Abstract
Toll-like receptor 9 (TLR9) is a cellular DNA-receptor of the innate immune system that is widely expressed in cancers. We demonstrated that low tumor TLR9 expression predicts poor disease-specific survival in triple negative breast cancer (TNBC) and renal cell carcinoma (RCC). We hypothesized that this is because TLR9 expression affects tumor immunophenotype. To begin to test this, we compared the number of tumor infiltrating CD8+ T lymphocytes with TLR9 expression in treatment naïve breast cancer (n = 197) and RCC (n = 94) cohorts with known TLR9 expression status. CD8+ T lymphocyte counts were assayed with image analysis after immunohistochemistry (IHC). Tumor TLR9 expression was not correlated with CD8+ T cell counts in breast cancer or RCC. CD8+ T cell counts were significantly associated with tumor proliferation index in TNBC, but not in non-TNBC. CD8+ T cell counts were also significantly associated with tumor grade in non-TNBC, but not in TNBC. In RCC, CD8+ T cell counts were significantly associated with tumor stage. CD8+ T cell counts were significantly associated with prognosis in TNBC and RCC, but the presence of CD8+ T cells in these tumors had opposite effects on disease-specific survival: High CD8+ counts were associated with better prognosis in TNBC and worse prognosis in RCC. Among TNBC patients, those with low tumor TLR9 and low CD8+ T cell counts had the poorest prognosis (log-rank p = 0.0002 vs. high tumor TLR9 and high CD8+ T cell count). In conclusion, pre-treatment tumor TLR9 status is not associated with tumor infiltrating CD8+ T lymphocytes in TNBC or RCC. The combination of TLR9 and CD8+ TIL count might be a novel composite prognostic marker in TNBC.
Abbreviations:
- BC, breast cancer
- CI, confidence interval
- CISH, chromogenic in situ hybridization
- DAB, diaminobenzidine
- DFS, disease-free survival
- ER, estrogen receptor
- HER, human epidermal growth factor
- IHC, immunohistochemistry
- MMP, matrix metallopeptidase
- Non-TNBC, non-triple negative breast cancer
- PR, progesterone receptor
- RCC, renal cell carcinoma
- TIFF, Tagged Image File Format
- TIL, tumor infiltrating lymphocyte
- TLR9, toll-like receptor 9
- TNBC, triple-negative breast cancer
Introduction
TLR9 is a cellular DNA receptor, which reacts to both microbial and vertebrate (self) DNA that enters cells during microbial infections or cell death.Citation1-3 DNA stimulation of TLR9 activates a robust, inflammatory reaction, with increased interleukin and cytokine production.Citation4,5 The outcome of this innate immune reaction is activation of the adaptive immunity in the whole organism and eventually, an immunological elimination of the invading microbe and the infected cells.Citation4,6
Although initially thought to be limited only to the innate immune system, it is now well established that TLR9 is widely expressed also in various cancer types, such as in breast, brain, gastric, lung, ovarian, prostate and kidney cancers as well as in cancers of the GI tract.Citation7-17 Stimulation of TLR9 expressing cancer cells with bacterial DNA-like, synthetic TLR9 ligands induces MMP-13-mediated invasion in vitro.Citation7,10,18,19 Based on these findings, it was expected that tumor TLR9 mediates invasion and metastasis and that high tumor TLR9 expression would be associated with worse survival. Such an association has indeed been made in brain, prostate and esophageal cancers.Citation9,14,16,20 However, in TNBC and in RCC, the prognostic value of tumor TLR9 expression is the opposite. In these cancers, low tumor TLR9 expression has been shown to predict poor prognosis and short disease-specific survival.Citation11,13 Among breast cancer patients, those with TNBC are considered to have the worst prognosis.Citation21,22 This is because of the generally aggressive behavior of such tumors and also because these tumors lack the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), which would allow targeted treatment.Citation21,22 In an attempt to understand why the absence of this protein is associated with poor prognosis, we have focused on the role of TLR9 in the pathophysiology of TNBC in our studies. We demonstrated recently in a pre-clinical model of TNBC that although the actual tumor response to doxorubicin treatment is independent of the tumor TLR9 status, the inflammatory response to this treatment may be significantly weaker in TNBC cells that lack TLR9 expression.Citation3 These findings lead us to think that tumor TLR9 expression status may affect the immunophenotype of TNBC tumors, and thereby, interfere with the immunologic response to standard cancer treatments.
It is becoming increasingly clear, that in addition to the direct cancer cell targeting effects of standard cancer treatments, their efficacy is also mediated via the immune system.Citation23-25 More specifically, cancer cell death induces the release of immunologically active molecules that activate anticancer immunity.Citation24,26-29 When successful, this results in an immunological destruction of residual disease and translates into cure.Citation30-32 The immunological destruction of cancer cells is mediated via oncolytic CD8+ T lymphocytes. However, the activity of the oncolytic cells is highly regulated by other types of tumor infiltrating lymphocytes (TILs), such as the regulatory Foxp3+ T cells (Tregs) and also by dendritic cells.Citation24,30 The balance of the oncolytic vs. tumor growth permitting TILs eventually depicts the fate of residual tumor cells. In addition to affecting treatment, pre-treatment TILs have also been shown to be predictive of treatment responses.Citation33,34 To begin to study the possible role of tumor TLR9 in defining tumor immunophenotype, we compared tumor TLR9 expression status with tumor CD8+ T cell infiltration, using treatment-naïve breast cancer and RCC cohorts that were previously characterized for TLR9 expression.Citation11,13
Results
Association of tumor TLR9 expression status and tumor infiltrating CD8+ T lymphocyte count in breast cancer
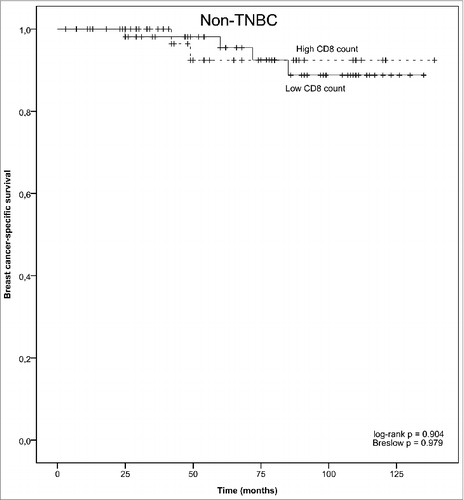
The baseline and clinicopathologic characteristics of the studied breast cancer patient population have been published by us previously.Citation13 The tumor TLR9 status was previously defined by sorting the TLR9 parameter into low (0-8) and high (> 8-16) TLR9 score groups.Citation13 Examples of high and low infiltrating CD8+ T cell staining patterns in breast cancer specimens are shown in . As expected, no cancer cells were stained with the CD8-antibody. There was not a statistically significant correlation between tumor TLR9 score and tumor infiltrating CD8+ T cell count in either the TNBC (n = 95) or non-TNBC (n = 102) groups (). When compared with standard tumor and patient characteristics, among the patients with TNBC tumors, CD8+ T cell counts were statistically significantly associated with the tumor proliferation rate and with borderline significance, also with the patient menopausal status (). Among the patients with non-TNBC tumors, CD8+ T cell counts were statistically significantly associated only with tumor grade ().
Figure 1. Immunohistochemical stainings of CD8+ T lymphocytes (brown) in breast cancer tissues representing (A) high and (B) low CD8+ TIL counts.
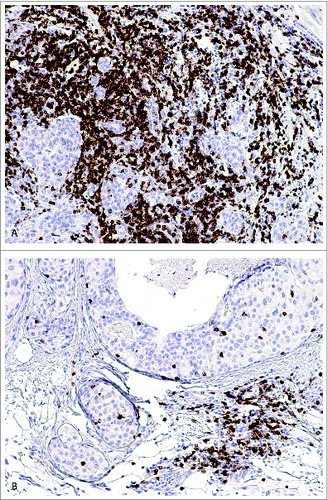
Table 1. CD8+ T-cell counts stratified by characteristics of disease in TNBC cases (n = 95)
Table 2. CD8+ T-cell counts stratified by characteristics of disease in ER+, PR+, HER2-cases (n = 102)
Association of CD8+ T cell counts and traditional prognostic factors with survival in TNBC
When traditional prognostic factors, T stage (T1 vs. 2-4), N stage (0 vs. 1-3), Ki67 (negative to moderate vs. high, determined as % of Ki67-stained tumor cells), grade (I-II vs. III) and CD8+ T lymphocyte count (below or above median) were taken into Cox regression analysis, tumor size (relative risk 6.8, 95% confidence interval (CI) 1.5-31.2), the presence of lymph node metastases (relative risk 6.9, 95% CI 2.22-21.52) but also low CD8+ T-cell count (relative risk 2.6, 95% CI 1.02-6.6) were predictors of worsened survival in TNBC ().
Table 3. Breast cancer-specific survival in TNBC
Association of CD8+ TIL with disease-specific survival in breast cancer patients
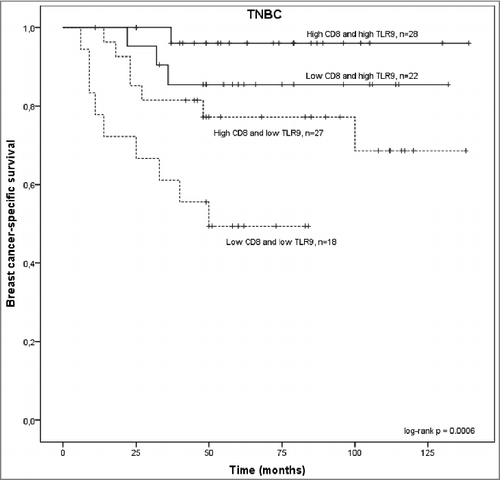
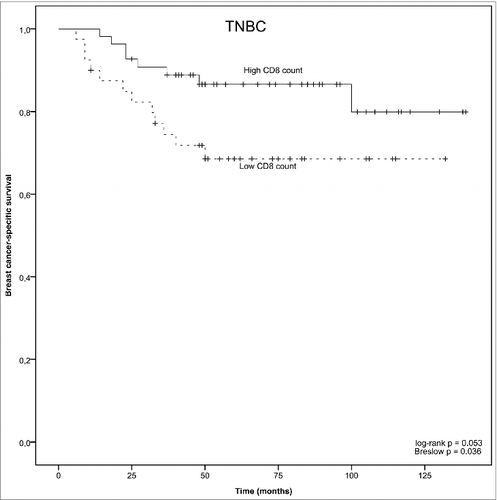
In the TNBC group, there was a significant association with tumor CD8+ TIL status and breast cancer-specific survival. Specifically, low CD8+ T cell count was associated with a significantly shorter disease-specific survival, as compared with TNBC tumors that had high CD8+ T cell counts (log-rank p = 0.053 and Breslow p = 0.036, ). No such association was detected among patients that had hormone-receptor positive breast cancer (non-TNBC group, ). Furthermore, among patients with TNBC tumors, breast cancer specific survival was the poorest with those patients whose tumors had low TLR9 score and low CD8+ T lymphocyte counts (, log-rank p = 0.0002, low TLR9 and low CD8+ TIL vs. high TLR9 and high CD8+ TIL group).
Figure 2. Kaplan–Meyer plot of breast-cancer specific survival in TNBC as stratified by predefined CD8+ T cell count cutoff points.
Association of CD8+ T cell infiltration with disease stage and survival in RCC
The baseline characteristics of the studied RCC cohort has been previously published by us.Citation11 Of the RCC specimens previously analyzed for TLR9 expression (n = 152), 94 were evaluable in this study for tumor infiltrating CD8+ T lymphocytes. The reasons for the lack of complete cohort included loss of the tumor blocks or folding of the specimens during antigen retrieval and/or staining. Examples of the CD8+ staining in RCC are shown in . As expected, no CD8+ staining was detected in cancer cells. Among the analyzed specimens, tumor infiltrating CD8+ T-cell count was significantly associated with disease stage. There were no statistically significant associations between tumor CD8+ T-cell counts and TLR9 expression status or other clinicopathological parameters in RCC (). However, CD8+ T cell counts were significantly associated with disease-specific survival. More specifically, high CD8+ T-cell counts were associated with significantly shorter disease-specific survival (log-rank p = 0.042) ().
Figure 5. Immunohistochemical stainings of CD8+ T lymphocytes (brown) in renal cell carcinoma tissues representing (A) high and (B) low CD8+ TIL counts.
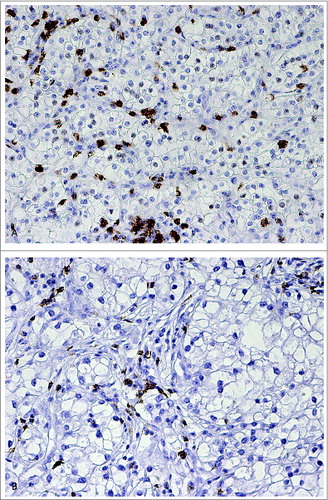
Figure 6. Kaplan–Meyer plots of renal cell carcinoma-specific survival, as stratified by predefined CD8+ T cell count cutoff points.
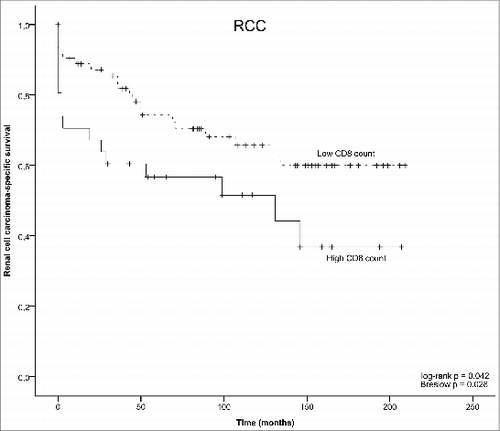
Table 4. CD8+ T-cell counts stratified by characteristics of disease in RCC cases (n = 94)
Discussion
Depending mostly on the tumor characteristics, tumors can either become destroyed by the immune system and therefore cured, or they can evade the immune attack, resulting in tumor outgrowth and clinical relapses.Citation24 Understanding the mechanisms of how tumor cells evade a normal immune attack has resulted in the development of several, novel immunotherapies during the past few years, especially for the treatment of melanoma.Citation35-37 Furthermore, accumulating evidence suggest that adaptive, anticancer immune responses contribute in an important manner to the success of chemotherapy.Citation28,30,38,39 Although breast cancers have not traditionally been considered immunologically active, recent findings suggest the contrary.Citation24 Specifically, strong immune cell filtrate in breast tumors before or after treatment have been shown to be associated with increased survival.Citation30,33,34,40-42 Furthermore, the specificities of the immune cell infiltrate matter.Citation43,44 Tumor characteristics that define breast cancer immunophenotype and the way they recruit immune cells are not currently very well understood.Citation35-37 Recent evidence, however, suggests that several factors, such as immune checkpoint proteins, COX-2 and CD73 may contribute to that. Citation45-47
We recently described a novel, poor prognosis subtype in TNBC, as characterized by low tumor TLR9 expression upon diagnosis.Citation13 Patients whose TNBC tumors had low TLR9 expression levels when diagnosed, had significantly worsened disease-specific survival, as compared with those TNBC patients whose tumors had higher TLR9 levels.Citation13 Our studies with a pre-clinical model of this disease suggested that tumor TLR9 expression may affect tumor immunophenotype and specifically, immunologic response to chemotherapy in TNBC.Citation3 Our results from the current study, however, suggest that tumor TLR9 expression status prior to treatment does not interfere with tumor infiltration of CD8+ T lymphocytes. Similar results were also discovered for RCC, another cancer type where low tumor TLR9 expression is associated with poor prognosis.Citation11 Although it appears that tumor TLR9 expression is not associated with CD8+ T cell accumulation in treatment naïve TNBC and RCC tumors, it is still possible that tumor TLR9 may contribute to tumor immunophenotype by affecting the pre- or post-treatment accumulation of other types of immune cells that are important in cancer immunity. Furthermore, our pre-clinical results suggest that TNBC tumor TLR9 expression status is rather more important in post-treatment immune response.Citation3 These issues require further studies both in animal models and with clinical samples.
Despite the lack of correlation with tumor TLR9 status, CD8+ TIL counts were, however, significantly associated with disease-specific survival both in TNBC and RCC. Interestingly however, the CD8+ TIL counts had opposite effects on survival in these diseases. In TNBC, high CD8+ T cell counts were associated with a significantly better survival. Our results in TNBC agree with those of Mahmoud et al., who also demonstrated that total CD8+ T cell counts were significantly associated with better breast cancer specific survival among patients with ER negative tumors.Citation48 Furthermore, we did not detect an association with CD8+ T cell counts in non-TNBC cases. Interestingly, among TNBC patients, those that had low tumor TLR9 expression combined with low CD8+ TIL counts upon diagnosis had a significantly worsened disease-specific survival, as compared with TNBC patients that had high tumor TLR9 score in combination with high CD8+ TIL count. Taken together, these findings suggest that the anticancer immune mechanisms mediated by tumor TLR9 and tumor infiltrating CD8+ T-cells play an important role in TNBC pathophysiology. Whether or not this combination could be a novel, prognostic composite marker in TNBC, requires further testing with a larger TNBC patient population. On the contrary to the findings in TNBC, high CD8+ T cell counts were associated with a significantly shortened disease-specific survival in RCC. Also this finding is in agreement with previous publications and further supports the previous notions that tumor infiltrating CD8+ T cells may be dysfunctional in RCC.Citation49-52
In conclusion, the importance of cancer immunotherapy is gaining appreciation and understanding the various mechanisms of tumor immunoevasion will likely result in better treatments of cancers that currently have poor prognosis. We show here that pretreatment tumor TLR9 expression does not affect CD8+ TIL recruitment into TNBC and RCC tumors. However, a combination of TLR9 and CD8+ stainings might be a novel prognostic, composite marker for TNBC. In general, understanding the possible TLR9-orchestrated immune response in TNBC and RCC might help in identifying patients that are likely to benefit from cancer immunotherapy.
Materials and Methods
Breast cancer specimens
Surgically resected, archival specimens of breast cancer (n =197, 95 of which were TNBCs and 102 non-TNBCs (ER+, PR+, HER2-)) were initially obtained from the Department of Pathology, Oulu University Hospital (Oulu, Finland) and the Department of Pathology and Forensic Medicine, University of Eastern Finland, (Kuopio, Finland). Specifically, we used the following definition of TNBC and non-TNBC: Tumors exhibiting nuclear estrogen and PR expression in more than 9% of invasive tumor cells were considered as steroid receptor-positive. The TNBC group did not express any ER or PR positivity. In other words, tumors expressing 1-9% steroid receptors were excluded from the study. Membranous HER2 expression was also studied by means of IHC and if a specimen exhibited a HER2 positive result (1+ to 3+ on a scale of 0 to 3+) in IHC, the Her2 gene amplification status was determined by means of chromogenic in situ hybridization (CISH). Breast cancers with 6 or more gene copies of the Her2 gene in cells were considered HER2 positive. The patients were treated at the Departments of Oncology, Oulu University Hospital or at the Kuopio University Hospital, between the years 2000 and 2008. All patients were Caucasian and they were treated and followed according to the prevailing, standard therapy including surgery, chemotherapy, radiation and anti-estrogens in case of hormone-receptor positive cancers. All patients had local or locally advanced disease. There were no statistically significant differences in tumor size, nodal status, grade, tumor proliferation rate (defined as % of cells showing immunopositivity for Ki67 staining), lymphatic or blood vessel invasion between the low and high TLR9 groups.Citation13 All the relevant patient data was gained from the hospital patient records and Statistics Finland. The research was approved by the Northern Ostrobothnia and Kuopio Hospital District Ethics committee and by the National Authority for Medicolegal Affairs (VALVIRA). Details of this cohort can be found in our previous publication.Citation13
Renal cell carcinoma specimens
Surgically resected, archival resection specimens of RCC (n = 94) were obtained from the Department of Urology, Oulu University Hospital (Oulu, Finland). The specimens were collected between the years 1990 and 1999. This retrospective clinical cohort consisted of patients (initially 152 patients with 77 (51%) females and 75 (49%) males) who underwent surgery for primary RCC at the Oulu University Hospital. There were no statistically significant differences between tumor size, tumor stage, nuclear grade or histological type of the tumors between the high and low TLR9 expression groups.Citation11 All clinical data and patient follow-up details were collected from patient records and re-evaluated by the same urologist. The study was approved by the Northern Ostrobothnia Hospital District Ethics committee. This cohort has been described in detail previously.Citation11 Both the breast cancer and renal cell cancer cohorts have been previously analyzed for TLR9 expression.Citation11,13
Immunohistochemical detection of CD8+ T lymphocytes
Tissue sections of 8 μm in thickness were cut with a microtome from the paraffin embedded tissue blocks of breast cancer or RCCs. The specimens were processed and stained for CD8+ using a 1:100 dilution of a monoclonal antibody to human CD8+ (NCL-CD8-4B11, Novocastra). IgG, instead of the primary antibody served as a negative staining control. The specificity of the antibody was confirmed by performing immunohistochemical stainings with the same protocol on paraffin-embedded human tissue sections that contained normal human appendix (data not shown). The bound antibodies were visualized using Envision Detection System (K500711; Dako, Carpinteria, Denmark A/S). Diaminobenzidine (DAB) was used as a chromogen. All staining was performed using the LabVision Autostainer™ (LabVision, Fremont, CA, USA).
CD8+ T lymphocyte quantification with image analysis
The quantification method used was the same for both the breast cancer and the RCC cohorts. First, a four field image capturing by QImaging MicroPublisher 5.0 RTV (QImaging, Canada) was done for the CD8+-stained specimens. The camera was attached to Nikon Eclipse E600 (Nikon Inc., Japan) microscope with a 20X objective. A total of 532 pictures were saved in Tagged Image File Format (TIFF) using a 2560 × 1920 resolution. Tumor infiltrating CD8+ T cells were counted from each tumor area captures and the total number per sample was determined by the sum of the counts. The tumor caption's CD8+ T cell quantity assessment was performed by a Java operating open source image analysis program ImageJ v1.44. 262 The quantification method has been previously described in detail.Citation53 The image analysis algorithm was kindly shared by the original developer of the method, Dr Juha Väyrynen (Dept. of Pathology, Oulu University Hospital, Oulu, Finland).
Statistical analysis
Statistical analyses of the data were performed by using the SPSS 17.0 for Windows (IBM SPSS Inc., USA). For the breast cancer specimens, the cases were sorted by triple negative status to TNBC (ER-, PR-, HER2-) group and non-TNBC groups (ER+, PR+, HER2-). The correlations between TLR9 scores and CD8+ T-cell counts in all groups were analyzed by using a nonparametric, independent samples test. The significance of associations between binominal variables was defined using two-sided Fisher's exact test. The hazard ratios and 95% CIs were provided for each covariate. Survival was analyzed by using Kaplan-Meier curves with the log-rank test. Disease-free survival (DFS) was calculated from the first operation date to the time of local relapse or detection of metastases, whichever came first. In the breast cancer group, CD8+ T cell count cut-off in the DFS analysis was 125.5 which was defined as an optimal DFS endpoint predictor by conducting a ROC analysis. CD8+ counts below this were considered low and counts above this, were considered high. In the RCC group, the CD8+ cut-off used in DFS analysis was 70, as also defined optimal threshold by ROC analysis. Similarly, CD8+ counts below this value were considered low and counts above this, were considered high. Cox regression analysis was used for the multivariate analysis.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study was supported by grants from Breast Cancer Research Foundation of Alabama (K.S.S.), Finnish Medical Fund and Finnish Urological Association (M.H.V.), Thelma Mäkikyrö Foundation, Finnish Cultural Foundation (M.M.), Cancer Society of Northern Finland (KV), the EVO funding of the Northern Savo Hospital District and the Cancer Society of Finland (Y.S.).
References
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000; 408:740-5; PMID:11130078; http://dx.doi.org/10.1038/35047123
- Lamphier MS, Sirois CM, Verma A, Golenbock DT, Latz E. TLR9 and the recognition of self and non-self nucleic acids. Ann N Y Acad Sci 2006; 1082:31-43; PMID:17145922; http://dx.doi.org/10.1196/annals.1348.005
- Tuomela J, Sandholm J, Kaakinen M, Patel A, Kauppila JH, Ilvesaro J, Chen D, Harris KW, Graves D, Selander KS. DNA from dead cancer cells induces TLR9-mediated invasion and inflammation in living cancer cells. Breast Cancer Res Treat 2013; 142:477-87; PMID:24212717; http://dx.doi.org/10.1007/s10549-013-2762-0
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol 2006; 117:979-87; quiz 88; PMID:16675322; http://dx.doi.org/10.1016/j.jaci.2006.02.023
- Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol 2007; 25:419-41; PMID:17378763; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104514
- Paul WE. Bridging innate and adaptive immunity. Cell 2011; 147:1212-5; PMID:22153065; http://dx.doi.org/10.1016/j.cell.2011.11.036
- Ilvesaro JM, Merrell MA, Swain TM, Davidson J, Zayzafoon M, Harris KW, Selander KS. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro. Prostate 2007; 67:774-81; PMID:17373717; http://dx.doi.org/10.1002/pros.20562
- Jukkola-Vuorinen A, Rahko E, Vuopala KS, Desmond R, Lehenkari PP, Harris KW, Selander KS. Toll-like receptor-9 expression is inversely correlated with estrogen receptor status in breast cancer. J Innate Immun 2009; 1:59-68; PMID:20375566; http://dx.doi.org/10.1159/000151602
- Kauppila JH, Takala H, Selander KS, Lehenkari PP, Saarnio J, Karttunen TJ. Increased Toll-like receptor 9 expression indicates adverse prognosis in oesophageal adenocarcinoma. Histopathology 2011; 59:643-9; PMID:22014045; http://dx.doi.org/10.1111/j.1365-2559.2011.03991.x
- Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, Rosenthal E, Chen D, Shackley B, Harris KW, Selander KS. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res 2006; 4:437-47; PMID:16849519; http://dx.doi.org/10.1158/1541-7786.MCR-06-0007
- Ronkainen H, Hirvikoski P, Kauppila S, Vuopala KS, Paavonen TK, Selander KS, Vaarala MH. Absent Toll-like receptor-9 expression predicts poor prognosis in renal cell carcinoma. J Exp Clin Cancer Res 2011; 30:84; PMID:21929816; http://dx.doi.org/10.1186/1756-9966-30-84
- Takala H, Kauppila JH, Soini Y, Selander KS, Vuopala KS, Lehenkari PP, Saarnio J, Karttunen TJ. Toll-like receptor 9 is a novel biomarker for esophageal squamous cell dysplasia and squamous cell carcinoma progression. J Innate Immun 2011; 3:631-8; PMID:21876325; http://dx.doi.org/10.1159/000329115
- Tuomela J, Sandholm J, Karihtala P, Ilvesaro J, Vuopala KS, Kauppila JH, Kauppila S, Chen D, Pressey C, Härkönen P et al. Low TLR9 expression defines an aggressive subtype of triple-negative breast cancer. Breast Cancer Res Treat 2012; 135:481-93; PMID:22847512; http://dx.doi.org/10.1007/s10549-012-2181-7
- Vaisanen MR, Vaisanen T, Jukkola-Vuorinen A, Vuopala KS, Desmond R, Selander KS, Vaarala MH. Expression of toll-like receptor-9 is increased in poorly differentiated prostate tumors. Prostate 2010; 70:817-24; PMID:20054821; http://dx.doi.org/10.1002/pros.21115
- Berger R, Fiegl H, Goebel G, Obexer P, Ausserlechner M, Doppler W, Hauser-Kronberger C, Reitsamer R, Egle D, Reimer D et al. Toll-like receptor 9 expression in breast and ovarian cancer is associated with poorly differentiated tumors. Cancer Sci 2010; 101:1059-66; PMID:20156214; http://dx.doi.org/10.1111/j.1349-7006.2010.01491.x
- Meng Y, Kujas M, Marie Y, Paris S, Thillet J, Delattre JY, Carpentier AF. Expression of TLR9 within human glioblastoma. J Neurooncol 2008; 88:19-25; PMID:18253698; http://dx.doi.org/10.1007/s11060-008-9536-2
- Zhang YB, He FL, Fang M, Hua TF, Hu BD, Zhang ZH, Cao Q, Liu RY. Increased expression of Toll-like receptors 4 and 9 in human lung cancer. Mol Biol Rep 2009; 36:1475-81; PMID:18763053; http://dx.doi.org/10.1007/s11033-008-9338-9
- Ilvesaro JM, Merrell MA, Li L, Wakchoure S, Graves D, Brooks S, Rahko E, Jukkola-Vuorinen A, Vuopala KS, Harris KW et al. Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular invasion. Mol Cancer Res 2008; 6:1534-43; PMID:18922969; http://dx.doi.org/10.1158/1541-7786.MCR-07-2005
- Nurmenniemi S, Kuvaja P, Lehtonen S, Tiuraniemi S, Alahuhta I, Mattila RK, Risteli J, Salo T, Selander KS, Nyberg P et al. Toll-like receptor 9 ligands enhance mesenchymal stem cell invasion and expression of matrix metalloprotease-13. Exp Cell Res 2010; 316:2676-82; PMID:20553713; http://dx.doi.org/10.1016/j.yexcr.2010.05.024
- Vaisanen MR, Jukkola-Vuorinen A, Vuopala KS, Selander KS, Vaarala MH. Expression of Toll-like receptor-9 is associated with poor progression-free survival in prostate cancer. Oncol Lett 2013; 5:1659-63; PMID:23761830; http://dx.doi.org/10.3892/ol.2013.1204
- Carotenuto P, Roma C, Rachiglio AM, Botti G, D'Alessio A, Normanno N. Triple negative breast cancer: from molecular portrait to therapeutic intervention. Crit Rev Eukaryot Gene Expr 2010; 20:17-34; PMID:20528735; http://dx.doi.org/10.1615/CritRevEukarGeneExpr.v20.i1.20
- Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest; 121:2750-67; PMID:21633166; http://dx.doi.org/10.1172/JCI45014
- Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology 2013; 2:e26536; PMID:24404424; http://dx.doi.org/10.4161/onci.26536
- de la Cruz-Merino L, Barco-Sanchez A, Henao Carrasco F, Nogales Fernandez E, Vallejo Benitez A, Brugal Molina J, Martínez Peinado A, Grueso López A, Ruiz Borrego M, Codes Manuel de Villena M et al. New insights into the role of the immune microenvironment in breast carcinoma. Clin Dev Immunol 2013; 2013:785317; PMID:23861693; http://dx.doi.org/10.1155/2013/785317
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51-72; PMID:23157435; http://dx.doi.org/10.1146/annurev-immunol-032712-100008
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/10.1038/nature10673
- Ladoire S, Hannani D, Vetizou M, Locher C, Aymeric L, Apetoh L, Kepp O, Kroemer G, Ghiringhelli F, Zitvogel L. Cell-Death-Associated Molecular Patterns As Determinants of Cancer Immunogenicity. Antioxid Redox Signal 2013; 20(7):1098-116; PMID:23394620; http://dx.doi.org/10.1089/ars.2012.5133
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729-41; PMID:23562161; http://dx.doi.org/10.1016/j.immuni.2013.03.003
- Kepp O, Tesniere A, Zitvogel L, Kroemer G. The immunogenicity of tumor cell death. Curr Opin Oncol 2009; 21:71-6; PMID:19125021; http://dx.doi.org/10.1097/CCO.0b013e32831bc375
- Andre F, Dieci MV, Dubsky P, Sotiriou C, Curigliano G, Denkert C, Loi S. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin Cancer Res 2013; 19:28-33; PMID:23258741; http://dx.doi.org/10.1158/1078-0432.CCR-11-2701
- Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, Spísek R. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res 2011; 71:4821-33; PMID:21602432; http://dx.doi.org/10.1158/0008-5472.CAN-11-0950
- Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ 2013; 21(1):69-78; http://dx.doi.org/10.1038/cdd.2013.72.
- Denkert C. Diagnostic and therapeutic implications of tumor-infiltrating lymphocytes in breast cancer. J Clin Oncol 2013; 31:836-7; PMID:23341523; http://dx.doi.org/10.1200/JCO.2012.47.1698
- Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology 2013; 2:e24720; PMID:24073365; http://dx.doi.org/10.4161/onci.24720
- Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol 2013; 94:41-53; PMID:23667165; http://dx.doi.org/10.1189/jlb.1212631
- O'Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer 2007; 110:2614-27; PMID:18000991; http://dx.doi.org/10.1002/cncr.23086
- Postow MA, Harding J, Wolchok JD. Targeting immune checkpoints: releasing the restraints on anti-tumor immunity for patients with melanoma. Cancer J 2012; 18:153-9; PMID:22453017; http://dx.doi.org/10.1097/PPO.0b013e31824b2404
- Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 2011; 224:389-400; PMID:21437909; http://dx.doi.org/10.1002/path.2866
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014; 14:135-46; PMID:24457417; http://dx.doi.org/10.1038/nrc3670
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31:860-7; PMID:23341518; http://dx.doi.org/10.1200/JCO.2011.41.0902
- Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjänen K. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer 1992; 28A:859-64; PMID:1524909; http://dx.doi.org/10.1016/0959-8049(92)90134-N
- Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarneri V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol 2014; 25(3):611-8; PMID:24401929; http://dx.doi.org/10.1093/annonc/mdt556
- Oda N, Shimazu K, Naoi Y, Morimoto K, Shimomura A, Shimoda M, Kagara N, Maruyama N, Kim SJ, Noguchi S. Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat 2012; 136:107-16; PMID:22986814; http://dx.doi.org/10.1007/s10549-012-2245-8
- Rathore AS, Kumar S, Konwar R, Srivastava AN, Makker A, Goel MM. Presence of CD3+ tumor infiltrating lymphocytes is significantly associated with good prognosis in infiltrating ductal carcinoma of breast. Indian J Cancer 2013; 50:239-44; PMID:24061465; http://dx.doi.org/10.4103/0019-509X.118744
- Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013; 19:5626-35; PMID:23983257; http://dx.doi.org/10.1158/1078-0432.CCR-13-0545
- Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, Overberg P, Rose I, Basu GD, Vranic S et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev 2014; 23:2965-70; PMID:25392179; http://dx.doi.org/10.1158/1055-9965.EPI-14-0654
- Markosyan N, Chen EP, Evans RA, Ndong V, Vonderheide RH, Smyth EM. Mammary carcinoma cell derived cyclooxygenase 2 suppresses tumor immune surveillance by enhancing intratumoral immune checkpoint activity. Breast Cancer Res 2013; 15:R75; PMID:24004819; http://dx.doi.org/10.1186/bcr3469
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949-55; PMID:21483002; http://dx.doi.org/10.1200/JCO.2010.30.5037
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001; 61:5132-6; PMID:11431351
- Cavalcanti E, Gigante M, Mancini V, Battaglia M, Ditonno P, Capobianco C, Cincione RI, Selvaggi FP, Herr W, Storkus WJ, et al. JAK3/STAT5/6 pathway alterations are associated with immune deviation in CD8 T cells in renal cell carcinoma patients. J Bbiomed Biotechnol 2010; 2010:935764; PMID:20339477; http://dx.doi.org/10.1155/2010/935764
- Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU. Intratumoral alterations of dendritic-cell differentiation and CD8(+) T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology 2012; 1:1451-3; PMID:23243626; http://dx.doi.org/10.4161/onci.21356
- Prinz PU, Mendler AN, Masouris I, Durner L, Oberneder R, Noessner E. High DGK-alpha and disabled MAPK pathways cause dysfunction of human tumor-infiltrating CD8+ T cells that is reversible by pharmacologic intervention. J Immunol 2012; 188:5990-6000; PMID:22573804; http://dx.doi.org/10.4049/jimmunol.1103028
- Vayrynen JP, Vornanen JO, Sajanti S, Bohm JP, Tuomisto A, Makinen MJ. An improved image analysis method for cell counting lends credibility to the prognostic significance of T cells in colorectal cancer. Virchows Arch 2012; 460:455-65; PMID:22527018; 10.1007/s00428-012-1232-0