Abstract
T cells are crucial players in the protection against cancer, and can be used in adoptive cell therapy to prevent or treat relapse. However, their state of differentiation determines their effectiveness, with early memory cells being the most favorable. Here, we discuss restraining of differentiation to engineer the ultimate tumor-reactive T cell.
Abbreviations:
- ACT: adoptive cell therapy
- Allo-SCT: allogeneic stem cell transplantation
- APC: antigen-presenting cells
- CAR: chimeric antigen receptor
- DCs: dendritic cells
- MiHAs: minor histocompatibility antigens
- Tcm: central memory T
- TCR: T-cell receptor
- Teff: effector T
- Tem: effector memory T
- TILs: tumor-infiltrating lymphocytes
- Tn: naive T
- Tscm: stem cell memory T
T Cells with Stem Cell Flavor
T cells are the soldiers of our immune system and crucial forces in the battle against solid and hematological malignancies. Adoptive cell therapy (ACT) exploiting tumor-reactive CD8+ T cells is an appealing strategy to treat advanced cancer. A crucial aspect for the functionality of these CD8+ T cells in antitumor immunity is their differentiation state.Citation1,2 Upon activation of naive T (Tn) precursor cells, proliferation and differentiation is initiated into the early memory subsets, stem cell memory T (Tscm) cells, and central memory T (Tcm) cells, and their later-arising subsets—effector memory T (Tem) cells and effector T (Teff) cells.Citation1,3 Terminally differentiated Teff cells show potent antitumor killing activity; however, most of them die after a single act of duty. In contrast, the early memory Tscm and Tcm cells not only possess stronger proliferative capacity, they are also capable of differentiating into effector T-cell subsets. Most importantly, they possess a high self-renewal capacity, which is less profound in the more differentiated T-cell subsets. The supremacy of these early memory T cells was demonstrated by Klebanoff et al., who described the evolvement of adoptive T-cell therapy in time.Citation2 Using the same pmel-1 melanoma mouse model, comparable antitumor effectiveness was achieved with 500 times less Tscm cells than the “old fashioned” interleukin (IL)-2-expanded Teff cells. This demonstrates the superiority of Tscm cells to fight off malignant cells, and makes early memory CD8+ T cells the most favorable subset for therapeutic approaches.
ACT of Early Memory T Cells
Various approaches to generate tumor-reactive CD8+ T cells for ACT in cancer patients have been explored. For most effective antitumor responses without severe side effects, it is imperative to target antigens that are specifically, or at least selectively, expressed by the malignant cells. These could be tumor-associated antigens, although these are generally self-peptides with overexpression on malignant cells compared to normal tissues. Neoantigens, on the other hand, appear on tumors as a consequence of tumor-specific mutations, and are highly immunogenic. In the setting of allogeneic stem cell transplantation (allo-SCT), donor T cells can recognize polymorphic minor histocompatibility antigens (MiHAs) expressed on patients’ malignant cells.Citation4 Therefore, ACT using donor-derived CD8+ T cells targeting MiHAs with restricted expression to the hematopoietic lineage is an attractive adjuvant therapy to prevent or treat tumor recurrence. However, the most widely explored ACT involves the isolation and ex vivo expansion of tumor-infiltrating lymphocytes (TILs). Subsequently, these tumor-reactive CD8+T cells are transferred back to the patient. An emerging strategy is to use tumor-reactive T-cell receptor (TCR)- or chimeric antigen receptor (CAR)-engineered T cells.Citation5 In particular, CAR T-cell therapy has shown spectacular responses in patients with acute lymphoblastic leukemia. An alternative approach for “T-cell engineering” is the ex vivo generation of tumor-reactive CD8+ T cells by expansion of their precursor cells using antigen-presenting cells (APCs), such as dendritic cells (DCs). Although various approaches are being explored, all induce T-cell activation resulting in differentiation into the less effective and less favorable Teff cells. This provides a strong rationale for monitoring and inhibiting T-cell differentiation during T-cell engineering for adoptive T-cell therapy.
Gattinoni et al. showed that, by mimicking canonical Wnt-signaling, T-cell differentiation can be effectively inhibited during the generation of TCR-engineered tumor-reactive T cells.Citation6 Interestingly, these TCR-engineered Tscm-like CCR7+CD62L+ T cells showed superior antitumor immunity. However, a firm inhibition in proliferation was observed during culture, rendering this strategy unsuitable for ex vivo priming and expansion of CD8+ T cells endogenously expressing tumor-reactive TCR, as proliferation is required in order to obtain sufficient numbers for adoptive transfer. Cieri et al. demonstrated that the presence of IL-7 and IL-15 favors the expansion of polyclonal-stimulated TCR-engineered CD62Llow T cells.Citation7 Alternatively, in our recent publication, we showed that pharmacological interference with the Akt-signaling pathway during ex vivo priming resulted in the expansion of MiHA-specific CD8+ T cells with an early CCR7+CD62L+ memory phenotype.Citation8 These Akt-inhibited MiHA-specific CD8+ T cells displayed effective proliferative capacity in vivo, and showed superior antitumor efficacy in multiple-myeloma-bearing mice. The superiority of Akt-inhibited T cells was also reported by Crompton et al. who showed that Akt inhibition of TILs, isolated from metastatic melanoma patients, promotes expansion of early CD62L+ memory T cells with improved persistence upon adoptive transfer in immune-deficient mice.Citation9 Moreover, adoptive transfer of Akt-inhibited pmel-1 CD8+ T cells resulted in decreased tumor growth and improved survival in B16 melanoma-bearing mice. These new findings demonstrates that pharmacological inhibition of Akt represents a feasible and attractive approach to generate tumor-reactive T cells with an early memory phenotype that possess superior antitumor immunity following adoptive transfer.
Upon infusion of tumor-reactive T cells, activation and support is essential to ensure potent long-lasting antitumor responses. This could be achieved via DC vaccination, using either monocyte-derived DCs or natural occurring DC subsets loaded with the tumor antigen of interest.Citation10 By transfer of tumor-specific T cells with an early memory phenotype, in combination with DC vaccination, an effective antitumor response can be elicited. Promoting immunologic memory of tumor-reactive T-cell responses may improve the curative potential of cancer immunotherapy. Taken together, inhibition of T-cell differentiation (e.g., via inhibiting Akt-signaling) during the generation of antigen-specific T cells could be broadly exploited for the development of superior tumor- or virus-reactive stem cell-like CD8+ T cells for adoptive immunotherapy in cancer and viral infections.
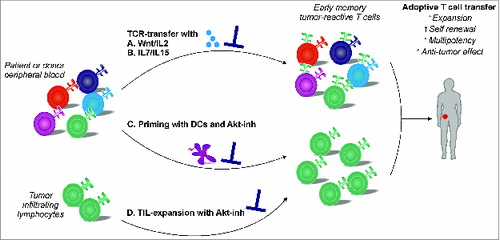
Figure 1. Strategies for the ex vivo generation of early memory tumor-reactive T cells for adoptive immunotherapy. Generation of early memory tumor-reactive T cells via different strategies. (A, B) T-cell receptor (TCR) transfer in the presence of Wnt signaling, or interleukin (IL)-7 and IL-15 stimulation. (C) Expansion of naive T-cell progenitors using dendritic cells in the presence of an Akt-inhibitor (Akt-inh). (D) Expansion of tumor-infiltrating lymphocytes (TILs) in the presence of an Akt-inhibitor.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nature Reviews Cancer 2012; 12: 671–84; PMID:22996603; http://dx.doi.org/10.1038/nrc3322
- Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J Immunotherapy 2012; 35: 651–60; http://dx.doi.org/10.1097/CJI.0b013e31827806e6
- Stemberger C, Neuenhahn M, Gebhardt FE, Schiemann M, Buchholz VR, Busch DH. Stem cell-like plasticity of naive and distinct memory CD8+ T cell subsets. Semin Immunol 2009; 21: 62–8; PMID:19269852; http://dx.doi.org/10.1016/j.smim.2009.02.004
- Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nature Rev Cancer 2004; 4: 371–80; http://dx.doi.org/10.1038/nrc1365
- June CH, Maus MV, Plesa G, Johnson LA, Zhao Y, Levine BL, Grupp SA, Porter DL. Engineered T cells for cancer therapy. Cancer Immunol Immunother: CII 2014; 63: 969–75; http://dx.doi.org/10.1007/s00262-014-1568-1
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nat Med 2011; 17: 1290–7; PMID:21926977; http://dx.doi.org/10.1038/nm.2446
- Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 2013; 121: 573–84; PMID:23160470; http://dx.doi.org/10.1182/blood-2012-05-431718
- van der Waart AB, van de Weem NM, Maas F, Kramer CS, Kester MG, Falkenburg JH, Schaap N, Jansen JH, van der Voort R, Gattinoni L, et al. Inhibition of Akt-signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood 2014; 124(23): 3490–500; PMID:25336630; http://dx.doi.org/10.1182/blood-2014-05-578583
- Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil R, Tran E, Hanada KI, Yu Z, Palmer DC, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res 2015; 75(2): 296–305; PMID:25432172; http://dx.doi.org/10.1158/0008-5472.CAN-14-2277
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15: e257-67; PMID:24872109; http://dx.doi.org/10.1016/S1470-2045(13)70585-0
