Abstract
Our preclinical data demonstrate that an intravenous injection of Modified Vaccinia virus Ankara induces CD8+ lymphocytes to infiltrate organs to control the growth of orthotopic renal carcinoma upon combination with a toll-like receptor 9 agonist. Such shaping of the tumor microenvironment could constitute the basis of more effective clinical protocols of tumor immunotherapy.
Introduction
Despite continuous efforts from public health authorities for the prevention and the early detection of cancer, this pathology has become one of the leading causes of death in developed countries.Citation1 Current therapies include (but are not limited to) surgery, chemotherapy, and radiotherapy. Nevertheless, patients suffering from advanced, recurrent, or aggressive cancers, such as of the lung, liver, or pancreas, have dismal perspectives. Active immunotherapy is based on the induction or the re-establishment of a specific CD8+ T-cell immune response that is able to destroy cancer cells. Despite promising preclinical results, objective clinical response rates to standalone active immunotherapy have remained below expectations.Citation2 An efficient active immunotherapy would require the proper generation of antigen-specific CD8+ T cells, trafficking, and homing of those cells to tumors where they are to specifically recognize and lyse tumor cells. Then, new mutated antigens released from the lytic process would ideally be processed to generate new, specific, T cells and, thus, create a virtuous and iterative cycle of successful immunotherapy.Citation3 Unfortunately, the access of the CD8+ T cell to the tumor bed is often insufficient and, tumor cells, in collaboration with their stroma, make up an immunosuppressive microenvironment that inhibits the cytotoxic activity of CD8+ T cells. Part of the current research in immunotherapy aims at overcoming these limitations.
In our study, we evaluated the immunotherapeutic efficiency of a Modified Vaccinia virus Ankara (MVA) expressing the tumor-associated antigen mucin 1 (MUC1) in an orthotopic model of renal carcinoma. MUC1 is a glycoprotein found aberrantly expressed and hypoglycosylated in numerous types of cancers, including renal carcinoma.Citation4 We hypothesized that an orthotopic model of cancer would be more representative of the patients’ situation than a subcutaneous ectopic tumor model in the study of the effects of antigen-specific immunotherapy. As the immunization route imprints the migratory capacities of the primed antigen-specific T cells,Citation5 we chose to compare the commonly used route for immunotherapeutics (subcutaneous; SC) to the intravenous (IV) route, which we thought would be more likely to generate MUC1-specific T cells that would migrate to a visceral organ such as the kidney. With an idea to generate higher frequencies of MUC1-specific T cells, we introduced a toll-like receptor 9 (TLR9) agonist (CpG ODN1826) in our immunotherapeutic scheme.
Our results indicate that significant prolongation of overall survival (OS) in our orthotopic model of renal carcinoma could be obtained, provided that MVA expressing MUC1 (MVA-MUC1) was administered via the IV route. ODN1826 greatly enhanced OS upon combination with IV MVA-MUC1 only.Citation6 In contrast to the SC injection, IV injection of MVA-MUC1 was associated with a massive infiltration of CD8+ T cells into the kidney bearing a tumor. This infiltration of CD8+ T cells was also detected in the contralateral kidney, liver, and lungs of animals. It was also induced by IV injection of an empty MVA vector and occurred in tumor-free animals, thus indicating that the observed phenomenon was neither dependent on the expression of MUC1 nor related to the presence of a tumor in a given organ. In tumor-bearing animals, the depletion of CD8+ T cells with specific antibodies emphasized the key role of those cells in this orthotopic model. Very low frequencies of MUC1 antigen-specific CD8+ T cells were detected after IV MVA-MUC1 immunization, but those were sufficient to control the growth of RenCa-MUC1 cells. As we characterized the amino acid sequences of the 2 H-2d–restricted human MUC1 epitopes, we observed that they presented a high level of homology with their mouse MUC1 counterparts. We hypothesized that the low frequencies of human MUC1-specific CD8+ T cells detected could be due to the thymic depletion of the most reactive mouse MUC1-specific CD8+ clones. Using a reporter gene, we observed that, in comparison to SC injection, IV administration of MVA resulted in a faster gene expression in the organs and antigen-presenting cells from the spleen. As a consequence, we observed that MUC1-specific CD8+ T cells were generated and detected faster than when MVA-MUC1 was injected IV. Infiltrating CD8+ T cells were characterized by an effector memory phenotype with expression of exhaustion markers. CpG ODN1826 by itself had neither an effect on OS in our model nor induced infiltration of CD8+ T cells in organs. In contrast, it induced infiltration of CD4+ T regulatory cells (Tregs) in kidneys bearing a tumor. Unexpectedly, the addition of ODN1826 to MVA-MUC1 immunotherapy decreased the frequency of MUC1-specific CD8+ T cells that we could detect from the spleen of the animals. Further, ODN1826 added to IV MVA-MUC1 modified the pattern of gene expression in the tumor-bearing kidney, skewing the tumor microenvironment toward the Th1 type (Tbx21, Ifng, Stat4, and Il27), the expression of co-stimulatory molecules (Cd40l), and favoring the recruitment of immune cells such as activated effector memory T cells (Cxcl11 and Ccl5), B cells (Cxcl13), and mature dendritic cells (Ccl19).
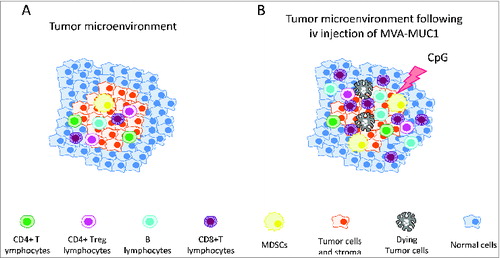
Important infiltration of tumor tissue by effector memory CD8+ T cells is a good prognostic indicator for disease outcome in most human cancers.Citation7,8 Our study suggests that, in the clinic, infiltration of organ-bearing tumors with such pivotal cells could be achieved with IV injection of MVA-MUC1 in pathologies that do benefit from an important CD8+ T-cell infiltrate. Once there, the effector activity of those MUC1-specific CD8+ T cells could be fostered by modification of the tumor microenvironment with compounds such as TLR9 ligands.Citation9,10 Concomitant or sequential combination with classical or emerging therapies would participate in the shaping of the tumor bed toward an immunoactive microenvironment capable of efficiently controlling and rejecting tumors ().
Figure 1. Tumors are made up of cancer cells and a stroma of carcinoma-associated fibroblasts and various immune cells defined as the tumor microenvironment. (A) Cancer cells and stromal cells interact to foster tumor growth while suppressing the activity of cytotoxic immune cells including that of tumor-associated antigen-specific CD8+ T cells. (B) Upon intravenous injection of MVA-MUC1, the subsequent infiltration of MUC1-CD8+ T cells modifies the ratio of suppressive cells to that of effector cells in favor of the latter. Further modification of the microenvironment with immunomodulating compounds such as CpG favors the cytotoxic activity of MUC1-specific CD8+ T cells to control tumor growth. Such a property of MVA could be used in a clinical setting to participate in the shaping of an immuno-efficient tumor microenvironment through combinations with existing and/or emergent therapies.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90; PMID:21296855; http://dx.doi.org/10.3322/caac.20107
- Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004; 10:909–15; PMID:15340416; http://dx.doi.org/10.1038/nm1100
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1–10; PMID:23890059; http://dx.doi.org/10.1016/j.immuni.2013.07.012
- Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nature Med 2000; 6:332–6; PMID:10700237; http://dx.doi.org/10.1038/73193
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nature Med 2002; 8:157–65; PMID:11821900; http://dx.doi.org/10.1038/nm0202-157
- Fend L, Gatard-Scheikl T, Kintz J, Gantzer M, Schaedler E, Rittner K, Cochin S, Fournel S, Préville X. Intravenous injection of MVA virus targets CD8+ lymphocyte to tumors to control tumor growth upon combinatorial treatment with a TLR9 agonist. Cancer Immunol Res 2014; 2(12):1163–74; PMID:25168392; http://dx.doi.org/10.1158/2326-6066.CIR-14-0050
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298–306; PMID:22419253; http://dx.doi.org/10.1038/nrc3245
- Becht E, Goc J, Germain C, Giraldo NA, Dieu-Nosjean MC, Sautes-Fridman C, Fridman WH. Shaping of an effective immune microenvironment to and by cancer cells. Cancer Immunol Immunother CII 2014; 63(10):991–7; http://dx.doi.org/10.1007/s00262-014-1590-3
- Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clinical Can Res 2011; 17:1765–75; http://dx.doi.org/10.1158/1078-0432.CCR-10-2672
- van den Boorn JG, Hartmann G. Turning tumors into vaccines: co-opting the innate immune system. Immunity 2013; 39:27–37; PMID:23890061; http://dx.doi.org/10.1016/j.immuni.2013.07.011
