?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Soil parent materials originating from different geologic settings represented broad differences in the forest nutrient environment, but few studies have been conducted on the relationships between soil parent materials and nutrient stocks in forest stands. This study was performed to compare the nutrient stocks of Japanese blue oak (Quercus glauca Thunb.) stands grown on forest soils inherited from two different parent materials, basalt and sandstone, in southern Korea. A total of 29 Japanese blue oak trees were destructively sampled (15 trees on basalt and 14 trees on sandstone) to compare the nutrient content of the tree components (stem wood, stem bark, branches, and leaves). Samples of the forest floor and a soil depth of 0–30 cm were collected to measure the nutrient stocks of the two parent materials. The mean nutrient concentrations of the tree components varied significantly between the basalt and sandstone parent materials. The mean carbon and potassium concentrations of stem wood were significantly higher in sandstone than in basalt, whereas the nitrogen concentration of stem wood and stem bark were lower in sandstone than in basalt (p < .05). A significantly higher carbon, nitrogen, potassium, and magnesium stocks of the forest floor were found in sandstone than in basalt. However, the soil carbon, nitrogen, calcium, and magnesium stocks at a depth of 0–30 cm were significantly higher in basalt than in sandstone. The results demonstrate that the aboveground nutrient concentration and belowground nutrient stocks of Japanese blue oak stands can be altered greatly by different parent materials.
Introduction
Soil parent materials originating from different geologic settings represent broad differences in the forest nutrient environment in forest ecosystems (Hahm et al. Citation2014; Kumbasli et al. Citation2017; Marek and Richardson Citation2020). The growth rates of individual trees and forest productivity can be attributed to parent materials inherited from different rocks because the mineral compositions of rocks strongly influence soil physical and chemical properties (Neff et al. Citation2006; Abella and Springer Citation2008; Leonard et al. Citation2015). Thus, tree growth and forest productivity in forest ecosystems appear to be directly or indirectly related to soil parent materials, which constitute the primary sources of plant nutrients (White et al. Citation2012; Augusto et al. Citation2017; Christophe et al. Citation2017; Marek and Richardson Citation2020). For example, the growth and mortality rates of the Douglas-fir, Pseudotsuga menziesii var. glauca (Beissn.) Franco, have been affected by different parent materials originating from different rocks (Shen et al. Citation2001). Furthermore, the mortality of western white pine (Pinus monticola Dougl. Ex D. Don) and Douglas-fir was higher in forests at sites over parent materials originating from meta-sedimentary rocks than on in those over parent materials originating from igneous rocks inland of Northwest, USA (Moore et al. Citation2004).
The nutrient stocks of forest stands play a key role in assessing the potential impacts of sustainable forest management and biogeochemical cycles in forest ecosystems (Leuschner et al. Citation2006; Neff et al. Citation2006; Augusto et al. Citation2008; Kim, Baek, et al. Citation2019). Thus, soils derived from different parent materials may influence tree growth and nutrient stocks differently due to differenes in reservoirs of inorganic nutrients and the release rates of soil nutrients (Vestin et al. Citation2013; Christophe et al. Citation2017). However, few studies have explored the relationships between parent materials inherited from different rocks and nutrient stocks in forest stands (White et al. Citation2012; Vestin et al. Citation2013).
The Japanese blue oak (Quercus glauca Thunb.) is distributed in a broad range of sites in the subtropical forests of Korea (Han et al. Citation2018; Kim, Kim, et al. Citation2019). Thus, this tree species was used to determine whether parent materials originating from different rock types could explain variations in the nutrient stocks of tree biomass, forest floor, and mineral soils. The aims of this study were to determine differences in the nutrient stocks of Japanese blue oak stands grown on parent materials inherited from different rock types. We hypothesized that the parent material types may affect the nutrient stocks of the Japanese blue oak stands.
Material and methods
Study site
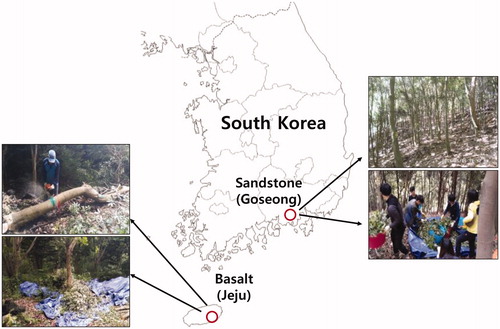
The study was conducted in Japanese blue oak stands grown on parent materials originating from two different rocks (basalt and sandstone) in the subtropical forest zone of southern Korea. The study sites were located in Jeju-do (basalt) and Goseong-gun (sandstone) (). The mean annual precipitation and temperature are higher in Jeju-do (1923 mm and 16.6 °C, respectively) with basalt parent materials than in Goseong-gun (1450 mm and 14.7 °C, respectively) with sandstone parent materials. The soils in Jeju-do are well-drained, highly fertile volcanic ash forest soils (Andisols, USDA Soil Taxonomy) originating from basalt with a loamy texture, whereas the soils in Goseong-gun are a dark reddish-brown forest soil of medium fertility (mostly Inceptisols, USDA Soil Taxonomy) originating from sandstone with a silty loam texture. Both study sites were composed of residual parent materials.
Nutrient content of tree components
The experimental design consisted of three 20 m × 20 m plots within each site. Five diameter classes based on DBH ranges were established for each site, and sample trees were randomly selected from each DBH class. To measure the nutrient concentrations of the tree components, 14 trees from sandstone and 15 trees from basalt (total, 29 trees), representing the DBH range of the stands were destructively sampled in late April and early July 2015, respectively. The trees were separated into tree components (i.e. leaves, branches, stem bark, and stem wood). The fresh weight of all the tree components was determined in the field by using portable electronic balances. All the investigations were performed in accordance with the technical standards of biomass measurement formulated by the Korea Forest Research Institute (Citation2010). Subsamples to determine the fresh-to-oven-dried biomass ratio were obtained from each tree component and oven-dried at 85 °C for one week. The dried samples were ground in a Wiley mill and passed through a 40-mesh stainless steel sieve. Carbon (C) and nitrogen (N) concentrations from the ground materials were determined using an elemental analyzer (Thermo Scientific Flash 2000, Milan, Italy). Phosphorus (P), potassium (K), calcium (Ca), and magnesium (Mg) concentrations were determined through dry ashing 0.5 g of the ground material at 470 °C for 4 h, digesting the ash with 3 mL of concentrated 5 M HCl, diluting the digest with 0.25 mL of concentrated HNO3 and 3 mL of concentrated 5 M HCl (Kalra and Maynard Citation1991), and measuring the concentrations via ICP-OES (Perkin Elmer Optima 5300DV, Shelton CT, USA). The nutrient content of each tree component was determined by multiplying the dry weight and nutrient concentration of each tree component.
The simple linear regression equation to estimate the nutrient content (C, N, P, K, Ca, and Mg) of each tree component was as follows;
where, Y is the nutrient content (g) of the respective tree component, DBH is the diameter at breast height (cm), and a and b are regression coefficients.
Nutrient content of forest floor and mineral soils
In April 2015, forest floor samples were collected from three random points of each plot by using a 900 cm2 steel template (30 cm × 30 cm). The forest floor samples were oven-dried at 85 °C, ground with a Wiley mill, and passed through a 40-mesh stainless sieve. Nutrient analysis of the forest floor was according to the same procedure for tree nutrient analysis.
Soil samples were collected from three randomly selected points in each plot. At each point, two soil samples were collected from three depths (0–10 cm, 10–20 cm, and 20–30 cm) by using 400 cm3 stainless core cans. The collected soil samples were oven-dried at 105 °C to measure bulk density, and air-dried to analyze the soil nutrients, respectively. The soil C and N concentrations were determined using an elemental analyzer (Vario Macro cube, Langenselbold, Germany). The soil P extracted with NH4F and HCl solutions (Kalra and Maynard Citation1991) was determined using a UV spectrophotometer (Jenway 6505, Staordshire, UK). Exchangeable K, Ca, and Mg concentrations extracted with NH4Cl solution (Kalra and Maynard Citation1991) and a mechanical vacuum extractor (Model 24VE, SampleTek, Science Hill, KY, USA) were determined with ICP-OES (Perkin Elmer Optima 5300DV, Shelton, CT, USA).
Soil nutrient stocks at each soil depth were calculated using the following formula:
where NS is the nutrient stocks at soil depth, NCi is the concentration of soil nutrients at each soil depth (i), BDi is soil bulk density at each soil depth, Di is each soil depth (cm), and VFi is volumetric coarse fragments content (%) at each soil depth.
Data analysis
The linear relationships between nutrient content and DBH were examined at p < .05 (SAS Institute Inc., 2003). The nutrient concentrations and stocks of aboveground and belowground for both parent materials were compared at p < .05 by using the PROC t-test procedure of SAS (SAS Institute Inc., 2003).
Results
Stand characteristics
The mean stand densities were higher for the sandstone (1610 trees ha−1) than for the basalt (967 trees ha−1) parent material, whereas the mean tree age for the sandstone parent material was slightly lower than that for the basalt parent materials (). The mean DBH and basal area were higher for the basalt (20.2 cm and 31.00 m2 ha−1) than for the sandstone (13.65 cm and 22.57 m2 ha−1) parent material. The mean tree height (10.2 m) was the same for both parent materials.
Table 1. General site and stand characteristics in Quercus glauca stands on different parent materials.
Nutrient concentration of tree components
The mean concentrations of C and K in the stem wood were significantly higher for sandstone than for basalt, whereas the N concentration in the stem wood was significantly lower for sandstone than for basalt (). The P, Ca, and Mg concentrations of the stem wood were not significantly different between the parent materials. The stem bark showed a trend similar to that of stem wood. In contrast to stem wood and stem bark, mean concentrations of P, K, and Mg in the branches were significantly higher for sandstone than for basalt. The nutrient content of all tree components and DBH was linearly related. The regression equations for the tree components were significant (p < .05), with DBH accounting for 32–88% of the variation in nutrient content ().
Table 2. Mean nutrient concentration of tree components in Quercus glauca on different parent materials type.
Table 3. Linear regression equations to estimate nutrient content (g) of tree components in a Quercus glauca on different parent materials type.
Nutrient concentration of forest floor and mineral soils
The N, P, and Ca concentrations of the forest floor were significantly higher for basalt than for sandstone, whereas the K concentration was significantly lower for basalt than for sandstone parent material (). The C and Mg concentrations of the forest floor were not affected by the parent materials. The organic C, total N, and exchangeable Ca2+ and Mg2+ at each soil depth were significantly higher for basalt than for sandstone parent material ().
Table 4. Nutrient concentration of the forest floor in Quercus glauca stands on different parent materials.
Table 5. Soil physical and chemical properties in Quercus glauca stands on different parent materials.
Nutrient stocks of tree components, forest floor, and mineral soils
The aboveground C, N, P, Ca, and Mg stocks of the tree components were not significantly different (p > .05) between the parent materials, whereas the K stock of the branches was significantly higher for sandstone than for basalt (). The nutrient stocks of the forest floor were significantly different between the parent materials, except for Ca stock (). However, the total nutrient stocks at mineral soil depth (0–30 cm) were significantly higher in the basalt parent material than in the sandstone parent material, except for the P and K stocks (). The soil K stocks at two soil depths (10–20 cm and 20–30 cm) were significantly different between the parent materials. However, P, K, and Ca stocks at the surface soil depth (0–10 cm) were not affected by the parent materials.
Table 6. Nutrient stocks of aboveground tree components in Quercus glauca stands on different parent materials.
Table 7. Nutrient stocks of the forest floor and at 30 cm of mineral soil depth in Quercus glauca stands on different parent materials.
Discussion
Nutrient concentrations of tree components, forest floor, and mineral soils
The study supports our hypothesis that forest soils derived from different parent materials have significantly different nutrient concentrations of tree components, forest floor, and mineral soils. The trees grown on basalt had a generally high nutrient uptake, except for leaf K concentration in the sandstone parent material. The higher mean nutrient concentrations of the stem wood and stem bark on basalt are likely due to the difference in soil nutrients between the parent materials. For example, soils derived from basalt tend to be richer in organic C and total N with high exchangeable cations (). In contrast, sandstone parent materials provide a poor nutrient and moisture environment for tree growth, with low nutrient concentration when compared with basalt parent material. Previous studies have found that trees growing on different rock types have different foliar nutrient concentrations, particularly P, K, and Ca (Shen et al. Citation2001; Moore et al. Citation2004; Christophe et al. Citation2017; Marek and Richardson Citation2020).
The high concentrations of N, P, and Ca in the forest floor of basalt when compared with sandstone parent material could be associated with the high nutrient concentration of the tree leaves, which are major litterfall components of the forest floor. Thus, high soil organic C concentration of the basalt parent material could be due to the increased inputs of organic matter obtained from litterfall decomposition with good soil properties. However, the difference in exchangeable cation concentrations in both parent materials may be attributable to the inherent mineralogical character and nutrient uptake throughout stand development (Binkley and Giardina Citation1998; Christophe et al. Citation2017; An et al. Citation2020; Marek and Richardson Citation2020). In addition, the accumulation of nutrients in the tree biomass of sandstone may be the main mechanism responsible for this low exchangeable cation concentration.
Nutrient stocks of tree components, forest floor, and mineral soils
The nutrient stocks of the tree components could be associated with the differences in tree density (Węgiel et al. Citation2018; Verma and Garkoti Citation2019), stand basal area, and nutrient concentrations of the tree components (Rodríguez-Soalleiro et al. Citation2018; Yang et al. Citation2018). In this study, the aboveground nutrient stocks of the tree components were not affected by the different parent materials, except for P and K stocks of the branches. The P and K stocks of the branches in sandstone parent material could be affected by high P (sandstone: 0.109%, basalt: 0.034%) and K concentrations (sandstone: 0.82%, basalt: 0.20%), but not the differences in stand basal area (sandstone: 22.57 m2 ha−1, basalt: 32.00 m2 ha−1) and stand density (sandstone: 1610 tree ha−1, basalt: 967 tree ha−1). In contrast to this result, Barron-Gafford et al. (Citation2003) have reported that the nutrient stocks of the tree biomass were significantly higher in more dense stands than in less dense stands as a result of greater acquisition of resources and biomass growth of the loblolly pine in the USA. The mean values of the aboveground C stocks were 110,027 kg C ha−1 for basalt and 73,071 kg C ha−1 for sandstone. Aboveground C stocks in basalt were slightly lower than the reported range (124,500–132,630 kg C ha−1) for Q. glauca stands in basalt in Jeju-do, Korea (Han et al. Citation2018), whereas the values in sandstone were considerably lower than the range. However, the mean values (basalt: 958 kg N ha−1, sandstone: 491 kg N ha−1) of the N stocks of the tree components were considerably higher than 226 kg N ha−1 for other coniferous forests in Korea (Kim Citation1999). The results indicate that Q. glauca exhibits higher nutrient uptake than other coniferous tree species in Korea.
The differences in the nutrient stocks of the forest floor could be related to the amount of forest floor, and not nutrient concentration of the forest floor. For example, the low C stocks in basalt could be due to rapid C mineralization, whereas the high accumulation in the forest floor of sandstone may have been due to slow decomposition rates as a consequence of low precipitation and temperature, which are major abiotic factors that regulate decomposition processes (Berg and Laskowski Citation2006). Furthermore, the concentrations of N, P, and Ca in the forest floor were lower in sandstone than in basalt. However, high P stocks in basalt could be due to P inputs through leaf litterfall from high P concentrations in >1-year-old leaves in the basalt parent material. The C stocks of the forest floor in this study were within 3610–6390 kg C ha−1 of the Q. glauca stands on basalt reported by Han et al. (Citation2018).
This study demonstrates that different parent materials have significant influences on the soil nutrient stocks at each soil depth. Significant effects of parent materials on soil nutrient stocks have been reported by Neff et al. (Citation2006) and Li et al. (Citation2017). Larger organic matter input in highly fertile soil would likely explain the higher soil C pool in basalt than in sandstone parent material. The influences of parent materials on soil N stocks may be due to their effects on soil organic C as soil nutrients because N is not a rock-derived element. However, similar P stocks in both parent materials could be due to similar soil acidity (basalt: pH 4.55–4.70; sandstone: pH 4.47–4.52) because plant-availability of P in forest soils depends on soil acidity (Augusto et al. Citation2017). In contrast to C, N, and P stocks, the higher soil K stocks in sandstone could be due to the differences in inherent mineral characteristics because K2O concentration in basalt is generally lower than that in sedimentary rocks (Moore et al. Citation2004). In addition, this result may be attributable to the higher leaching losses in basalt regions because of the difference in annual precipitation (basalt: 1923 mm; sandstone: 1450 mm). Tripler et al. (Citation2006) reported that exchangeable K+ is soluble and easily leached from forest soils. Although, the Ca2+ and Mg2+ concentrations in both parent materials showed significant differences (p < .05) at the three soil depths, the nutrient stocks of these cations did not differ in the parent materials. The significantly low bulk density in the basalt parent material () may be an additional factor that probably influences the differences in soil nutrient stocks. Therefore, the bulk density difference between basalt and sandstone parent materials could be an important controlling factor for nutrient stocks than the actual nutrient concentration in the soil.
Conclusions
This study quantitatively demonstrated broad differences in the nutrient environments represented by parent materials originating from different bedrocks in Japanese blue oak stands. The Japanese blue oak stands developed from basalt parent material exhibited greater nutrient accumulation than those developed from sandstone parent material. Although both parent materials may have different mechanisms for the nutrient cycle because of different stand characteristics, the parent materials accounted for the consequence of nutrient stocks due to the difference in inherent bedrocks. Thus, parent materials can be a useful variable for explaining forest nutrition responses throughout stand development processes.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abella SR, Springer JD. 2008. Canopy-tree influences along a soil material gradient in Pinus ponderosa-Quercus gambelii forests, northern Arizona. J Torr Bot Soc. 135(1):26–36.
- An J, Chang H, Han SH, Khamzina A, Son Y. 2020. Changes in basic soil properties and enzyme activities along an afforestation series on the dry Aral Sea Bed, Kazakhstan. For Sci Technol. 16(1):21–31.
- Augusto L, Achat DL, Jonard M, Vidal D, Ringeval B. 2017. Soil parent material-a major driver of plant nutrient limitations in terrestrial ecosystems. Glob Chang Biol. 23(9):3808–3824.
- Augusto L, Meredieu C, Bert D, Trichet P, Porté A, Bosc A, Lagane F, Loustau D, Pellerin S, Danjon F, et al. 2008. Improving models of forest nutrient export with equations that predict the nutrient concentration of tree compartments. Ann for Sci. 65(8):808–808.
- Barron-Gafford GA, Will RE, Burkes EC, Shiver B, Teskey RO. 2003. Nutrient concentrations and contents, and their relation to stem growth, of intensively managed Pinus taeda and Pinus elliottii stands of different planting densities. For Sci. 49(2):291–300.
- Berg B, Laskowski R. 2006. Litter decomposition: guide to carbon and nutrient turnover. Adv Ecol Res. 38:227–261.
- Binkley D, Giardina C. 1998. Why do tree species affect soils? The warp and woof of tree-soil interactions. Biogeochemistry. 43:89–106.
- Christophe C, Gil K, Laurent SA, Paul-Oliver R, Marie-Pierre T. 2017. Relationship between soil nutritive resources and the growth and mineral nutrition of a beech (Fagus sylvatica) stand along a soil sequence. CATENA. 155:156–169.
- Hahm WJ, Riebe CS, Lukens CE, Araki S. 2014. Bedrock composition regulates mountain ecosystems and landscape evolution. Proc Natl Acad Sci USA. 111(9):3338–3343.
- Han YS, Lee EP, Park JH, Lee SY, Lee SI, You YH. 2018. Organic carbon distribution and cycling in the Quercus glauca forest at Gotjawal wetland, Jeju island Korea. J Ecol Environ. 42:8.
- Kalra YP, Maynard DG. 1991. Methods manual for forest soil and plant analysis. Northwest region, information. Report. NOR-X-319. Edmonton, AB, Canada: Northern Forestry Centre.
- Kim C. 1999. Aboveground nutrient distribution in pitch pine (Pinus rigida) and Japanese larch (Larix leptolepis) plantations. J Kor For Soc. 88(2):266–272.
- Kim C, Baek G, Yoo BO, Jung SY, Lee KS, An KW. 2019. Comaprison of allometric equations and biomass expansion factors for six evergreen broad-leaved trees in subtropical forests in southern Korea. J Sus For. 38 (3):199–212.
- Kim H, Kim S, Lee J, Chang H, Roh Y, An J, Son Y. 2019. Development of a forest carbon and nitrogen model: pilot application for a Pinus densiflora forest in Central Korea. For Sci Technol. 15(4):202–209.
- Korea Forest Research Institute. 2010. Survey manual for biomass and soil carbon. Seoul, Korea: KFRI.
- Kumbasli M, Makineci E, Keten A, Beskardes V, Özdemir E. 2017. Effects of parent material, stand type and oak species on defoliation of coppice-originated oak (Quercus spp.) forests in Northern Turkish Thrace. Bosque. 38(2):299–306.
- Leonard JM, Medina AL, Neary DG, Tecle A. 2015. The influence of parent material on vegetation response 15 years after the Dude fire Arizona. Forests. 6(12):613–635.
- Leuschner C, Meier IC, Hertel D. 2006. On the niche breadth of Fagus sylvatica: soil nutrient status in 50 central European beech stands on a broad range of bedrock types. Ann For Sci. 63(4):355–368.
- Li D, Wen L, Zhang W, Yang L, Xiao K, Chen H, Wang K. 2017. Afforestation effects on soil organic carbon and nitrogen pools modulated by lithology. For Ecol Manag. 400:85–92.
- Marek RS IV, Richardson JB. 2020. Investigating surficial geologic control on soil properties, inorganic nutrient uptake, and northern hardwood growth in western Massachusetts USA. J Soil Sci Plant Nutr. 20: 901–911.
- Moore JA, Hamilton DA Jr, Xiao Y, Byrne J. 2004. Bedrock type significantly affects individual tree mortality for various conifers in the inland Northwest, USA. Can J For Res. 34(1):31–42.
- Neff JC, Reynolds R, Sanford RL Jr, Fernandez D, Lamothe P. 2006. Controls of bedrock geochemistry on soil and plant nutrients in southeastern Utah. Ecosystems. 9(6):879–893.
- Rodríguez-Soalleiro R, Eimil-Fraga C, Gómez-García E, García-Villabrille JD, Rojo-Alboreca A, Muñoz F, Oliveira N, Sixto H, Pérez-Cruzado C. 2018. Exploring the factors affecting carbon and nutrient concentrations in tree biomass components in natural forests, forest plantations and short rotation forestry. For Ecosyst. 5:35.
- SAS Institute Inc. 2003. SAS/STAT Statistical software. Version 9.1. Cary, NC: SAS Publishing.
- Shen G, Moore JA, Hatch CR. 2001. The effect of nitrogen fertilization, rock type, and habitat type on individual tree mortality. For Sci. 47(2):203–213.
- Tripler CE, Kaushal SS, Likens GE, Walter MT. 2006. Patterns in potassium dynamics in forest ecosystems. Ecol Lett. 9(4):451–466.
- Verma AK, Garkoti SC. 2019. Population structure, soil characteristics and carbon stock of the regenerating banj oak forests in Almore Central Himalaya. For Sci Technol. 15(3):117–127.
- Vestin JLK, Söderberg U, Bylund D, Nambu K, van Hees PAW, Haslinger E, Ottner F, Lundström US. 2013. The influence of alkaline and non-alkaline parent material on Norway spruce tree chemical composition and growth rate. Plant Soil. 370(1–2):103–113.
- Węgiel A, Bielinis E, Polowy K. 2018. Macronutrient stocks in Scots pine stands of different densities. Forests. 9(10):593.
- White KP, Coleman M, Page-Dumroese DS, Gessler PE, Kimsey M, Shaw T. 2012. Examining soil parent material influence over Douglas-fir stem growth response of fertilization: taking advantage of information from spatiotemporally distributed experiments. For Ecol Manag. 286:10–107.
- Yang K, Zhu J, Xu S, Zheng X. 2018. Conversion from temperate secondary forests into plantations (Larix spp.): impact on belowground carbon and nutrient pools in northeastern China. Land Degrad Dev. 29(11):4129–4139.