Abstract
This study was conducted to clarify the effects of begging call playbacks on the growth of great tit, Parus major, nestlings in artificial nest boxes. The mean body mass of nestlings significantly differed between the control (60 dB playback intensity), playback 1 (80 dB), and playback 2 (100 dB) groups. However, mean tarsus length was not different based on treatment. The daily body mass and tarsus length gain of nestlings in playback 2 were higher than in the control and playback 1 groups in this study. Further long-term research is needed to understand the relationship between begging behavior of nestlings and parental care in the great tit.
Introduction
Begging behavior of nestlings is common in various taxa (including vertebrates and invertebrates) and is used as a model system to understand animal communication and conflicts between individuals (Kilner and Johnstone Citation1997; Tarwater et al. Citation2009). The adaptive advantages for nestlings of advertising their desires and for parents to respond seem fairly obvious (Leonard and Horn Citation1996). Begging behavior consists of a number of potential cues to which parents might respond, but nestling begging vocalizations are easily assessed by parents and easily manipulated by biologists (Burford et al. Citation1998).
Begging calls may result in a fitness cost to nestlings in terms of energy expenditure and/or predator attraction (Haskell Citation1994; Leech and Leonard Citation1996; Kölliker et al. Citation1998). Smaller or younger nestlings within broods appear to beg at a greater rate for a given level of food intake (Lotem Citation1998). Such effects may be due to different competitive begging strategies (Price et al. Citation1996; Wright et al. Citation2002).
Benefits to begging nestlings may vary over time. Parents may increase provisioning in response to short-term increases in begging but may be less flexible over longer periods (Price Citation1998). The rate at which one parent supplies food can change the provisioning behavior of its partner, independent of the nestling's behavior. With conflicts of interests, each parent faces the challenge of meeting the needs of its young without being exploited by excessively demanding offspring or lazy partners (Hinde and Kilner Citation2007).
The young of altricial birds are entirely dependent on their parents to feed them from hatching to fledging. Chick begging is a stimulus to parental feeding, and the begging level of the brood increases with deprivation. It seems that the begging behavior of nestlings acts as an important factor in the proximate control of parental feeding intensity (Cotton et al. Citation1996). Nestlings respond to the arrival of their parents at the nest with food by producing a vigorous begging display that involves calling, posturing and gaping. Parents respond to variation in the display by feeding the most intensive beggars of their offspring (Nolan and Ketterson Citation2001).
In this study, we experimentally manipulated the intensity of begging call playbacks in the great tit, Parus major, to investigate the effects of begging calls on the growth of nestlings. We collected data on the body mass and tarsus length of nestlings.
Methods
We conducted our experiment in mixed forests at the Ansung Campus, Chung-Ang University (37°00′N, 127°13′E), South Korea, from March to July of 2011 and 2012. We selected a study site measuring 120 × 240 m in each deciduous and coniferous forest. The dominant tree species were Mongolian oak Quercus mongolica and serrata oak Q. serrata in deciduous forest, and pitch pine Pinus rigida was in coniferous forests. The study site was divided into 30 × 30 m grids marked with flags, facilitating the accurate identification of the nest box location. A total of 45 wooden artificial nest boxes (length × width × height, 16 × 15 × 30 cm) with 1.5 cm thick walls were placed 1–2 m above the ground in trees (Rhim et al. Citation2011; Kwak et al. Citation2012; Son et al. Citation2012).
To survey the breeding ecology in each of the nest boxes, we visited 4–5 times per week. Briefly, the date of egg appearance in the nest and clutch size were recorded in each nest box. After egg laying, we visited the artificial nest boxes every day to determine clutch size, egg size and breeding parameters (hatching date, unhatched and hatched eggs, dead nestlings, and fledglings) (Aslan and Yavuz Citation2010; Son et al. Citation2012).
We recorded the begging calls of nestlings that were 4–5 days old with a digital recording device (PMD-650, Marantz) and microphone (MKH 416P48, RF Condenser MIC). The mean intensity of begging calls was 62.14 ± 10.69 dB in the nest boxes. We determined the control (60.00 ± 5.00 dB), treatment 1 (80.00 ± 5.00 dB), and treatment 2 (100.00 ± 5.00 dB) groups based on the intensity of begging calls. We played back these calls in the artificial nest boxes. For the birds in each nest (N = 15 nests), we measured the body mass and tarsus length every day. Nestlings were weighed (mass to nearest 0.1 g) and tarsus was measured (length to nearest 0.1 mm) (Kim Citation2013). We surveyed 15 breeding nest boxes for control and both treatments. Laying date, clutch size, and brood size were similar in the nest boxes.
We used repeated measures ANOVA (analysis of variance) to determine whether treatment influenced growth of nestlings. Tukey's post-hoc tests were used to determine pair-wise differences between treatments. Additionally, Kruskall–Wallis tests were used to determine whether treatment influenced daily body mass and tarsus length gains. Significance was declared at P < 0.05 for all statistical tests.
Results
The mean body mass of great tit nestlings in any group did not increase with the intensity of begging call playback until day 8 after hatching, except at day 2. However, mean body mass was significantly different after day 9, except at day 14 after hatching. The final mean body mass of nestlings in the playback 2 group was significantly higher than in the control and playback 1 groups (ANOVA, F = 1.97, P < 0.01) ().
Table 1. Body mass (grams, mean ± SD) of great tit nestlings in artificial nest boxes each day after hatching for the control (60 dB), playback 1 (80 dB), and playback 2 (100 dB) groups.
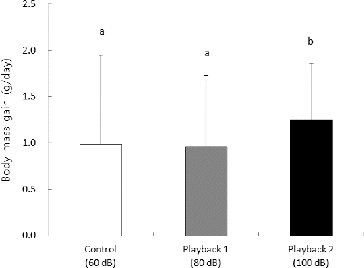
The daily body mass gain of nestlings was significantly different between control, playback 1, and playback 2 treatments (Kruskal–Wallis test, χ2 = 22.29, df = 2, P < 0.001). Also, daily body mass gain of nestlings in playback 2 was higher than those in the control and playback 1 groups (Tukey's post-hoc test, P < 0.05) ().
Figure 1. Daily body mass gain (g/day) of great tit nestlings in artificial nest boxes for the control (60 dB), playback 1 (80 dB), and playback 2 (100 dB) groups. Values are presented as mean and standard deviation (SD). Different letters indicate significant differences between the mean values for a given treatment (P < 0.05).
Tarsus lengths of great tit nestlings were measured from the fifth day after hatching . On each day, there were no differences in mean tarsus length of nestlings between the control and playback treatments (P > 0.05). The tarsus length of nestlings was approximately 20 mm at day 15 after hatching ().
Table 2. Tarsus length (mm, mean ± SD) of great tit nestlings in artificial nest boxes each day after hatching for the control (60 dB), playback 1 (80 dB), and playback 2 (100 dB) groups.
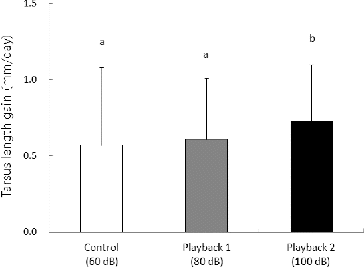
Tarsus length gain of nestlings differed between control and playback treatments (χ2 = 8.55, df = 2, P < 0.05). Moreover, the tarsus length gain of nestlings was significantly higher in playback 2 compared with the control and playback 1 groups (P < 0.05) ().
Figure 2. Daily tarsus length gain (mm/day) of great tit nestlings in artificial nest boxes for the control (60 dB), playback 1 (80 dB), and playback 2 (100 dB) groups. Values are presented as mean and SD. Different letters indicate significant differences between the mean values for a given treatment (P<0.05).
Discussion
Whether begging behavior is an honest signal or a manipulative one, parents should adjust their feeding in response to begging level. Nestling fitness should depend on provisioning if begging behavior is to be beneficial to nestlings (Burford et al. Citation1998). We found significant differences between control and treatment playbacks in body mass and tarsus length. Thus, it can be surmised that intensity of begging call playback affected feeding.
Parents are thought to be more responsive to the provisioning behavior of their partner than they are to offspring begging calls when adjusting nest food supply (Hinde and Kilner Citation2007). Feeding is only one aspect of parental care, and the estimated parental effort should also include resources allocated to other forms of parental care such as brooding, deterring predators and territorial defense (Verhulst and Tinbergen Citation1997). Three main factors play a part in the regulation of parental effort: the working capacity of the parents, the hunger level of the nestlings and environmental conditions such as temperature, humidity and food (Bengtsson and Rydén Citation1983).
Parents mostly feed one single nestling during any given visit, use highly predictable feeding locations (Kölliker et al. Citation1998) and give priority to nestlings at positions close to parental feeding position (Kölliker and Richner Citation2004). The quality and quantity of food brought to nestling by parents are factors of crucial importance for the nestlings’ chances of survival and growth (Bengtsson and Rydén Citation1983).
It should be made clear that nestlings did not demonstrate indisputable evidence of resource matching (i.e. ideal free distributions) in this experiment (Budden and Wright Citation2005; Shin and Lee Citation2008; Kim et al. Citation2010). It is difficult to gather empirical evidence for the costs of increasing begging call intensity in the field, and we cannot exclude this as a possible explanation of the patterns in nestling body mass (Wright et al. Citation1998). Further long-term research is needed to understand the relationship between begging behavior of nestlings and parental care in the great tit.
References
- Aslan A, Yavuz M. 2010. Clutch and egg size variation, and productivity of the house sparrow (Passer domesticus): effects of temperature, rainfall, and humidity. Turk J Zool. 34:255–266.
- Bengtsson H, Rydén O. 1983. Parental feeding rate in relation to begging behavior in asynchronously hatched broods of the great tit Parus major: an experimental study. Behav Ecol Sociobiol. 12:243–251.
- Budden AE, Wright J. 2005. Learning during competitive positioning in the nest: do nestlings use ideal free ‘foraging’ tactics? Behav Ecol Sociobiol. 58:227–236.
- Burford JE, Friedrich TJ, Yasukawa K. 1998. Response to playback of nestling begging in the red-winged blackbird, Agelaius phoeniceus. Anim Behav. 56:555–561.
- Cotton PA, Kacelnik A, Wright J. 1996. Chick begging as a signal: are nestling honest? Behav Ecol. 2:178–182.
- Haskell D. 1994. Experimental evidence that nestling begging behavior incurs a cost due to nest predation. Proc R Soc Lond B 257:161–164.
- Hinde CA, Kilner RM. 2007. Negotiations within the family over the supply of parental care. Proc R Soc B 274:53–60.
- Kilner R, Johnstone RA. 1997. Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol Evol. 12:11–15.
- Kim KJ. 2013. Influence of begging call playbacks on parents and nestling in great tit (Parus major) [ MSc. thesis]. Seoul: Chung-Ang University.
- Kim SN, Kee WK, Shin KI, Kafatos M, Seo DJ, Kwak HB. 2010. Comparison of spatial interpolation techniques for predicting climate factors in Korea. For Sci Tech. 6:97–109.
- Kölliker M, Richner H. 2004. Navigation in a cup: chick positioning in great tit, Parus major, nests. Anim Behav. 68:941–948.
- Kölliker M, Richner H, Werner I, Heeb P. 1998. Begging signals and biparental care: nestling choice between parental feeding locations. Anim Behav. 55:215–222.
- Kwak DA, Lee WK, Son Y, Choi S, Yoo S, Chung DJ, Lee SH, Kim SH, Choi JK, Lee YJ, Byun WH. 2012. Predicting distributional change of forest cover and volume in future climate of South Korea. For Sci Tech. 8:105–115.
- Leech SM, Leonard ML. 1996. Is there an energetic cost to begging in nestling tree swallows (Tachycineta bicolor)? Proc R Soc Lond B 263:983–987.
- Leonard M, Horn A. 1996. Provisioning rules in tree swallow. Behav Ecol Sociobiol. 38:341–347.
- Lotem A. 1998. Differences in begging behavior between barn swallow, Hirundo rustica, nestlings. Anim Behav. 55:809–818.
- Nolan Jr. V, Ketterson E. 2001. Current ornithology Vol. 16. New York: Kluwer Academic.
- Price K. 1998. Benefits of begging for yellow-headed blackbird nestlings. Anim Behav. 56:571–577.
- Price K, Harvey H, Ydenberg R. 1996. Begging tactics of nestling yellow-headed blackbirds, Xanthocephalus xanthocephalus, in relation to need. Anim Behav. 51:421–425.
- Rhim SJ, Son SH, Kim KJ. 2011. Breeding ecology of tits Parus spp. using artificial nest boxes in a coniferous forest over a five-year period. For Sci Tech. 7:141–144.
- Shin MY, Lee DK. 2008. Long-term prediction of forest sustainability of the natural deciduous forest in Pyungchang area of Gangwon province, Korea. For Sci Tech. 4:28–34.
- Son SH, Kim KJ, Hwang HS, Rhim SJ. 2012. Relationship between atmospheric conditions and breeding ecology of tits in artificial nest boxes. J Anim Vet Adv. 11:3682–3686.
- Tarwater CE, Kelley JP, Brawn JD. 2009. Parental response to elevated begging in a high predation, tropical environment. Anim Behav. 78:1239–1245.
- Verhulst S, Tinbergen JM. 1997. Clutch size and parental effort in the great tit Parus major. Ardea 85:111–126.
- Wright J, Both C, Cotton PA, Bryant D. 1998. Quality vs. quantity: energetic and nutritional trade-offs in parental provisioning strategies. J Anim Ecol. 67: 620–634.
- Wright J, Hinde C, Fazey I, Both C. 2002. Begging signals more than just short-term need: cryptic effects of brood size in the pied flycatcher (Ficedula hypoleuca). Behav Ecol Sociobiol. 52:74–83.
