Abstract
Tumor-associated antigens such as NY-ESO-1 are expressed in a variety of solid tumors but absent in mature healthy tissues with the exception of germline cells. The immune system anti-cancer attack is mediated by cell lysis or induction of growth arrest through paralysis of tumor cells, the latter of which can be achieved by tumor-specific CD4+, IFNγ-producing THelper type 1 (TH1) cells. Translation of these immune-mediated mechanisms into clinical application has been limited by availability of immune effectors, as well as the need for complex in vitro protocols and regulatory hurdles. Here, we report a procedure to generate cancer-testis antigen NY-ESO-1-targeting CD4+ TH1 cells in vitro for cancer immunotherapy in the clinic. After in vitro sensitization by stimulating T cells with protein-spanning, overlapping peptide pools of NY-ESO-1 in combination with IL-7 and low dose IL-2, antigen-specific T cells were isolated using IFNγ capture technique and subsequently expanded with IL-2, IL-7 and IL-15. Large numbers of NY-ESO-1-specific CD4+ T cells with a TH1 cytokine profile and lower numbers of cytokine-secreting CD8+ T cells could be generated from healthy donors with a high specificity and expansion potential. Manufactured CD4+ T cells showed strong specific TH1-responses with IFNγ+, TNFα+, IL-2+ and induced cell cycle arrest and apoptosis in tumor cells. The protocol is GMP-grade and approved by the regulatory authorities. The tumor-antigen specific CD4+ TH1 lymphocytes can be adoptively transferred as a T-cell therapy to boost anticancer immunity and this novel cancer treatment approach is applicable to both T cells from healthy allogeneic donors as well as to autologous T cells derived from cancer patients.
Introduction
T-cell responses have the potential to manifest powerful antitumor effects. Adoptive T-cell therapy (ACT) has shown promise as a treatment option for patients afflicted with a variety of malignancies.Citation1-4 Investigations of adoptive T-cell transfer have previously focused on CD8+ cytotoxic T lymphocytes (CTLs), tumor-antigen specific T-cell clones or tumor-infiltrating lymphocytes (TILs). Mounting data from studies in humans and mice suggest that tumor-specific CD4+ THELPER type 1 (TH1) cells are highly effective in constraining cancer, so we sought to develope a manufacturing protocol to generate predominantly tumor antigen-specific, CD4+ TH1 lymphocytes. Although solid tumors arise from tissues that usually do not express MHC Class II molecules, mice models have shown that primed CD4+ T cells are able to completely eliminate MHC Class II negative tumors.Citation5 Even more, CD4+ T cells are not simply required as helpers for CD8+ T cells but yet have the ability to eradicate tumors without the presence of CD8+ T cells during lymphopenia.Citation6 The tumor antigen specific TH1-cells were detected in the tumor environment, where they inhibit tumor growth through cytokine signals.Citation7-11 The secretion of interferon γ (IFNγ) and tumor necrosis α (TNFα) by TH1 lymphocytes has been shown to be crucial for efficient tumor control involving tumor-destructive, tumor-silencing and antiangiogenic mechanisms.Citation8,9,12-14 This concept is further supported by a published report of a patient with metastatic melanoma wherein adoptive transfer of a NY-ESO-1-specific TH1-cell clone induced complete regression of the tumor.Citation15 The TH1-cell clone had a highly polarized TH1 phenotype and regression of the tumor was complete, although not all tumor cells expressed NY-ESO-1. Nevertheless, effective immunotherapies have been limited by many factors, such as the limited availability of effector cells, regulatory hurdles, tolerogenic factors (e.g., IL-4 and IL-10), immunosuppressive regulatory T cells (Tregs)Citation16 and MHC downregulation on malignant tissue.Citation17
NY-ESO-1 belongs to the family of cancer-testis antigens which are among the most attractive targets for cancer immunotherapy as their expression is normally restricted to germline cells and NY-ESO-1 is aberrantly expressed in many tumor entities.Citation18-21 According to the prioritization of cancer antigens performed by the National Cancer Institute, NY-ESO-1 is a favored antigen to be addressed in clinical studies.Citation22 Priming of T-cell responses against NY-ESO-1 has been described in cancer-free individuals for MHC Class II epitopesCitation23 and in cancer patients in which the presence of NY-ESO-1-specific T-cell responses in the blood improved survival.Citation24 However, these endogenous T-cell responses have not been powerful enough to prevent progression/relapse in all patients. Therefore, adoptive T-cell transfer is a reasonable approach to improve the efficacy of antitumor immune responses in vivo. Previous work has shown an association between NY-ESO-1 antibody production and CD4+ T-cell recognition of MHC Class-II restricted NY-ESO-1 epitopes.Citation25 Generation of NY-ESO-1-specific CD4+ and CD8+ T cells in parallel was shown for the first time by Zeng et al. using a single peptide with dual specificity to HLA-A2 and HLA-DP4.Citation26
According to these considerations, we have set up a fast and simple good manufacturing protocol (GMP)-protocol using overlapping 15-mer peptides encompassing the entire NY-ESO-1 protein to induce specific and potent CD4+ and CD8+ T-cell response with a strong TH1 profile. This approach has been approved by the authorities and could be applied to all HLA types, thus allowing a standardized protocol for every eligible patient.
Results
Induction of T-cell responses against NY-ESO-1 in PBMC of healthy donors
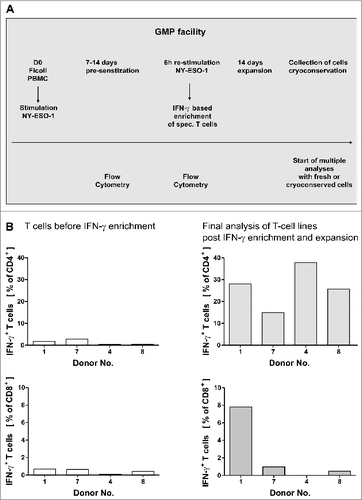
For analysis of NY-ESO-1-responding T cells in healthy individuals, the peripheral blood mononuclear cells (PBMCs) of 12 healthy volunteers were stimulated at day 0 with NY-ESO-1 overlapping peptides. IFNγ exocytosis was subsequently analyzed after re-stimulating T cells with the same antigen 14 d later by cytokine catch assays (). The frequency of responding CD4+ T cells of exceeded 0.1% IFN-γ+−secreting T cells detected in 11 out of 12 donors (1.1 ± 0.9%, in responders, mean ± SD) with the highest response in donor 7 (2.8% IFNγ+) whereas CD8+ responses above 0.1% IFN-γ+ were only detected in 9 out of 12 donors (1.0 ± 1.3% in responders, mean ± SD). Only one donor had no detectable T-cell response to NY-ESO-1. These results demonstrate that NY-ESO-1-specific T cells are detectable in healthy donors after a pre-sensitization period. In pilot small-scale experiments we found that donors with at least 0.1% specific IFNγ+ T cells, the magnetic enrichment of tumor antigen-specific T cells was feasible (data not shown).
Table 1 Frequency of NY-ESO-1 responding T cells from the peripheral blood of healthy donors (A) NY-ESO-1-specific T cells after 14 d priming
Table 1 (B) NY-ESO-1-specific T cells after GMP-grade isolation and expansion
GMP-grade generation of NY-ESO-1 specific T cells from healthy donors
Our aim was to establish a rapid and simple GMP-conforming approach to generate NY-ESO-1-specific T cells from healthy donors (). Healthy donors with frequencies higher than 0.1% specific IFNγ+ T cells were asked to give a higher amount of blood for large-scale generation of T cells and 4 donors gave their consent. T-cell lines were generated from the 4 donors (after informed consent) in a GMP facility including all required quality assessment and quality controls. Loss of antigen through sterile filtration was excluded by HPLC (data not shown). Analysis of T cells during the process showed NY-ESO-1-specific CD4+ and CD8+ T-cell responses in some donors after only one week of pre-sensitization (data not shown). Thus we decided to enrich T cells of those donors as early as one week following pre-sensitization while T-cell lines of other donors required 14 d pre-sensitization before enriching IFNγ+ cells. For all donors, the isolation of a CD3+ IFNγ+ population was successful with purities ranging from 44.6–82.5% and final IFNγ+ cell counts (following isolation) ranging from 2.4 × 106 and 20 × 106 (). As the absolute cell count after IFNγ enrichment was quite low, a subsequent expansion of T cells was carried out with autologous feeder cells in the presence of interleukin (IL)-2, IL-7 and IL-15 without further addition of antigen. A 19-to 351-fold expansion of IFNγ+ T cells was achieved after enrichment of IFNγ+ cells. For 3 of the 4 experiments the absolute cell count was extrapolated using the expansion factor () as not all cells from the IFNγ+ cells (positive fraction) were used for expansion. For all large-scale validations, T cell viability after expansion was higher than 90% and all preparations were free of microbial contamination and mycoplasma (data not shown). T-cell lines were analyzed by intracellular staining and cytofluorimetric analysis for IFNγ expression using mature autologous dendritic cells (DCs) pulsed with NY-ESO-1 overlapping peptide pools. T-cell responses to NY-ESO-1 could be increased to encompass 15–37.8% of CD4+ T cells ( and ). In contrast, NY-ESO-1 specific lymphocyte purity was relatively lower (max. 7.8%) among CD8+ T cells ( and ). The overall frequency of CD3+ cells after in vitro expansion varied between 22–98%, revealing the presence of co-expanded natural killer (NK) cells.
Figure 1. GMP grade isolation and expansion of NY-ESO-1-specific T cells. (A and B) Peripheral blood mononuclear cells (PBMCs) from healthy donors were pre-sensitized with NY-ESO-1 overlapping peptides and IFNγ-secretion was analyzed after re-stimulation with NY-ESO-1 (as indicated below) followed by IFNγ-based enrichment and expansion using good manufacturing processes (GMP). (A) Time schedule of the protocol for generating NY-ESO-1-specific T cells using overlapping peptide pools of NY-ESO-1. (B) Frequencies of IFNγ+CD4+ and IFNγ+ CD8+ T cells directly before IFNγ enrichment and in the final T-cell product (after IFNγ enrichment and 14 d of expansion). Analyses were performed using overlapping peptide pools and determined by intracellular IFNγ staining after 6 h of re-stimulation with NY-ESO-1 antigen-pulsed or actin S control antigen (ACTS) dendritic cells (T cells: DC 10:1). Background values induced by stimulation with overlapping ACTS control peptide pools were subtracted. GMP grade large scale T-cell generation was done from 4 donors as proof of principle.
NY-ESO-1-specific CD4+ T-cell lines retain high proliferation capacity
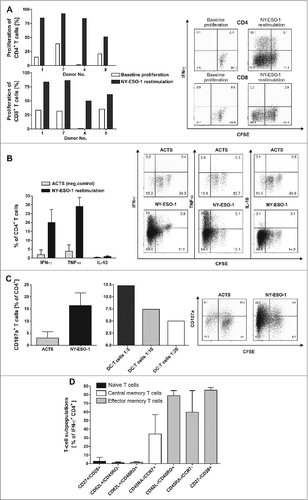
Adoptive T-cell transfer immunologic success requires infusion of T cells with expansion potential to induce a sustained response in vivo. In all 4 T-cell lines generated, a proliferation response to NY-ESO-1 overlapping peptides pools was observed among CD4+ cells (30.3–82.2%) as well as CD8+ T cells, albeit to a lesser extent (). CD8+ T cell responses to NY-ESO-1 were comparatively lower (26.1–54.9%) than those of CD4+ cells, but were significantly above background proliferation to actin S (ACTS) in all cases.
Figure 2. NY-ESO-1-specific proliferation, cytokine expression and CD107a expression of T cells with no alloreactive immune responses. (A) CD4+ and CD8+ T cells show a specific proliferation in response to NY-ESO-1. T cells of the final in vitro expanded T-cell products from 4 healthy donors were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and stimulated with NY-ESO-1 or control actin peptide (ACTS) overlapping peptide pool-pulsed dendritic cells (DCs), respectively. After 6 d cells were re-stimulated for 6 h with the same antigens and analyzed by flow cytometry. Bars represent NY-ESO-1-specific proliferation and background proliferation (ACTS) for each donor ; representative CFSE staining combined with IFNγ analysis of T cells from donor 4. (B) T cells show a TH1 driven CD4 response to NY-ESO-1. CFSE-stained CD4+ T cells were analyzed for production of the cytokines IFNγ, TNFα and IL-10 by intracellular staining and flow cytometry (n = 4 donors). Results are mean +/− SD; representative staining of T cells from donor 1. (C) CD4+ T cells show cytolytic responses to NY-ESO-1. T-cell products of donors 1 and 4 were analyzed in 4 independent experiments for CD107a expression after 6 h of re-stimulation with NY-ESO-1 or ACTS overlapping peptide pools pulsed DCs. Bars show mean results of 4 experiments +/− SD and the results of different DC:T-cells ratios during re-stimulation are shown; representative staining of T cells from donor 4. (D) For final analysis of T-cell lines, T cells from donor 1, 7 and 4 were re-stimulated for 6 h with overlapping peptide (NY-ESO-1, ACTS) pool-pulsed dendritic cells (DCs) and analyzed by immunostaining and multispectral fluorescence cytometry. Bars represent mean values of double positive cells gated on CD4+/IFNγ+ T cells. T-cell subpopulations were defined as naïve T cells (CD27+/CD28+, CD62L+/CD45RO−), central memory T cells (CD62L+/CD45RO+, CD45RA−/CCR7+) and effector memory T cells (CD62L/CD45RO+, CD45RA−/CCR7− and CD27−CD28+).
TH1 cytokines predominate the CD4+ T-cell response to NY-ESO-1
The polarization of the cytokine profile among CD4+ T cells as well as proliferation rates were analyzed in the final T-cell products by multispectal cytofluorimetry. Intracellular staining and analysis via flow cytometry demonstrated that among expanded T cells from healthy (n = 4) donors, CD4+ cells show a TH1 cytokine profile characterized by the presence of IFNγ (20.1 ± 7% mean ± SD) and TNFα (29.1 ± 5%), but no IL-10 secreting T cells in response to NY-ESO-1 overlapping peptides pools ().
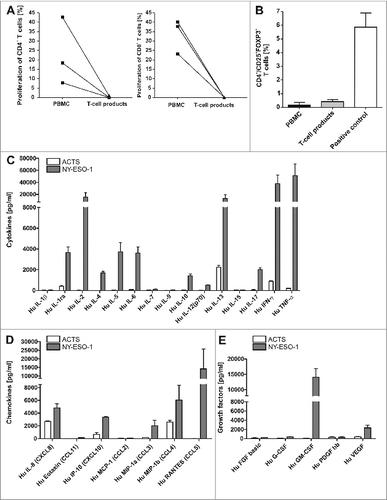
Figure 3. T-cell lines show effector memory phenotype with TH1 cytokine profile that do not include regulatory (T)cells. (A) There was no induction of alloreactivity when T cells of the final T-cell products and PBMCs of the same donor from donor 1, 7 and 4 were stimulated with allogeneic PBMCs and proliferation was analyzed via CFSE staining. Background proliferation using stimulation with autologous PBMCs was subtracted. (B) The final T-cell products were evaluated by immunostaining and flow cytometry for the frequency of regulatory T cells (Tregs) in comparison to peripheral blood mononuclear cells (PBMCs). T cells stimulated with 1000 U/mL IL-2 and CD3/CD28 beads were used as a positive control for appropriate gating of Tregs. Bars represent the mean results +/− SD of CD25+ FOXP3+ T cells from 3 different donors. (C–E) To quantify TH1 cytokines (C), chemokines (D) and growth factors (E) in the supernatants of T cells, the T-cell line from donor 1 was re-stimulated for 6 h with NY-ESO-1-or ACTS-pulsed DCs and supernatants were analyzed in a multiplex magnetic cytokine assay. All multiplex analyses represent mean results of 4 wells from 2 undiluted and 2 diluted (1:2, so values multiplied by 2) +/− SD of a T-cell line from donor 1.
NY-ESO-1-specific CD4+ T cells show a cytolytic response to NY-ESO-1
Since cytokine producing CD4+ T cells were the major specific T-cell population in our GMP generated NY-ESO-1 targeted T-cell lines we next investigated direct cytotoxic effects of CD4+ T cells against NY-ESO-1 pulsed targets. To address this question CD4+ T-cell lines from donor 1 and donor 4 were re-stimulated for 6 h with DCs pulsed with pools of overlapping NY-ESO-1 peptides in the presence of CD107a antibody (). CD4+ T cells showed pronounced cytolytic responses to NY-ESO-1 correlating with the effector-to-target cell ratio.
CD4+ IFNγ+ T cells differentiate into a central-and effector memory phenotype
As shown in , multispectral fluorescence cytometry for T-cell maturation markers revealed a predominance of T cell subsets of early and late differentiation stages among donor-derived T cell lines (n = 3). Only a small population of naïve T cells were detected, defined as CD27+/CD28+ cells (2.9 ± 4% mean ± SD) and CD62L+/CD45RO− cells (1.0 ± 1%). A higher percentage of T cells were central memory T cells identified as CD45RA−/CCR7+ (34.5 ± 22%). The majority of T cells were effector memory T cells defined as CD62L−/45RO+ (78.9 ± 5%) Equivalent results were found using the markers CD45RA, CCR7, CD27 and CD28 ().
Safety assessment of the final T-cell product
Adoptive T-cell transfer in allogeneic settings should be highly specific without any alloreactivity. Alloreactivity was analyzed in mixed lymphocyte reactions using CFSE-based proliferation assays. Results revealed neither IFNγ secretion after re-stimulation with irradiated allogeneic PBMC (data not shown) nor any specific alloreactive proliferation in response to allogeneic PBMC (Fig. 3A). In contrast, unselected T cells from the same donor contained alloreactive T cells between 9 and 45 percent.
Regulatory T cells (Treg) may hamper the efficacy of adoptive T-cell transfer. Since IL-2 can potentially induce Treg enrichment, we next set out to analyze FOXP3/CD25 double positive CD4+ T cells (n = 3) to detect Tregs in our GMP generated NY-ESO1-specific T-cell lines. Although high frequencies of cells expressing the IL-2 receptor (CD25) were detected, no remarkable upregulation of FOXP3 was found () relative to levels among PBMCs and in comparison to the positive control (Treg induction by high dose IL-2 and CD3/CD28 beads).
To exclude T-cell responses against impurities, we cross-analyzed NY-ESO-1-specific T-cell lines with pools of overlapping NY-ESO-1 peptides from a different commercial supplier. T-cell specificities were confirmed, as a similar frequency of NY-ESO-1-specific T cells was detected independent of the supplier of the pools of overlapping NY-ESO-1 peptides (Fig. S1).
Response pattern of cytokines, chemokines and growth factors
To further confirm these results and to address the question whether other cytokines and chemokines may be involved in the antigen-specific response, we repeated the generation of NY-ESO-1-specific T cells from donor 1. Culture supernatants of the final T cell product were collected after restimulation with antigen-pulsed DCs (overlapping petide pools of NY-ESO-1 or ACTS, respectively) and multiplex magnetic cytokine analysis was performed. High amounts of TNFα, IFNγ and IL-2 were detected in the culture supernatants (Fig. 3C, 3D and 3E). Moreover the chemokine MIP-1β (CCL4), the growth factor GM-CSF and Rantes (CCL5) were enhanced in supernatants of T cells re-stimulated with NY-ESO-1. Regarding TH2 cytokines only IL-13 was strongly induced after re-stimulation with NY-ESO-1. T-cell analyses from 3 more NY-ESO-1 T-cell lines showed the same cytokine pattern with high levels of IFNγ, TNFα and IL-2 in NY-ESO re-stimulated T cells, although naturally the absolute concentrations of cytokines differed from donor to donor. The growth factor GM-CSF was strongly induced in half of the 4 samples and Rantes could be induced only in T cells from this donor after restimulation with NY-ESO-1 (data not shown). Experimental values above the highest standard in Figure 3C-E were set to the level of the highest standard as they could not be extrapolated (see Materials and Methods), occurring for IL-2, IFNγ, MIP-1β, Rantes and GM-CSF for at least one condition in T cells stimulated with NY-ESO-1.Values set according to the lowest standard value (as levels were below the standard) were observed for IL-2, IL-4, IL-12, IL-17, Eotaxin, Rantes, basic FGF and G-CSF for at least one condition only in T cells stimulated with ACTS. In the case of PDGF, all values in ACTS_stimulated T cells as well as in NY-ESO-1 stimulated T cells were below the lowest standard value. These results confirm that the generated T-cell products could induce a proinflammatory micromilieu.
Peptide and protein specificity of NY-ESO-1 T cells
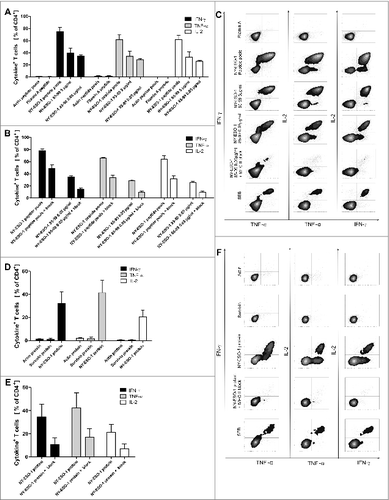
To further elucidate the specificity of NY-ESO-1-specific CD4+ T cells we analyzed NY-ESO-1 T cells from the repeated T-cell generation from donor 1 (78.4% IFNγ+ CD4+ NY-ESO-1-specific T cells, ) that had been utilized for cytokine analyses in supernatants (). Peptide specificity was confirmed with a HLA-Class II binding single peptide epitope NY-ESO-185–99 that was chosen using SYFPEITHI database. NY-ESO-185–99 is predicted to bind to HLA-DRB1*1501, the HLA-II type of donor 1. We found that 39.3% of CD4+ T cells from the donor 1 T-cell line secreted IFNγ in response to peptide-loaded DC (5 μg/mL NY-ESO-185–99; ). This provides indirect evidence suggesting exclusion of clonality of the T-cell lines, since about half of the responding T cells respond to a single peptide and the remaining presumably respond to others. In blocking experiments we proved that T-cell activation is HLA-dependent. More than 50% of IFNγ, TNFα and IL-2 cytokine secretion could be blocked by an anti-HLA-DR, DB, DQ antibody (). To analyze if the T cells recognize peptides after natural processing by antigen presenting cells, dendritic cells were pulsed with recombinant NY-ESO-1 protein, co-cultured with T-cell lines, and analyzed 16 h later. shows that 34.7% of CD4+ T cells secreted IFNγ in response to the processed protein. Again, the reaction could be blocked efficiently down to 11% IFNγ+ CD4+ T cells using HLA-DR, DB, DQ blocking antibodies ().
Figure 4. Recognition of a HLA-DR-binding peptide and naturally processed peptides. (A) As generation of NY-ESO-1-specific T cells was performed with an antigen independent of HLA types, we assessed MHC-binding specificity with a HLA Class II peptide predicted to bind HLA-DRB1*1501 according to SYFPEITHI database and synthesized this peptide (NY-ESO-185–99). NY-ESO-1-specific T cells from of donor 1 were re-stimulated with dendritic cells (DCs) pulsed with 5 µg/mL and 0.05 µg/mL NY-ESO-1 85–99 or control antigens (overlapping pools of NY-ESO-1, Class II matched background control filamin A peptide, positive control SEB) for 6 h. Specific responses were analyzed through expression of TNFα, IFNγ and IL-2. Recognition of the peptide is demonstrated by the bars depicting the mean values of 4 independent experiments +/− SD) (B) To confirm MHC Class II dependent recognition, HLA-DR, DB, DQ blocking antibodies were added to 3 of the T cell-stimulations described above. Bars show the specific blocking of T-cell peptide recognition using anti-HLA-DR, DB, DQ antibodies as mean values of 3 independent experiments +/− SD. (C) Immunostaining and cytofluorimetric analysis for IFNγ, TNFα and IL-2 to assay MHC Class II dependent peptide-specificity. (D) Recognition of naturally processed peptides: NY-ESO-1-specific T cells from donor 1 were re-stimulated with DCs in the presence of recombinant NY-ESO-1 protein or appropriate controls (negative = recombinant proteins Actin C and Survivin; positive control = SEB) for 16 h. Analysis for protein specificity represent 4 independent experiments +/− SD. Specific responses were analyzed through expression of TNFα, IFNγ and IL-2. (E) Blocking of the protein recognition by HLA-DR, DB, DQ antibodies: Bars demonstrate mean cytokine secretion of 3 independent experiments +/− SD. (F) Immunostaining and cytofluorimetric analysis for IFNγ, TNFα and IL-2 to assay MHC-Class II dependent peptide-specificity.
Antitumor responses induced by NY-ESO-1-specific T cells
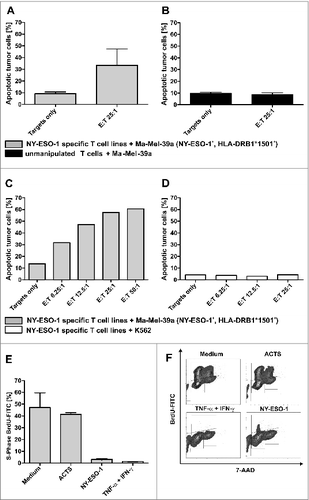
T cells generated from donor 1 were further used to analyze antitumor responses. First, induction of apoptosis was tested with the melanoma cell line Ma-Mel-39a that was matched to HLA-DRB1*1501 of the T-cell donor and expressed NY-ESO-1. The expression of NY-ESO-1 was confirmed by immunhistochemistry on cytospins, HLA-DRB1*1501 typing was assessed by PCR and MHC II expression was confirmed by immunostaining and quantitative flow cytometry (Quifikit Assay; data not shown). The rate of melanoma apoptosis increased after 4 h of co-culture with NY-ESO-1 specific T-cell lines to 35.7% compared to 9.6% in the medium control (mean value of 4 independent experiments) at an effector to target (E:T) cell ratio of 25:1 (). In contrast, unmanipulated T cells from 3 healthy donors did not induce apoptosis after co-culture with Ma-Mel-39a cells (). A representative titration of T cells from donor 1 further illustrates these results (Fig. 5C). No apoptosis induction was observed after 4 h co-incubation of NY-ESO-1 T cells with K562 cells, thus excluding HLA-independent, non-specific, soluble toxic effects (). To further characterize antitumor responses with regard to effects mediated by secreted soluble agents, such as cytokines, cell culture supernatants were generated after 6 h of re-stimulation with NY-ESO-1, as described above. Cell cycle assays revealed that supernatants could induce a predominant G0/G1 arrest and apoptosis in the primary melanoma tumor cell line WM115 after 5 d of co-incubation comparable to the positive control consisting of 100 ng/mL IFNγ and 20 ng/mL TNFα (). In contrast supernatants generated using negative control antigen Actin S as stimulus, had no effect on the cell cycle and results were comparable to the medium control.
Figure 5. Antitumor responses induced by NY-ESO-1 specific T cells. (A) Cell-mediated antitumor response were assessed by co-culturing donor-derived T cell lines with melanoma cells. NY-ESO-1_specific T cells from donor 1 were re-stimulated for 4 h with the NY-ESO-1 expressing cell line Ma-MEL-39a, that was partially matched to the T-cell donor (HLA-DRB1*1501). Analysis of 4 independent experiments show an induction of apoptosis at an effector to target (E:T) cell ratio of 25:1. (B) Un-manipulated T cells from donor 1 did not induce apoptosis of Ma-Mel-39a cells, demonstrating the specific recognition of MaMel-39a by NY-ESO-1 cells. (C) Titration of T cells demonstrates a T-cell dependent increase of apoptosis in Ma-Mel-39a. (D) Absent induction of apoptosis in K562 cells by NY-ESO-1-specific T cells excludes non-specific toxic effects. (E) Supernatant-mediated anti-tumor response: NY-ESO-1-specific cell lines from donor 1 were re-stimulated with overlapping pools of peptides from NY-ESO-1 or Actin S (ACTS). Supernatants were collected 6 h later and co-incubated for 5 d with the primary melanoma cell line WM115 and BrdU incorporation was analyzed by flow cytometry. Controls were performed using medium alone and 100 ng/mL IFNγ + 20 ng/mL TNFα. DNA amount was analyzed by counterstaining with 7-AAD. Analyses of 2 independent experiments demonstrate a cell cycle arrest in the cytokine melanoma cell line WM115. (F) Representative result of the flow cytometry analysis after BrdU and 7-AAD staining.
Discussion
We present a protocol conforming to GMP to generate NY-ESO-1-specific T cells that will enable clinicians to treat malignancies expressing this tumor-associated antigen. Attacking tumor cells with tumor antigen-specific CD4+ TH1 cells is a novel and promising approach. It was shown recently, that TH1 cells are able to mediate direct induction of senescence in tumor cells through TH1 cytokine signals. Thus, adoptive T-cell transfer (ACT) of tumor antigen-specific CD4+ TH1 cells could become a novel and highly efficient antitumor therapeutic avenue. To date, adoptive T-cell transfer has been performed against selected malignancies, however the protocols currently used for clinical grade generation of T-cell lines are extremely labor intensive and restricted to scarce centers. Therefore, we established a simple, rapid and broadly applicable method to generate polyclonal, antigen-specific, TH1-driven CD4+ and CD8+ T cells from peripheral blood lymphocytes. NY-ESO-1 was chosen as model tumor antigen since it is expressed in many tumor entities, and efficacy in soliciting T-cell responses has already been proven in the setting of immunotherapies.Citation15,22,27 Moreover precursor T cells specific for NY-ESO-1 have been previously described in healthy individualsCitation23 and their presence has been associated with beneficial clinical courses in cancer patients.Citation24 Indeed, we demonstrated for 11 of 12 healthy donors that specific T-cell responses can be detected after 14 d of pre-sensitization with NY-ESO-1 without the use of dendritic cells.
Using PBMCs from four donors we have shown here that large-scale generation of NY-ESO-1-specific T cells according to GMP regulations is feasible. The polyclonal T-cell products so generated show robust TH1 cytokine (i.e., IFNγ and TNFα) responses with a preponderance of CD4+ helper T cells. The expression of IFNγ and TNFα in CD4+ cells defines the helper T cell population as TH1 cells.Citation28,29 IFNγ has been described as a mediator of antitumor reactions alone or synergistically with TNFα.Citation30-32 Research efforts of the last years have demonstrated the importance of CD4+ TH1 cells for tumor rejection in mouse models. CD4+ T cells are presumed to have direct cytotoxic effects,Citation33,34 as well as arrest cell growth and induce senescence leading to tumor dormancy through secretion of IFNγ and TNFα.Citation32,35 Finally, CD4+ T cells are important for invasion of CD8+ T cells into the tumor.Citation36 In addition, TH1 cytokines lead to an upregulation of MHC expression on tumor cellsCitation17,37 and enable CD8+ attack. Clinical responses have been described in single patients with melanoma through adoptive transfer of NY-ESO-1-specific CD4+ T cells.Citation15 CD4+ antitumor responses in mice appear to be even more efficient than CD8+ T cells, particularly CD4+ T cells partnered with NK cellsCitation11 in adoptive transfer. In humans, it has been shown that genetically engineered lymphocytes targeting NY-ESO-1 induce objective response rates in patients afflicted with metastatic synovial cell sarcoma and melanoma.Citation38 Very recently, Tran et al. impressively demonstrated that epithelial cancer can be cured by polyclonal, polyfunctional TH1 CD4+ T cells. Specifically, a patient with cholangiocarcinoma exhibited tumor regression in response to T cells against a mutated antigen encompassing more than 95% of CD4+ TH1 T cells.Citation14
In contrast to many other approaches of T-cell transfer we present a protocol that is focused on polyclonal, polyfunctional CD4+ T cells. Multiplex cytokine array confirmed secretion of the TH1 cytokines IFNγ, TNFα and IL-2 and excluded tolerogenic cytokines.
We demonstrated antigen-specificity by 2 approaches. First, we examined whether NY-ESO-1-specific T cells can recognize intracellular processed protein. For this purpose we pulsed dendritic cells with recombinant NY-ESO-1 and confirmed specific T-cell activation of NY-ESO-1-specific T cells. Second, we defined a new single MHC Class-II binding NY-ESO-1 epitope predicted to be immunodominant. NY-ESO-1-specific T cells were subsequently analyzed and about half of T cells were proven to be directed against this epitope. Of note this is the first report demonstrating the recognition of the MHC Class II NY-ESO-1-specific T-cell epitope SRLLEFYLAMPFATP matched to the frequent MHC allele HLA-DR B1*1501.
Adoptive T-cell transfer approaches aim at a sustained immunity in vivo. Therefore, repopulating capacity and hence the T-cell phenotype have a strong influence on the success of T-cell transfer.Citation39,40 The GMP-protocol presented here, shows a strong potential of proliferation of both CD4+ and CD8+ T cells after repeated exposure to the antigen. The polyclonal IFNγ+ / CD4+ T cells consist of a mixture of central memory and effector memory T cells. However the expansion rate was variable in our protocol, but even in the case of the worst expansion rate, 343 million T cells were retrieved at the end of the protocol. In case of the lowest starting cell frequency, 67 million T cells were generated during the protocol. It remains to be determined if these numbers of ex vivo expanded immune cells are sufficient for efficacious treatment and precisely how many T cells are necessary for a successful immunotherapy. Future clinical trials will address these questions.
To evaluate the anticancer effect of in vitro generated NY-ESO-1-specific TH1 CD4+ cells, we performed co-culture experiments with tumor cells. NY-ESO-1-specific TH1 CD4+ cells induce a G0/G1 arrest in tumor cells mediated by IFNγ and TNFα as shown by cell cycle assays. In addition, induction of apoptosis through NY-ESO-1 specific TH1 CD4+ cells was proven using a NY-ESO-1 positive tumor cell line. The cytopathic mechanisms of NY-ESO-1-specific TH1 CD4+ cells include soluble factors as well as cell-to-cell contact, as shown by supernatant mediated effects and CD107a assays.
Several studies propagate the use of lymphodepletion or stem cell transplantation prior to administration of tumor antigen-specific T cells. Allogeneic stem cell transplantation has been successfully applied in the treatment of hematologic malignancies.Citation41-44 Treatment of solid tumors with allogeneic stem cell transplantation has not fulfilled clinical promise and remains controversial. However, relapse after allogeneic stem cell transplantation has been shown to be associated with absence of T-cell responses against tumor antigens, leading to the assumption that these responses are involved in graft-versus-tumor effects.Citation45 In accordance with these results, tumor elimination has been demonstrated using allogeneic T cells against gp100 and TRP-1.Citation46 Therefore, we excluded alloreactive responses in our T-cell lines to enable their use after allogeneic SCT in patients that were pretreated with this regimen.
In conclusion, TH1-driven, tumor-associated-antigen specific T cells from healthy donors were successfully generated from peripheral blood for adoptive transfer using a clinical grade protocol and according to current GMP regulations. Generated T-cell lines are functional and specific comprising a predominance of TH1 CD4+ T cells. Recognition and paralysis of tumor cells by NY-ESO-1-specific TH1 CD4+ cells holds great promise for tumor immunotherapy. Furthermore, the preparation of NY-ESO-1-specific T cells in the GMP facility has already been approved as an advanced therapeutic medicinal product by regulatory authorities. The availability of T-cell cancer immunotherapies derived from peripheral blood will broaden possible applications. Future outlooks could encompass combining T-cell therapy with vaccination strategies against NY-ESO-1 to boost T-cell responses post-adoptive transferCitation47 and combining T-cell therapy with immune checkpoint inhibitors to sustain T-cell responses.
Materials and Methods
Culture media and additives
RPMI 1640 low endotoxin (Biochrom), supplemented with 1% L-glutamine (Biochrom) and 10% human pooled AB-serum (DRK Tübingen, Germany and DRK Ulm, Germany) was used for all cell cultures, except for maintaining tumor cells where medium was supplemented with 10% fetal calf serum (Biochrom). IL-4, TNFα, GM-CSF, IL-6 and IL-1β were provided by Cellgenix in research quality, and IL-7 and IL-15 in GMP quality (Cellgenix). IL-2 (Proleukin) was obtained from Novartis. For cell-cycle assays, IFNγ (Imukin→) was provided by Boehringer, and TNFα by R&D Systems. Prostaglandin E was purchased from Sigma Aldrich.
Peripheral blood mononuclear cells and tumor cell lines
Peripheral blood mononuclear cells (PBMCs) from healthy donors were isolated by Ficoll/Paque (Biochrom) density gradient centrifugation from whole blood or leukapheresis after informed consent. PBMCs were used for determination of NY-ESO-1 frequency and from some donors for additional generation of NY-ESO-1 specific T-cell lines. MHC typing was performed by PCR in the Institute of Transfusion Medicine, University of Tübingen. The NY-ESO-1+/HLA-DRB1*1501+ melanoma cell line Ma-Mel-39a was supplied by EST DAB Database Tübingen, Germany and the melanoma cell line WM115 ATCC®1675 (kindly provided by Birgit Schittek, University Department of Dermatology, Tübingen) and identity was approved by STR profiling in the DZMS Leipzig.
Antigens
NY-ESO-1 protein-spanning, overlapping peptide pools of 15 amino acid length with 11 amino acid overlap (JPT or Miltenyi Biotec) were used for T-cell generation. Sterile filtration of antigens was done before use in the GMP lab. Integrity of antigen after sterile filtration was confirmed by high-performance liquid chromatography (HPLC). Specificity analyses were done with the overlapping pools of NY-ESO-1, recombinant NY-ESO-1 protein (Fisher Scientific), and NY-ESO-1 derived MHC Class II peptide SRLLEFYLAMPFATP predicted to bind HLA-DRB1*1501 according to SYFPEITHI database (synthesized in the Department of Immunology, Tübingen, Germany). As negative controls to define the background stimulation of T cells, overlapping peptide pools of Actin S (JPT), recombinant survivin and recombinant protein actin C (both Abnova) and the filamin-A derived MHC Class II peptide ETVITVDTKAAGKGK (synthesized in the Department of Immunology) were used. Staphylococcus enterotoxin B (SEB) (Sigma-Aldrich) was used as positive control in T-cell stimulations.
Antibodies
Flow cytometry of cells was performed using the following antibodies: anti-CD3 BV-510, anti-CD56-BV711, anti-CD45RO-BV785 (Biolegend), anti-CD4-AF-700 (Exbio), anti-CD3-PE-Cy7 (clone SK7), anti-CD4-APC-H7, anti-CD4-PerCP, anti-CD8-FITC, anti-CD8-APC-H7, anti-CD3-APC, anti-CD45RA-FITC, anti-CD45RO-PE, anti-CD27-FITC, anti-CD27-PE-CF594, anti-CD107a-APC, anti-IFN-γ-APC, anti-IFN-γ-PE (all BD Bioscience) anti-CD62L-FITC, anti-CD28-PE, anti-CD80-PE, anti-CD83-FITC (PharMingen BD), anti-CD3-FITC (clone MEM 57) anti-CD25-PE (both Exbio) anti-CD8-PB (Dako), anti-CCR7-PE (R&D Systems), anti-TNFα-APC, anti-CD28-PECy7 (eBioscience), anti-IFNγ-PE, anti-IL-10-APC (both Miltenyi Biotech). Dead cells were excluded by staining with the amine-reactive dye Alexa Fluor 350 (AF350 NHS Succinimidyl Ester; Invitrogen,) ; Annexin V conjugated with Alexa Fluor 647 (AF647-Annexin V) was used to determine apoptosis in tumor cells (Biolegend). Regulatory T cells were analyzed using anti-FOXP3-AF647 antibody-kit (Biolegend). Blocking experiments were done using HLA-DR, DB, and DQ antibodies, natrium acid (sodium azide) free, (BD Bioscience) at a final concentration of 10 μg/mL.
Generation of NY-ESO-1-specific T cells
Large scale generation of NY-ESO-1-specific T cells according to GMP guidelines was performed in a GMP facility of the University Children´s Hospital Tübingen. The protocol consisted of 3 steps: 1) Antigen-specific pre-sensitization using overlapping pools of NY-ESO-1 in the presence of IL-2 and IL-7; 2) IFNγ based magnetic enrichment of antigen-specific T cells; and 3) Expansion of T cells using autologous feeder cells in the presence of IL-2, IL-7 and IL-15. A detailed protocol for large-scale GMP generation of NY-ESO-1-specific T cells is specified in the supplement and an overview of the procedure is depicted in . Briefly, PBMC from healthy donors were isolated by Ficoll/Paque density gradient centrifugation During pre-sensitization 1 × 107 cells/mL PBMCs () were stimulated with pools of NY-ESO-1 overlapping peptides at 1 μg/mL overnight in RPMI1640, 10% human AB-serum and 1% L-glutamine in culture flasks (Cellstar, Greiner Bio-One) diluted to 5 × 106 cells/mL in the presence of 10 U/mL recombinant human IL-2 (Proleukin®) and 10 ng/mL GMP-grade IL-7. Medium and cytokines were replaced every second to third day. When specific T cells raised to >0.1% within 7–14 days, enrichment of IFNγ+ cells was done after re-stimulation with NY-ESO-1 peptide pool for 6 h using CliniMACS® (Miltenyi Biotec) technique as described previouslyCitation48. The negative fraction was irradiated (30 Gy) and used as feeder cells in ratios of 50–100:1 (feeder:responder) at a concentration of 5 × 106cells/mL in the presence of 10 ng/mL IL-7, 10 ng/mL IL-15 and 50 U/mL IL-2 (Proleukin®) for 14 d. Culture splitting and replacement of medium and cytokines was done every second to third day as needed.
Detection of NY-ESO-1-specific T cells in flow cytometry
For screening of healthy donors for NY-ESO-1-specific T cells and to define the time point of magnetic enrichment during pre-sensitization, IFNγ+ T cells were detected via cytokine secretion assay (CSA-Assay;Miltenyi Biotec) according to manufacturer´s instruction. Peptide stimulation of 2.5 × 106 PBMC from pre-sensitized cultures were re-stimulated for 6 h using overlapping pools of NY-ESO-1 or controls. The stimulated PBMCs were stained using fluorochrome labeled anti-CD3-, anti-CD4-, anti-CD8 antibodies (see above). To analyze the cytokine expression on CD3, CD4 and CD8 T-cell products, 1 × 106 T cells were re-stimulated with different NY-ESO-1 derived antigens using 1 × 106 dendritic cells and intracellular cytokine staining (see above for fluorophore-conjugated antibodies) was performed using the Fix and Perm Kit (AnDerGrub) as previously described. For some analyses T-cell differentiation markers CD45RO, CD62L, CD28, CD27 and CCR-7 were co-analyzed by flow cytometry. All fluorescence cytometry analyses were performed on a LSRII flow cytometer or FACS-Calibur and using FACS-DIVA version 6.1.3 or Cellquest software (all BD Bioscience).
Generation of monocyte-derived dendritic cells
Dendritic cells (DC) were generated as described previouslyCitation49 with monocytes from PBMCs enriched by an adhesion step. Cell culture was performed in the presence of 100 ng/mL GM-CSF and 40 ng/mL IL-4 for 6 d followed by a maturation step with 10 ng/mL IL-1β, 10 ng/mL TNFα, 10 ng/mL IL-6 and 1 μg/mL Prostaglandin E2 for 24 h. Cells were analyzed by flow cytometry to determine the percentage of double positive CD80/CD83 cells. DCs were used for subsequent analysis if double positive cells exceeded 70%.
Functional analysis of NY-ESO-1-specific T-cell lines
In vitro proliferation was detected with carboxyfluorescein diacetate succinimidyl ester (CFSE) as described previouslyCitation50. In brief, cells were labeled with 1.6 μM CFSE (Molecular Probes; Invitrogen) and seeded (1 × 106 cells/well) in 48-well plates with 1 × 105 antigen-pulsed DC. Six d later, the cells were re-stimulated by peptide, stained using appropriate antibodies and staining methods (as described above) for detection of intra-and extracellular markers/cytokines, and analyzed by flow cytometry. Alloreactivity was analyzed using 1 × 106 CFSE-stained T cells in co-culture with 1 × 106 irradiated autologous or allogeneic PBMC for 5 d. Cells were re-stimulated with autologous or allogeneic irradiated PBMCs for 16 h and analyzed as described above. Cytotoxicity was analyzed by CD107a expression in flow cytometry after stimulation of 1 × 106 T cells with different amounts of antigen-pulsed DCs after 6 h in the presence of a CD107a-APC antibody and MonensinA (both BD Bioscience). Tumor-cell apoptosis was determined by flow cytometry. T cells were stained with 0.4 μM CFSE (Molecular Probes; Invitrogen) for discrimination from tumor cells and co-incubated with tumor cells for 4 h in 15 mL tubes. Cells were stained with AF350 NHS Succinimidyl Ester to assess dead cells and Annexin-V-647 to determine apoptosis (Biolegend) according to manufacturer´s instructions. Cytokine levels were quantified in supernatants of co-cultures from 2.5 × 106 T cells and 2.5 × 105 antigen-pulsed DC for 6 h and analyzed with the Bio-Plex magnetic cytokine assay (Bio-Rad-Laboratories) using the BioPlex-reader and-manager software (all Bio-Rad). Values higher than the highest standard value or lower than the lowest standard value were set on lowest or highest standard value, respectively.
Cell cycle analysis
Supernatants from T-cell lines were generated as described for detection of cytokine levels and co-incubated with the primary melanoma cell line WM115. Cell cycle analysis was performed following 5 d of co-incubation of tumor cells with T-cell line supernatants or 20 ng/mL TNFα + 100 ng/mL IFNγ using a FITC-BrdU Flow Kit (BD Bioscience) after 20 h BrdU incorporation. Cells were stained with FITC-conjugated anti-BrdU antibody and 7-actinomycin D (7-AAD) and analyzed by flow cytometry.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1002723_Supplementary_Materials.zip
Download Zip (671.9 KB)Acknowledgments
We thank Sylvia Klein, Christiane Braun, Adelheid Hauler, Marie-Luise Schwesinger, Roksana Wojcik and Sarah Bühler for their excellent technical assistance and Marco Sterk for kindly providing regulatory T cells as positive controls. We thank the Department of Pathology, University of Tübingen for kindly performing NY-ESO-1 immunhistochemistry of tumor cell lines and the Institute of Transfusion Medicine, University Hospital Tübingen for HLA typing and Miltenyi Biotec for providing CliniMACS materials free of charge.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB685), the German Federal Ministry of Science and Education (BMBF, iVAC-ALL) and Fortüne-program (University of Tübingen, 1892–0–0 and 1977–0–1), the European Social Fund in Baden-Württemberg and the Institutional Strategy of the University of Tübingen (Deutsche Forschungsgemeinschaft, ZUK 63).
References
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol 2005; 23:2346–57; PMID:15800326; http://dx.doi.org/10.1200/JCO.2005.00.240
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008; 26:5233–9; PMID:18809613; http://dx.doi.org/10.1200/JCO.2008.16.5449
- Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17:4550–7; PMID:21498393; http://dx.doi.org/10.1158/1078-0432.CCR-11-0116
- Rapoport AP, Aqui NA, Stadtmauer EA, Vogl DT, Fang HB, Cai L, Janofsky S, Chew A, Storek J, Akpek G, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood 2011; 117:788–97; PMID:21030558; http://dx.doi.org/10.1182/blood-2010-08-299396
- Kagamu H, Shu S. Purification of L-selectin(low) cells promotes the generation of highly potent CD4 antitumor effector T lymphocytes. J Immunol 1998; 160:3444–52.
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651–67; PMID:20156973; http://dx.doi.org/10.1084/jem.20091921
- Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol 2008; 180:3122–31; PMID:18292535; http://dx.doi.org/10.4049/jimmunol.180.5.3122
- Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 2008; 13:507–18; PMID:18538734; http://dx.doi.org/10.1016/j.ccr.2008.04.001
- Rocken M. Early tumor dissemination, but late metastasis: insights into tumor dormancy. J Clin Invest 2010; 120:1800–3; PMID:20501952; http://dx.doi.org/10.1172/JCI43424
- Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 2010; 207:651–67; PMID:20156973; http://dx.doi.org/10.1084/jem.20091921
- Perez-Diez A, Joncker NT, Choi K, Chan WF, Anderson CC, Lantz O, Matzinger P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007; 109:5346–54; PMID:17327412; http://dx.doi.org/10.1182/blood-2006-10-051318
- Egeter O, Mocikat R, Ghoreschi K, Dieckmann A, Rocken M. Eradication of disseminated lymphomas with CpG-DNA activated T helper type 1 cells from nontransgenic mice. Cancer Res 2000; 60:1515–20; PMID:10749115
- Ziegler A, Heidenreich R, Braumuller H, Wolburg H, Weidemann S, Mocikat R, Rocken M. EpCAM, a human tumor-associated antigen promotes Th2 development and tumor immune evasion. Blood 2009; 113:3494–502; PMID:19188665; http://dx.doi.org/10.1182/blood-2008-08-175109
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344:641–5; PMID:24812403; http://dx.doi.org/10.1126/science.1251102
- Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 2008; 358:2698–703; PMID:18565862; http://dx.doi.org/10.1056/NEJMoa0800251
- Johnson BD, Jing W, Orentas RJ. CD25+ regulatory T cell inhibition enhances vaccine-induced immunity to neuroblastoma. J Immunother 2007; 30:203–14; PMID:17471167; http://dx.doi.org/10.1097/01.cji.0000211336.91513.dd
- Wolfl M, Jungbluth AA, Garrido F, Cabrera T, Meyen-Southard S, Spitz R, Ernestus K, Berthold F. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol Immunother 2005; 54:400–6; PMID:15449039; http://dx.doi.org/10.1007/s00262-004-0603-z
- Chen YT, Boyer AD, Viars CS, Tsang S, Old LJ, Arden KC. Genomic cloning and localization of CTAG, a gene encoding an autoimmunogenic cancer-testis antigen NY-ESO-1, to human chromosome Xq28. Cytogenet Cell Genet 1997; 79:237–40; PMID:9605863; http://dx.doi.org/10.1159/000134734
- Nakada T, Noguchi Y, Satoh S, Ono T, Saika T, Kurashige T, Gnjatic S, Ritter G, Chen YT, Stockert E, et al. NY-ESO-1 mRNA expression and immunogenicity in advanced prostate cancer. Cancer Immun 2003; 3:10
- Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res 2003; 63:6076–83; PMID:14522938
- Ayyoub M, Taub RN, Keohan ML, Hesdorffer M, Metthez G, Memeo L, Mansukhani M, Hibshoosh H, Hesdorffer CS, Valmori D. The frequent expression of cancer/testis antigens provides opportunities for immunotherapeutic targeting of sarcoma. Cancer Immun 2004; 4:7
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323–37; PMID:19723653; http://dx.doi.org/10.1158/1078-0432.CCR-09-0737
- Valmori D, Souleimanian NE, Hesdorffer CS, Old LJ, Ayyoub M. Quantitative and qualitative assessment of circulating NY-ESO-1 specific CD4+ T cells in cancer-free individuals. Clin Immunol 2005; 117:161–7; PMID:16103015; http://dx.doi.org/10.1016/j.clim.2005.07.004
- Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A, et al. Functional T cells targeting NY-ESO-1 or melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol 2012; 30:1835–41; PMID:22529253
- Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci U S A 2001; 98:3964–9; PMID:11259659; http://dx.doi.org/10.1073/pnas.061507398
- Zeng G, Li Y, El-Gamil M, Sidney J, Sette A, Wang RF, Rosenberg SA, Robbins PF. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res 2002; 62:3630–5; PMID:12097265
- Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ. NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 2006; 95:1–30; PMID:16860654; http://dx.doi.org/10.1016/S0065-230X(06)95001-5
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol 2002; 2:933–44; PMID:12461566; http://dx.doi.org/10.1038/nri954
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today 1996; 17:138–46; PMID:8820272; http://dx.doi.org/10.1016/0167-5699(96)80606-2
- Tu SP, Quante M, Bhagat G, Takaishi S, Cui G, Yang XD, Muthuplani S, Shibata W, Fox JG, Pritchard DM, et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res 2011; 71:4247–59; PMID:21512143; http://dx.doi.org/10.1158/0008-5472.CAN-10-4009
- Liu F, Hu X, Zimmerman M, Waller JL, Wu P, Hayes-Jordan A, Lev D, Liu K. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PloS One 2011; 6:e16241; PMID:21264227; http://dx.doi.org/10.1371/journal.pone.0016241
- Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494:361–5; PMID:23376950; http://dx.doi.org/10.1038/nature11824
- Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637–50; PMID:20156971; http://dx.doi.org/10.1084/jem.20091918
- Brown DM. Cytolytic CD4 cells: direct mediators in infectious disease and malignancy. Cell Immunol 2010; 262:89–95; PMID:20236628; http://dx.doi.org/10.1016/j.cellimm.2010.02.008
- Wieder T, Braumuller H, Kneilling M, Pichler B, Rocken M. T cell-mediated help against tumors. Cell Cycle 2008; 7:2974–7; PMID:18838866; http://dx.doi.org/10.4161/cc.7.19.6798
- Wong SB, Bos R, Sherman LA. Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J Immunol 2008:3122–31; http://dx.doi.org/10.4049/jimmunol.180.5.3122
- Keller CW, Fokken C, Turville SG, Lunemann A, Schmidt J, Munz C, Lunemann JD. TNF-alpha induces macroautophagy and regulates MHC class II expression in human skeletal muscle cells. J Biol Chem 2011; 286:3970–80; PMID:20980264; http://dx.doi.org/10.1074/jbc.M110.159392
- Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011; 29:917–24; PMID:21282551; http://dx.doi.org/10.1200/JCO.2010.32.2537
- Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, Greil J, Albert MH, Schwinger W, Nathrath M, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol 2013; 31:39–48; PMID:23169501; http://dx.doi.org/10.1200/JCO.2011.39.8495
- Feuchtinger T, Opherk K, Bicanic O, Schumm M, Grigoleit GU, Hamprecht K, Jahn G, Handgretinger R, Lang P. Dendritic cell vaccination in an allogeneic stem cell recipient receiving a transplant from a human cytomegalovirus (HCMV)-seronegative donor: induction of a HCMV-specific T(helper) cell response. Cytotherapy 2010; 12:945–50; PMID:20230226; http://dx.doi.org/10.3109/14653241003587645
- Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, Ljungman P, Ferrant A, Verdonck L, Niederwieser D, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86:2041–50; PMID:7655033
- Handgretinger R, Chen X, Pfeiffer M, Schumm M, Mueller I, Feuchtinger T, Hale G, Lang P. Cellular immune reconstitution after haploidentical transplantation in children. Biol Blood Marrow Transplant 2008; 14:59–65; PMID:18162222; http://dx.doi.org/10.1016/j.bbmt.2007.10.015
- Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K, Rubnitz JE, Sandlund JT, Ribeiro RC, Srinivasan A, et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 2011; 118:223–30; PMID:21613256; http://dx.doi.org/10.1182/blood-2011-01-333070
- Bethge WA, Faul C, Bornhauser M, Stuhler G, Beelen DW, Lang P, Stelljes M, Vogel W, Hagele M, Handgretinger R, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis 2008; 40:13–9; http://dx.doi.org/10.1016/j.bcmd.2007.07.001
- Kapp M, Stevanovic S, Fick K, Tan SM, Loeffler J, Opitz A, Tonn T, Stuhler G, Einsele H, Grigoleit GU. CD8+ T-cell responses to tumor-associated antigens correlate with superior relapse-free survival after allo-SCT. Bone Marrow Transplant 2009; 43:399–410; PMID:19139738; http://dx.doi.org/10.1038/bmt.2008.426
- Boni A, Muranski P, Cassard L, Wrzesinski C, Paulos CM, Palmer DC, Gattinoni L, Hinrichs CS, Chan CC, Rosenberg SA, et al. Adoptive transfer of allogeneic tumor-specific T cells mediates effective regression of large tumors across major histocompatibility barriers. Blood 2008; 112:4746–54; PMID:18799724; http://dx.doi.org/10.1182/blood-2008-07-169797
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med 2014; 6:232ra51; PMID:24739759; http://dx.doi.org/10.1126/scitranslmed.3008068
- Feuchtinger T, Richard C, Joachim S, Scheible MH, Schumm M, Hamprecht K, Martin D, Jahn G, Handgretinger R, Lang P. Clinical grade generation of hexon-specific T cells for adoptive T-cell transfer as a treatment of adenovirus infection after allogeneic stem cell transplantation. J Immunother 2008; 31:199–206; PMID:18481389; http://dx.doi.org/10.1097/CJI.0b013e31815ef862
- Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997; 27:3135–42; PMID:9464798; http://dx.doi.org/10.1002/eji.1830271209
- De Rosa SC, Brenchley JM, Roederer M. Beyond six colors: a new era in flow cytometry. Nat Med 2003; 9:112–7; PMID:12514723; http://dx.doi.org/10.1038/nm0103-112
