Abstract
Tryptophan catabolism by indoleamine 2,3-dioxygenase (IDO1) is a physiological immunoregulatory mechanism often hijacked by tumors. Our recent extensive study of IDO1 protein expression in human tissues showed expression in mature dendritic cells and in pulmonary and placental endothelial cells. IDO1 was also expressed in 56% of tumors, either by tumoral, stromal, or endothelial cells. These results and reagent will guide the clinical development of IDO1 inhibitors for cancer therapy.
Introduction
In recent years, tryptophan catabolism by indoleamine 2,3-dioxygenase (IDO1) has emerged as a powerful mechanism of tumor-induced immunosuppression, favoring the growth of established tumors by dampening proliferation and function of antitumor effector T cells, while also promoting regulatory T-cell differentiation and inflammation-associated tumorigenesis. Although the exact mechanisms involved remain incompletely characterized, IDO1 is now recognized as a valid target for cancer therapy, based on promising preclinical results. IDO1 inhibitors have been developed and some of them have already been tested in clinical trials, either alone or in combination with other immunotherapies.
Although numerous studies have reported IDO1 expression in the context of mouse and human tumors, there is no consensus on the nature and exact location of IDO1 expression. Several studies reported IDO1 expression in the tumor-draining lymph nodes (TDLNs), mostly in mouse tumor models, whereas others observed IDO1 expression at the tumor site, either constitutively or in the context of inflammation, which induces IDO1 expression through the production of interferon gamma (IFNγ). Although these different facets of IDO1 expression may each represent a biological reality, part of these discrepancies may have resulted from the use of anti-IDO1 antibodies that lacked a careful validation for specificity, particularly for usage in immunohistochemistry. Other discrepancies may result from species-specific differences, inasmuch as, for example, constitutive IDO1 expression is commonly observed in human but not in mouse tumor cells.
To clarify these issues in the context of ongoing clinical trials with IDO1 inhibitors, our group recently developed and fully validated a new monoclonal antibody recognizing human IDO1, which provides a highly specific signal in immunohistochemical staining of paraffin sections. It allowed us to conduct an extensive analysis of IDO1 expression in normal and tumoral human tissues, which was reported in a recent publication.Citation1
In normal human tissues, IDO1 expression was observed in mature dendritic cells (DCs) located in secondary lymphoid organs, in some epithelial cells of the female genital tract, in endothelial cells of term placenta, and, surprisingly, in pulmonary endothelial cells. The biological function of IDO1 in lung endothelia is unclear. It is unlikely that tryptophan catabolism by cells exposed to the blood flow can impose an immunosuppressive flavor to the microenvironment. Therefore, a cell-intrinsic function is more likely. In that regard, it is interesting to note that the density of the pulmonary vasculature is reduced in IDO1-deficient as compared to that in wild-type mice, suggesting a role for IDO1 in lung vascular development.Citation2 In mice, inflammation-induced IDO1 expression in endothelial cells was also reported to induce vasodilation and contribute to reduced blood pressure during severe inflammation.Citation3
In secondary lymphoid organs, approximately 50% of mature conventional DCs expressed IDO1, whereas neither plasmacytoid DCs nor any other cell type did. This parallels the previously observed IDO1 induction during in vitro maturation of monocyte-derived DCs, which we observed during the terminal phase of the DC maturation program and might represent a negative feedback mechanism of retro-control of the immune response, aimed at protecting the organism from immunopathology.Citation1 In contrast, we observed that TDO2 and IDO2, 2 other tryptophan-catabolizing enzymes, were not expressed by monocyte-derived DC—neither before nor after maturation.Citation1
Published work reported an enriched IDO1 expression in TDLNs as compared to normal lymph nodes, mostly in mouse tumor models but also in human tumors, suggesting an important role of TDLNs in shaping tumor immune tolerance.Citation4 However, our results did not confirm an enrichment of IDO1 expression in a series of 30 human TDLNs obtained from human melanomas and breast carcinomas: these TDLNs expressed IDO1 at the same level as normal lymph nodes.Citation1 These results suggest that, at least in humans, the IDO1 expression that is relevant to tumor immunosuppression is located at the tumor site rather than in TDLNs. This is in line with recent findings showing that the use of IDO1 inhibitors in a mouse model barely affected the priming of new antitumor T cells but strongly reactivated effector T cells in situ at the tumor site.Citation5
We also tested a series of 866 human tumors of 15 common types: approximately 56% expressed IDO1.Citation1 Three distinct cellular expression patterns emerged, individually or in combination: IDO1 was expressed by tumor cells (20% of the samples), by interstitial cells in lymphocyte-rich areas in the tumor stroma (46% of the samples), or by endothelial cells (14% of the samples). Part of the IDO1 expression by tumor cells might result from an ongoing immune response involving T lymphocytes producing IFNγ, a strong inducer of IDO1 expression. This is exemplified by cervical carcinoma, where IDO1-positive tumor cells are often located at the periphery of tumor nodules, which are surrounded by T lymphocytes ().Citation1 This is reminiscent of the expression profile observed for PD-L1, another protein involved in tumoral immune resistance, which is also induced by IFNγ and often observed in T–cell-infiltrated tumors.Citation6 This PD-L1 expression profile, indicative of an adaptive resistance mechanism, was found to predict clinical responses to PD1/PD–L1-blocking reagents.Citation7 In a similar manner, IDO1 expression in inflamed tumors might also result from an adaptive resistance mechanism. In line with this, IDO1 expression in human melanoma was found to correlate with T-cell infiltration.Citation8 The IDO1 expression we often observed in the tumor stroma also likely results from an adaptive resistance mechanism. Such a pattern was dominant, for example, in colorectal carcinomas.
Figure 1. IDO1 Protein expression in human tumors assessed by immunohistochemistry. Illustrative images from formalin-fixed paraffin-embedded tissue microarray sections of cervical (A, B) and endometrial carcinomas (C, D) stained with the anti-IDO1 antibody 4.16H1. Tumoral (T), stromal (S), and lymphocyte-enriched (L) areas are indicated. Immunolabeled cells are stained dark red.
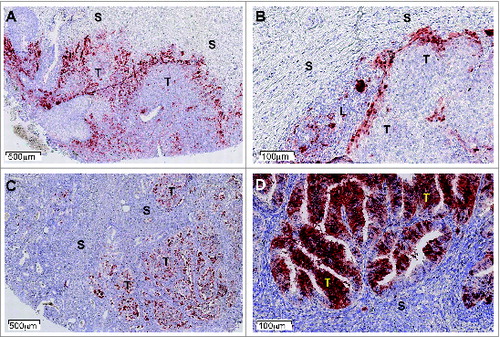
In contrast, a subset of tumors expressed IDO1 within tumor cells in the absence of any inflammation.Citation1, 9 This is the case in many endometrial carcinomas, which often contain IDO1-expressing tumor cells scattered within tumor nodules in the absence of obvious T-cell infiltration ().Citation1 Constitutive IDO1 expression was also observed in a number of human tumor lines,Citation9 and is likely triggered by oncogenic events whose characterization will be of great interest. Tumor-intrinsic constitutive IDO1 expression might contribute to tumoral immune resistance by preventing T-cell infiltration, a mechanism conceptually different from adaptive resistance, where IDO1 expression would represent a negative feedback mechanism induced by the T-cell response.
The last pattern of IDO1 expression, which was particularly striking in kidney cancer, is restricted to endothelial cells. As discussed earlier, the biological function of endothelial IDO1 is unclear at the present time. Intriguingly, endothelial IDO1 expression in kidney tumors was reported to be associated with a better prognosis, whereas in most other tumor types, IDO1 expression is associated with a worse clinical outcome.Citation10 Notably, as opposed to many other tumor types, T-cell infiltration of kidney tumors is associated with a bad prognosis. Further studies will be required to understand those unexpected features of kidney tumors. The new antibody reported in this study will be a useful translational tool not only to address such mechanistic issues, but also to select patients more likely to benefit from IDO1 inhibitors currently under clinical development.
Disclosure of Potential Conflicts of Interest
Benoît J Van den Eynde has ownership interest in, and is consultant/Advisory Board Member for, iTeos Therapeutics.
References
- Théate I, van Baren N, Pilotte L, Moulin P, Larrieu P, Renauld JC, Hervé C, Gutierrez-Roelens I, Marbaix E, Sempoux C, et al. Extensive profiling of the expression of the indoleamine 2,3-dioxygenase 1 protein in normal and tumoral human tissues. Cancer Immunol Res 2015; 3(2):161–72; PMID: 25271151; http://dx.doi.org/10.1158/2326-6066.CIR-14-0137
- Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, et al. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov 2012; 2:722–35; PMID:22822050; http://dx.doi.org/10.1158/2159-8290.CD-12-0014
- Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med 2010; 16:279–85; PMID:20190767; http://dx.doi.org/10.1038/nm.2092
- Lee JR, Dalton RR, Messina JL, Sharma MD, Smith DM, Burgess RE, Mazzella F, Antonia SJ, Mellor AL, Munn DH. Pattern of recruitment of immunoregulatory antigen-presenting cells in malignant melanoma. Lab Invest 2003; 83:1457–66; PMID:14563947; http://dx.doi.org/10.1097/01.LAB.0000090158.68852.D1
- Spranger S, Koblish HK, Horton B, Scherle PA, Newton R, Gajewski TF. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer 2014; 2:3; PMID:24829760; http://dx.doi.org/10.1186/2051-1426-2-3.
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127ra37; PMID:22461641; http://dx.doi.org/10.1126/scitranslmed.3003689.
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568–71; PMID:25428505; http://dx.doi.org/10.1038/nature13954.
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID:23986400; http://dx.doi.org/10.1126/scitranslmed.3006504.
- Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 9:1269–74; PMID:14502282; http://dx.doi.org/10.1038/nm934.
- Riesenberg R, Weiler C, Spring O, Eder M, Buchner A, Popp T, Castro M, Kammerer R, Takikawa O, Hatz RA, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Can Res 2007; 13:6993–7002; http://dx.doi.org/10.1158/1078-0432.CCR-07-0942.
