Abstract
Epithelial–mesenchymal transition and immunosuppression are crucial for cancer metastasis and treatment resistance. The mechanism by which these distinct processes are co-opted remains incompletely understood. Our recent work has exposed the “dirty affairs” of the 2 at the tumor site, thus calling for a combined therapy to break such a dangerous liaison.
Introduction
In advanced tumors, multiple mechanisms of resistance emerge during treatment that affect both tumor cell properties and the immune microenvironment.Citation1 For example, an increasing number of studies suggest that radiotherapy and chemotherapy induce an epithelial–mesenchymal transition (EMT), and that the mesenchymal tumor cells have stem cell properties, including resistance to killing by chemotherapeutics and radiotherapy.Citation2,3 Immunotherapies can also create selective pressures that lead to outgrowth of resistant tumor cells through immunoediting.Citation1 Obviously, the interplay between tumor cells and immune cells is a common feature during tumor progression and therapy resistance, but its biological basis has remained obscure. In a recent issue of Nature Communications, Chen and colleaguesCitation4 delineated the point using a K-rasLA1/+p53R172HΔg/+non–small cell lung cancer (NSCLC) murine model to compare the differential effects of epithelial and mesenchymal cancer cells on tumor-infiltrating CD8+ T cells. In this study, they identified a novel mechanism of tumor-intrinsic regulation of PD-L1 via the microRNA (miRNA)-200–ZEB1 axis, thus linking an EMT regulatory program to CD8+tumor-infiltrating lymphocyte (TIL) exhaustion and, further, find that immunosuppression is critical to tumor metastasis. Importantly, by using integrative analysis of mRNA and miRNA expression and immune profiling in 2 large independent NSCLC patient datasets (The Cancer Genome Atlas and Profiling of Resistance Patterns & Oncogenic Signaling Pathways in Evaluation of Cancers of the Thorax and Therapeutic Target Identification), their data demonstrate that EMT is highly associated with a profound inflammatory tumor microenvironment in lung adenocarcinoma, showing the elevation of multiple immune checkpoint molecules, such as PD-L1, PD-1, TIM-3, BTLA, CTLA-4, and B7-H3, high levels of tumor-infiltrating Foxp3+ regulatory T cells, and immunosuppressive cytokines such as transforming growth factor β (TGFβ), interleukin (IL)-6, and IL-10 (unpublished data).Citation4 Taken together, the preferential accumulation of immunosuppressive cells within mesenchymal lung tumors appears to be a conserved mechanism between human and mouse models. These findings also suggest that an EMT signature or immunosuppression signature may have potential utility as a biomarker to select patients who will benefit from immunotherapies in NSCLC and, possibly, in a broad range of other cancers. This work emphasizes the need to conduct biomarker-driven clinical studies to identify and validate markers of response and resistance in patients.
The authors previously reported that miR-200 expression is repressed in highly metastatic cancer cells and that forced miR-200 expression reversed the EMT phenotype, abrogating tumor invasion and metastasis.Citation5 The significance of these findings is supported by the evidence that low miR-200 expression is part of a miRNA expression signature in primary tumors that predicts disease recurrence in patients with early-stage lung cancer.Citation6 The miR-200 family includes 5 members arranged in 2 genomic clusters (miR-200a/200b/429 and 200c/141) that directly target EMT-inducing transcription factors, including zinc-finger E-box-binding homeobox 1 (ZEB1). In turn, the ZEB1 can directly repress the transcription of both miR-200 family loci. In cancer cells, the miR-200–ZEB1 axis controls the expression of multiple genes involved in migration, invasion, and metastatic growth at distant sites. Thus, miR-200 and ZEB1 form a double-negative feedback loop and function as a key regulatory axis of the EMT program. This new study demonstrates a novel function of the EMT regulatory axis, by which PD-L1 expression on tumor cells is regulated, producing CD8+ T-cell dysfunction, enhanced tumor growth, and metastasis.Citation4
Do immune cells induce EMT? The answer is yes, in that immune cells are known to produce a diverse array of EMT inducers/mediators. For instance, CD4+ T-regulatory cells produce TGFβ, IL-6, IL-10, and tumor necrosis factor α (TNFα). TNFα is also produced by chronically stimulated effector CD8+ T cells, activated macrophages, and proinflammatory T cells.Citation7 Other immune suppressive cells such as myeloid-derived suppressor cells, tumor-associated macrophages, and tumor-associated neutrophils can also produce very strong EMT inducers.Citation8-10 Overall, the evidence suggests that immune effectors can induce EMT following an acute or chronic inflammatory response, or alternatively, can produce other cytokines or chemokines (e.g., MCP-1) that can attract other immune effectors (e.g., macrophages) to the tumor that provide the stimuli.Citation7 From these studies, we gain insight on how the suppressive immune microenvironment transforms and/or maintains the cancer cells in a mesenchymal state. With the aim of simplifying the mechanisms based on our data, the working model is shown in .
Figure 1. Dangerous liaison between epithelial–mesenchymal transition and immunosuppression. The microRNA-200–ZEB1 axis instigates the interplay between cancer cells and immune cells in the tumor microenvironment.
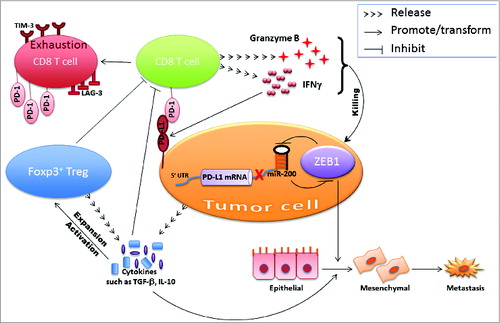
Although the complexity of the interplay between EMT and immunosuppression needs to be further clarified, there appears to be a mutual regulation between EMT and immunosuppression (double-positive feed-forward loop) during tumor development. Cancer cell EMT synergizes with a suppressive immune microenvironment to promote tumor progression and therapy resistance. Breaking this refractory loop could prevent therapy resistance and subsequent cancer relapse. Indeed, the study also shows that mice with subcutaneously transplanted mesenchymal tumors demonstrated greater sensitivity to the anti–PD-L1 antibody treatment than epithelial tumors.Citation4 This is a striking result because we have previously reported that the mesenchymal cancer cell subpopulation accounts for aggressive growth, invasion, metastasis, and therapeutic resistance.
Despite promising results in the treatment of many different malignancies with immunotherapy alone or in combination with conventional therapy (radiotherapy, chemotherapy, and targeted therapy), only a select group of patients respond to these interventions. Primary or acquired resistance to these therapies limits improvement in response rates and patient survival. Understanding the underlying mechanisms that define the target vulnerabilities of mesenchymal cancer cells will outline optimal combinations of chemotherapy and immunotherapy that should be tested.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Radvanyi L. Immunotherapy exposes cancer stem cell resistance and a new synthetic lethality. Mol Ther 2013; 21:1472–4; PMID:23903573; http://dx.doi.org/10.1038/mt.2013.160
- Shintani Y, Okimura A, Sato K, Nakagiri T, Kadota Y, Inoue M, Sawabata N, Minami M, Ikeda N, Kawahara K, et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg 2011; 92:1794–804; discussion 1804; PMID:22051275; http://dx.doi.org/10.1016/j.athoracsur.2011.07.032
- Li T, Zeng ZC, Wang L, Qiu SJ, Zhou JW, Zhi XT, Yu HH, Tang ZY. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene Ther 2011; 18:617–26; PMID:21637307; http://dx.doi.org/10.1038/cgt.2011.29
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014; 5:5241; PMID:25348003; http://dx.doi.org/10.1038/ncomms6241.
- Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, Thilaganathan N, Du L, Zhang Y, Pertsemlidis A, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev 2009; 23:2140–51; PMID:19759262; http://dx.doi.org/10.1101/gad.1820209
- Patnaik SK, Kannisto E, Knudsen S, Yendamuri S. Evaluation of microRNA expression profiles that may predict recurrence of localized stage I non-small cell lung cancer after surgical resection. Cancer Res 2010; 70:36–45; PMID:20028859; http://dx.doi.org/10.1158/0008-5472.CAN-09-3153
- Reiman JM, Knutson KL, Radisky DC. Immune promotion of epithelial-mesenchymal transition and generation of breast cancer stem cells. Cancer Res 2010; 70:3005–8; PMID:20395197; http://dx.doi.org/10.1158/0008-5472.CAN-09-4041.
- Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9:e1001162; PMID:21980263; http://dx.doi.org/10.1371/journal.pbio.1001162
- Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013; 93:844–54; PMID:23752129; http://dx.doi.org/10.1038/labinvest.2013.69.
- Palena C, Hamilton DH, Fernando RI. Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncol 2012; 8:713–22; PMID:22764769; http://dx.doi.org/10.2217/fon.12.59.
