Abstract
The immunosuppressive tumor microenvironment (TME) is a major obstacle in cancer immunotherapy. Therefore, it has gained attention as a target site. mRNA emerged as a versatile drug class for cancer therapy. We reported that intratumoral administration of mRNA encoding the fusokine Fβ2 supports tumor-specific T-cell immunity. This study provides proof of concept of the use of mRNA to modulate the TME.
Abbreviations:
Cancer Immunotherapy: Opportunities and Obstacles
The idea that the immune system can be exploited to combat cancer originated in the nineteenth century when it was observed that tumors occasionally shrunk when infected.Citation1 Ever since, scientists have studied the immune system, searching for a means to harness the body's defense mechanisms against cancer. Breakthroughs such as the identification of tumor-associated antigens (TAAs), dendritic cells (DCs), major histocompatibility complex (MHC) I-restricted antigen presentation to CD8+ T cells, and the production of synthetic antibodies have shaped the cancer immunotherapy field. Based on these findings, therapies such as cancer vaccines, adoptive T-cell transfer, and antibodies were developed and these are now extending the lives of patients.Citation2 Although the number of patients that benefit from these therapies is growing, there are still a number of obstacles to overcome.
A major hurdle is the immunosuppressive tumor microenvironment (TME). Here, tumor cells and immune cells such as myeloid-derived suppressor cells (MDSCs), macrophages, and regulatory T cells cooperate to dampen antitumor immune responses using a plethora of inhibitory mechanisms. Several drugs have been developed to revert the suppressive TME, including pattern-recognition receptor agonists, stimulatory cytokines, decoy receptors that capture immunosuppressive cytokines, and monoclonal antibodies that target immune-checkpoint molecules. The targets of these immunomodulatory drugs can be found within the TME.Citation3,4
Intratumoral Delivery of Immunomodulatory Drugs
In 1890, William Coley injected bacterial toxins into primary tumors, showing tumor regression in a number of patients.Citation1 Nonetheless, for decades, drugs were preferentially administered systemically, because such administration was contended to induce strong systemic antitumor immune responses capable of rejecting primary and metastasized tumors.Citation5 However, the limitations encountered with systemic delivery of immunomodulatory drugs, of which toxicity is the most pressing, together with the growing appreciation that many of their targets are present within the TME has revived the concept of intratumoral therapy delivery.
A multitude of studies analyzing the activation of cytotoxic T lymphocytes (CTLs) and inhibition of regulatory factors evidenced that local delivery of cancer immunotherapies has several advantages. These include stimulation of systemic immune responses with enhanced breadth and simultaneous reduction of immunosuppression that, together, enable therapeutic antitumor immunity with little or no toxicity.Citation4 The broad effects elicited by single agents are explained by the intricate communication between cells and the suppressive mechanisms they exert in the TME. This implies that the modulation of 1 cell population or suppressive mechanism also impacts others.
mRNA: An Interesting Technology Platform for Intratumoral Therapy
Weide et al.Citation6 were the first to inject naked tumor mRNA into the dermis of melanoma patients, showing increased humoral immune responses in several patients. This pioneering work has put mRNA on the map as a promising drug class for cancer immunotherapy. Ever since, in vitro-transcribed mRNA has been evaluated as an investigational medicinal product (IMP) for the delivery of TAAs and/or cell-reprogramming proteins into DCs.Citation7,8 Importantly, mRNA as an IMP is safe, stable prior to administration, readily biodegraded, inexpensive to produce, and well-defined chemically, thus facilitating quality control and ensuring reproducible manufacturing and activity.Citation7 Therefore, delivering immunomodulatory drugs such as antibodies, cytokines, and decoy receptors under the form of mRNA represents an attractive approach that circumvents the cumbersome time- and money-consuming approach involved in producing recombinant proteins according to Good Manufacturing Practices regulations.
A prerequisite for the use of mRNA (as IMP) to modulate the TME is its uptake and translation by cells within the tumor. We demonstrated, in several mouse tumor models using mRNA encoding firefly luciferase, that mRNA can be delivered to the tumor and the expression of firefly luciferase can be detected for up to 5 d post-delivery. Moreover, we demonstrated, by using the Batf3−/− model, that CD8α+ cross-presenting DCs are mainly responsible for the uptake of naked mRNA.Citation9 This finding opens the possibility of exploiting tumor-infiltrating DCs (TiDCs) to produce immunomodulating proteins locally.
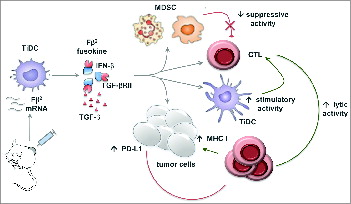
As a proof of concept, we delivered mRNA encoding a fusokine consisting of interferon β (IFNβ) fused to the ectodomain of the transforming growth factor β (TGFβ) receptor II, referred to as Fβ2. The rationale was that IFNβ would exert an immunostimulatory function, whereas the ectodomain of the TGFβ receptor II would reduce the TGFβ-mediated immunosuppression. We showed that Fβ2 reduced the suppressive activity of MDSCs, while it enhanced the stimulatory capacity of DCs and the lytic activity of CTLs. Moreover, Fβ2 enhanced the expression of MHC I on tumor cells, thus enhancing recognition and killing by CTLs. Nonetheless, delivery of Fβ2 mRNA to the tumor only resulted in a transient delay in tumor growth. Further analysis showed that Fβ2 induced a high expression of PD-L1 on tumor cells and that a combination of Fβ2 mRNA delivery and PD-1/PD-L1 blockade enhanced the potential of this local therapy ().Citation10
Figure 1. Intratumoral delivery of Fβ2 mRNA results in its selective uptake by tumor-infiltrating DCs (TiDCs) followed by its production and secretion in the TME. Fβ2 inhibits the suppressive activity of myeloid-derived suppressor cells (MDSCs) on cytotoxic T lymphocytes (CTLs), whereas it boosts the CTL-stimulatory activity of TiDCs. Moreover, Fβ2 enhances the lytic activity of CTLs while simultaneously enhancing the expression of major histocompatibility complex (MHC) I molecules and, as such, the presentation of tumor-associated antigens (TAAs) on tumor cells. Consequently, tumor cells become a target for CTLs. However, Fβ2 also upregulates the expression of PD-L1 on tumor cells. The latter enables tumor cells to counteract the CTL-mediated attack. Notably, several questions regarding the exact mechanisms through which Fβ2 mediates these effects remain. Comparison of the intratumoral delivery of IFNβ, soluble TGFβ receptor II, and Fβ2 mRNA could shed light on these mechanisms.
To our knowledge, this is the first study showing the use of mRNA encoding secreted proteins to engineer the TME. The finding that TiDCs can be exploited as local producers of mRNA-encoded proteins opens new avenues to deliver stimulatory cytokines, decoy receptors, or combinations thereof, as well as molecules developed to block inhibitory or to activate stimulatory immune checkpoints. In addition, the uptake of mRNA by TiDCs offers the opportunity to develop antigen-independent immunization strategies, as TiDCs carry TAAs. Stimulating TiDCs to drain to lymph nodes and activate CTLs, for example, through the delivery of TriMix mRNA – a mix of 3 mRNA molecules encoding the CD40 ligand, constitutively active TLR4, and CD70 — is an attractive strategy.Citation9 In addition mRNA-mediated TiDC-stimulation followed by mRNA-based engineering of the TME could be an attractive approach to activate CTLs and preserve their function in the tumor nest.
Conclusion
The growing appreciation of intratumoral delivery of cancer immunotherapy agents, as pioneered by William Coley in the nineteenth century, together with the revival of the use of mRNA as an anticancer drug, as pioneered in the clinic by Weide et al. in the twentieth century, will most likely lead to better outcomes with anticancer therapy in the 21st century.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were declared.
Funding
The Laboratory of Molecular and Cellular Therapy is supported by grants from the Interuniversity Attraction Poles Program-Belgian State-Belgian Science Policy, the National Cancer Plan of the Federal Ministry of Health, the Stichting tegen Kanker, the Vlaamse Liga tegen Kanker, the FWO-Vlaanderen, the Scientific Fund Willy Gepts of the University Hospital Brussels, SRP research funding of the Vrije Universiteit Brussel, an IWT-TBM program, and an Integrated Project and EU FP7-funded Network of Excellence. Kevin Van der Jeught is a doctoral fellow of the IWT.
References
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006; 26: 154–8; PMID:16789469
- Wayteck L, Breckpot K, Demeester J, De Smedt SC, Raemdonck K. A personalized view on cancer immunotherapy. Cancer Lett 2014; 352: 113–25; PMID:24051308; http://dx.doi.org/10.1016/j.canlet.2013.09.016
- Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res 2014; 20: 1747–56; PMID:24691639; http://dx.doi.org/10.1158/1078-0432.CCR-13-2116
- Van der Jeught K, Bialkowski L, Daszkiewicz L, Broos K, Goyvaerts C, Renmans D, Van Lint S, Heirman C, Thielemans K, Breckpot K. Targeting the tumor environment to enhance antitumor immune responses. Oncotarget 2015; 6(3):1359–1381.
- Eggermont AM. Advances in systemic treatment of melanoma. Ann Oncol 2010; 21 Suppl 7: vii339–44; PMID:20943639
- Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, Rammensee HG, Garbe C, Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother 2008; 31: 180–8; PMID:18481387; http://dx.doi.org/10.1097/CJI.0b013e31815ce501
- Van Lint S, Renmans D, Broos K, Dewitte H, Lentacker I, Heirman C, Breckpot K, Thielemans K. The ReNAissanCe of mRNA-based cancer therapy. Expert Rev Vaccines 2015; 14(2): 235–251.
- Benteyn D, Heirman C, Bonehill A, Thielemans K, Breckpot K. mRNA-based dendritic cell vaccines. Expert Rev Vaccines 2015: 14(2): 161–167.
- Van Lint S. Development of a local treatment modality for the systemic cure of cancer using mRNA as a medicinal product. 1st ed. Brussels: VUB Press; 2014.
- Van der Jeught K, Joe PT, Bialkowski L, Heirman C, Daszkiewicz L, Liechtenstein T, Escors D, Thielemans K, Breckpot K. Intratumoral administration of mRNA encoding a fusokine consisting of IFN-β and the ectodomain of the TGF-β receptor II potentiates antitumor immunity. Oncotarget 2014; 5: 10100–13; PMID:25338019
