported. Moreover, among the EP patients, fever was surprisingly less frequent in the mortality group than in the survival group (25 vs. 71%, p = .002). In EP, pancreatic infection is usually common, and fever is thought to be caused not only by SIRS but also by infection and even sepsis. Inability to present fever implies disrupted thermoregulatory response, impaired immunity, or underlying defective inflammatory response due to pathogen- or host-specific factorsCitation35. Higher mortality and morbidity were reported in afebrile bacteremia patients than in those with feverCitation35,Citation36. Yo et al. surveyed the risk and outcome of afebrile bacteremia patients and found that afebrile patients were older than febrile patients (p = .007). Older age (age ≥ 85), nonhematologic malignancy, necrotizing fascitis, spontaneous bacterial peritonitis, and pneumonia were positive independent predictors of afebrile bacteremia, excluding pancreatitis, whereas E. coli infection, the most common pathogen of EP in our study, was found to be an independent negative predictor of afebrile bacteremia. Underlying comorbidities, including diabetes mellitus, ESRD, liver cirrhosis, human immunodeficiency virus infection, and hematologic malignancy were not significantly different between patients with afebrile and febrile bacteremiaCitation36. Nadkarni et al. showed a successful outcome with conservative management of 9 EP patients. All patients were not elderly, without initial shock, and were febrileCitation19. Kvinlaug et al. also demonstrated 5 EP patients with successful non-operative treatment; of these, 2 patients were elderly, and all 5 patients had feverCitation18. Thus, the relationship between absence of fever and increased mortality in EP patients suggested that afebrile EP may be a severe form of IPN. Moreover, the afebrile status in EP may be attributed to impaired immunity and inflammatory response rather than to old age, comorbidities, or microbial pathogens.
Shock
Theoretically, in the early phase of AP, pancreatic necrosis and peripancreatic fluid are sterile. Primary organ failure in the early phase of AP is mostly due to severe SIRS and increases the risk of subsequent IPNCitation37. Immunosuppression, decreased anti-inflammatory response, and subsequent infection occur and result in secondary organ failure in the late phaseCitation37,Citation38. Mortality in patients with primary organ failure plus IPN was 49.5 vs. 36% in those with IPN and secondary organ failure (p = .06) and 4% in those with IPN but without organ failure (p < .001)Citation37. Given that many studies have reported a low mortality rate, it is reasonable to follow the current consensus in treating IPNCitation18,Citation25,Citation26. However, in the mortality group of our study cohort, as high as 60% (12/20) of the patients experienced early shock and died within the first 2 days of admission, in which emergent surgical necrosectomy was almost the first choice and some even died before any intervention was attempted. This implies that an aggressive and fulminant subtype of EP, with concurrent severe SIRS and IPN-related septic shock in the early phase, might existCitation39. For these EP patients in the most severe condition, with the limited timeframe for decision making, the most appropriate approach of intervention was not extensively studied. More investigations and trials enrolling larger databases may be required to improve the survival for these EP patients with the worst outcomes.
Outcome
In those researches demonstrating a low mortality rate of EP (0–15.6%), most of the included patients were relatively stable or more likely to follow the “traditional” disease course of IPNCitation16,Citation18,Citation19,Citation26. On the contrary, the high overall mortality rate reported, ranging from 32.8 to 36.4% that was comparable to the 34.5% obtained in our study, probably attributed to the participants with the “fulminant EP” courseCitation25,Citation29. Reported factors associated with mortality were old age, hypotension, gas outside the pancreas on computed tomography, initial surgical evacuation, lack of initial percutaneous drainage, and development of multiorgan failureCitation29.
The length of stay is 24.4 days in the overall cohort, but it was significantly shorter in the mortality group than in the survival group (12.3 vs. 30.8 days, p = .006), owing to the high number of fulminant EP cases with extra-fast mortality.
The study has several limitations. First, the sample size is relatively small in our study, owing to the rareness and limited studies obtained in the literature search of EP. EP patients with the benign course may not be reported, and the published articles overemphasized on the fulminant cases. Two articles were studies with a relatively high number of enrolled patients, but they were not unfortunately included in the analysis. Second, many enrolled articles were brief case reports or small case series with incomplete records and course mentioned, which resulted in many missing data and statistical bias. By using the mean value, we tried our best to overcome the interference from the missing values and to demonstrate the impact on clinical care of patients with EP.
Conclusions
EP carries a high mortality rate of up to 34.5%. Preexisting DM poses no significant risk to mortality in EP, although this is more commonly found in EP than in the overall AP patients. Following the trend of the step-up approach in treating IPN, minimally invasive procedures are increasingly adopted prior to open surgery. In our study of EP patients, older age, afebrile status, and presence of shock are associated with higher mortality. Although surgical interventions were performed more often in the mortality group than in the survival group, surgery did not result in a significant risk to mortality in EP patients. Despite improved outcome in the selected groups of patients, there is little consensus on the management of patients with fulminant type of EP. To improve the survival of this aggressive group, further prospective investigation involving a larger sample size is warranted.
Transparency
Declaration of funding
There is no funding to disclose for this article.
Declaration of financial/other interests
The authors declare that they have no conflicts of interest.
Author contributions
Su YJ was in charge of the study design, data analysis, research team discussion, and correspondence. Chou CY wrote the draft, drew the figures in the study, and assisted in data search and collection. Yang HW assisted in data search, collection, integration, and processing. Chang CW was responsible for the statistical analyses.
Previous presentations
This work was presented as a poster in the annual meeting of the Taiwan Society of Emergency Medicine, 2019.
Acknowledgements
We are grateful to the Taylor & Francis Editing Services.
References
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111.
- Zilio MB, Eyff TF, Azeredo-Da-Silva ALF, et al. A systematic review and meta-analysis of the aetiology of acute pancreatitis. HPB (Oxford). 2019;21(3):259–267.
- Rawla P, Sunkara T, Thandra KC, et al. Hypertriglyceridemia-induced pancreatitis: updated review of current treatment and preventive strategies. Clin J Gastroenterol. 2018;11(6):441–448.
- Kahaleh M, Freeman M. Prevention and management of post-endoscopic retrograde cholangiopancreatography complications. Clin Endosc. 2012;45(3):305–312.
- Rawla P, Raj JP. Doxycycline-induced acute pancreatitis: a rare adverse event. Gastroenterol Res. 2017;10(4):244–246.
- Rosendahl J, Witt H, Szmola R, et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet. 2008;40(1):78–82.
- Gerson LB, Tokar J, Chiorean M, et al. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7(11):1177–1182.
- Ko CW, Sekijima JH, Lee SP. Biliary sludge. Ann Intern Med. 1999;130(4):301–311.
- Khoo TK, Vege SS, Abu-Lebdeh HS, et al. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab. 2009;94(6):2115–2118.
- Rawla P, Bandaru SS, Vellipuram AR. Review of infectious etiology of acute pancreatitis. Gastroenterol Res. 2017;10(3):153–158.
- Jalaly NY, Moran RA, Fargahi F, et al. An evaluation of factors associated with pathogenic PRSS1, SPINK1, CTFR, and/or CTRC genetic variants in patients with idiopathic pancreatitis. Am J Gastroenterol. 2017;112(8):1320–1329.
- Chen HZ, Ji L, Li L, et al. Early prediction of infected pancreatic necrosis secondary to necrotizing pancreatitis. Medicine (Baltimore). 2017;96(30):e7487.
- Harris HW, Barcia A, Schell MT, et al. Necrotizing pancreatitis: a surgical approach independent of documented infection. HPB (Oxford). 2004;6(3):161–168.
- Doctor N, Philip S, Gandhi V, et al. Analysis of the delayed approach to the management of infected pancreatic necrosis. WJG. 2011;179(3):366–371.
- Darrivere L, Lapidus N, Colignon N, et al. Minimally invasive drainage in critically ill patients with severe necrotizing pancreatitis is associated with better outcomes: an observational study. Crit Care. 2018;22(1):321.
- Jain S, Mahapatra SJ, Gupta S, et al. Infected pancreatic necrosis due to multidrug-resistant organisms and persistent organ failure predict mortality in acute pancreatitis. Clin Transl Gastroenterol. 2018;9(10):190.
- Grayson DE, Abbott RM, Levy AD, et al. Emphysematous infections of the abdomen and pelvis: a pictorial review. Radiographics. 2002;22(3):543–561.
- Kvinlaug K, Kriegler S, Moser M. Emphysematous pancreatitis: a less aggressive form of infected pancreatic necrosis? Pancreas. 2009;38(6):667–671.
- Nadkarni N, D’Cruz S, Kaur R, et al. Successful outcome with conservative management of emphysematous pancreatitis. Indian J Gastroenterol. 2013;32(4):242–245.
- Esparza L, Díaz AI, Anguiano MP. [Emphysematous pancreatitis]. Med Intensiva. 2012;36(1):65. Spanish.
- Alonso Calderón E, Pérez González C, Prieto Calvo M, et al. [Emphysematous pancreatitis. Fulminant course]. Cir Esp. 2017;95(10):611. Spanish.
- Xi Terence LY, Jia GY, Madhavan K. Fulminant emphysematous pancreatitis. Clin Gastroenterol Hepatol. 2019;17(3):A32.
- Werge M, Novovic S, Schmidt PN, et al. Infection increases mortality in necrotizing pancreatitis: a systematic review and meta-analysis. Pancreatology. 2016;16(5):698–707.
- Anderson CM, Kerby JD, Perry WB, et al. Pneumoretroperitoneum in two patients with Clostridium perfringens necrotizing pancreatitis. Am Surg. 2004;70(3):268–271.
- Wig JD, Kochhar R, Bharathy KG, et al. Emphysematous pancreatitis. Radiological curiosity or a cause for concern? JOP. 2008;9(2):160–166.
- Barreda L, Targarona J, Pando E, et al. Medical versus surgical management for emphysematous pancreatic necrosis: is gas within pancreatic necrosis an absolute indication for surgery? Pancreas. 2015;44(5):808–811.
- Frey C, Zhou H, Harvey D, et al. Co-morbidity is a strong predictor of early death and multi-organ system failure among patients with acute pancreatitis. J Gastrointest Surg. 2007;11(6):733–742.
- Koziel D, Gluszek-Osuch M, Suliga E, et al. Elderly persons with acute pancreatitis - specifics of the clinical course of the disease. CIA. 2018;14:33–41.
- Bul V, Yazici C, Staudacher JJ, et al. Multiorgan failure predicts mortality in emphysematous pancreatitis: a case report and systematic analysis of the literature. Pancreas. 2017;46(6):825–830.
- Mole DJ, Gungabissoon U, Johnston P, et al. Identifying risk factors for progression to critical care admission and death among individuals with acute pancreatitis: a record linkage analysis of Scottish healthcare databases. BMJ Open. 2016;6(6):e011474.
- Chen HJ, Wang JJ, Tsay WI, et al. Epidemiology and outcome of acute pancreatitis in end-stage renal disease dialysis patients: a 10-year national cohort study. Nephrol Dial Transplant. 2017;32(10):1731–1736.
- Nawaz H, O’Connell M, Papachristou GI, et al. Severity and natural history of acute pancreatitis in diabetic patients. Pancreatology. 2015;15(3):247–252.
- Shen HN, Lu CL, Li CY. Effect of diabetes on severity and hospital mortality in patients with acute pancreatitis: a national population-based study. Diabetes Care. 2012;35(5):1061–1066.
- Bohidar NP, Garg PK, Khanna S, et al. Incidence, etiology, and impact of fever in patients with acute pancreatitis. Pancreatology. 2003;3(1):9–13.
- Drewry AM, Ablordeppey EA, Murray ET, et al. Monocyte function and clinical outcomes in febrile and afebrile patients with severe sepsis. Shock. 2018;50(4):381–387.
- Yo CH, Lee MG, Hsein YC, et al. Risk factors and outcomes of afebrile bacteremia patients in an emergency department. Diagn Microbiol Infect Dis. 2016;86(4):455–459.
- Padhan RK, Jain S, Agarwal S, et al. Primary and secondary organ failures cause mortality differentially in acute pancreatitis and should be distinguished. Pancreas. 2018;47(3):302–307.
- Zerem E. Treatment of severe acute pancreatitis and its complications. WJG. 2014;20(38):13879–13892.
- Komatsu H, Yoshida H, Hayashi H, et al. Fulminant type of emphysematous pancreatitis has risk of massive hemorrhage. Clin J Gastroenterol. 2011;4(4):249–254.