Abstract
Stimulation of CD40 on dendritic cells to expand and activate tumor-specific T cells and generate anticancer immunity is an attractive therapeutic approach. Since CD40 agonists exert their effects upstream of checkpoint inhibitors, including PD-1 or PD-L1 antagonists, they are ideal candidates for combination regimens.
Keywords:
Immunotherapy will play an important role in oncology in the foreseeable future on the basis of impressive long-term survival data for the checkpoint inhibitors ipilimumab (targeting CTLA-4), pembrolizumab (PD-1), and nivolumab (PD-1) in metastatic melanoma and other indications such as non-small cell lung cancer and renal cell carcinoma.Citation1 Furthermore, it appears that these truly game-changing effects may be further augmented by combining different immunotherapeutic drugs.Citation1 As a consequence, the main driver for drug development within the area of immunotherapy is to develop compounds that act in a complementary or synergistic manner with checkpoint inhibitors to facilitate and enhance the steps of the cancer-immunity cycle.Citation2 However, in order to establish immunotherapy for the earlier stages of cancer, it will be essential not only to increase the response rate but also to decrease toxicity. This can be achieved through optimization of drug combinations and dosing regimens and through the identification of predictive biomarkers for efficacy and toxicity.
CD40 is ranked as one of the most important targets for immunotherapy of cancer, second only to PD-1 (Cancer Immunotherapy Trial Network, CITN). Activation of CD40 on dendritic cells increases cross-presentation of tumor antigens and consequently the number of activated tumor-directed T effector cells (). CD40 agonistic antibodies mainly exert their effects upstream of the checkpoint inhibitors and are ideal candidates for combination regimens including, for example, PD-1 or PD-L1 antagonists. Clinical precedence with anti-CD40 agonistic antibodies shows a 20% overall response rate, clearly justifying further clinical trials with CD40 agonists.Citation4 To this end, Alligator Bioscience has developed a potent and fully human CD40 agonistic antibody, ADC-1013, that has completed preclinical development and has now entered clinical Phase I. In a recent publication, we demonstrate that ADC-1013 activates dendritic cells and generates a strong antitumor effect on established bladder cancer tumors in a human CD40 transgenic mouse model.Citation3
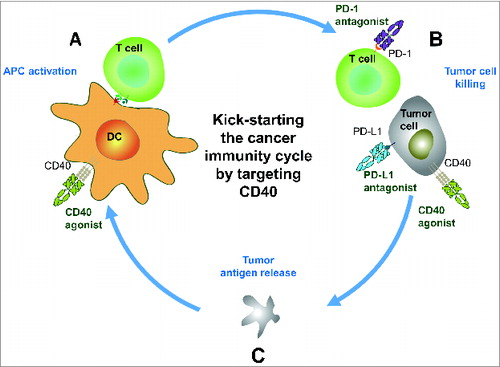
Figure 1. Kick-starting the cancer-immunity cycle by targeting CD40. (A) ADC-1013 activates CD40 receptors on antigen presenting cells such as dendritic cells (DCs), resulting in upregulation of co-stimulatory molecules. T cells are primed and activated, resulting in an expansion of activated T cells. (B) The activated tumor-specific T cells traffic to tumors and kill tumor cells. CD40 agonists have the potential to be used as monotherapy; however, there is a great opportunity to further enhance the effect by combining CD40 treatment with antibodies targeting the PD-1/PD-L1 axis. Furthermore, CD40 agonists can induce direct killing of CD40+ tumor cells through the induction of apoptosis, antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and programmed cell death (PCD). (C) This results in release of tumor associated antigens, which has the potential to augment the uptake and presentation of tumor antigens on DCs to T cells thus expanding the repertoire of tumor-specific T cells.
In order to fully exploit the potential of CD40 stimulation in combination therapies, a number of factors will need to be addressed, including (i) route of administration, (ii) antibody format and properties, and (iii) clinical dosing regimen. In all clinical trials to date with CD40 antibodies, the intravenous route has been used to administer the drug. To improve the risk/benefit ratio of CD40 agonistic antibodies, we argue that it may be more beneficial to administer CD40 agonists either subcutaneously or intratumorally. Subcutaneous administration will reduce the Cmax and delay Tmax, which may reduce acute immune-related adverse effects. Intratumoral administration will in addition result in the preferential activation of dendritic cells in the tumor microenvironment, as demonstrated in preclinical models.Citation5-8 This is expected to reduce immune-related adverse effects and possibly increase efficacy.
The ultimate therapeutic goal of CD40 agonistic antibodies is to induce antitumor immunity through dendritic cell-mediated activation of tumor-specific T effector cells. It is still not known how to best achieve this in the clinical setting, neither in terms of antibody format nor in terms of functional properties such as affinity or level of agonistic activity.Citation9 The functional properties of antibodies that are or have been in clinical development vary both with respect to Fc dependency of the agonistic effects and with respect to isotype (IgG1 or IgG2). IgG1 antibodies induce antibody-dependent cellular cytotoxicity (ADCC) against CD40-positive tumors, which can augment the antitumor immune response through the release of tumor antigens and further boost the cancer-immunity cycle ().Citation2 A potential risk of CD40 agonistic IgG1 antibodies is the induction of ADCC against dendritic cells; however, preclinical and clinical data show that dendritic cells are activated, rather than depleted, by IgG1 CD40 agonists.Citation4 Antibodies of the IgG2 isotype lack the additional effector function on CD40-expressing cells.
It is reasonable to assume that CD40 agonistic antibodies will eventually be used in combination with other immunotherapies such as checkpoint inhibitors or vaccines. One example of possible synergy is the combination of CD40 agonists with PD-1 or PD-L1 blocking agents, since CD40 agonists may induce upregulation of the PD-1/PD-L1 pathway, thus making these patients more likely to respond to subsequent PD-1/PD-L1 therapy.Citation10
One additional aspect to consider when combining a CD40 agonist with a checkpoint inhibitor is the differences in kinetics. For checkpoint inhibitors it seems that continuous receptor blockade is important for therapeutic effects, whereas continuous exposure to a CD40 agonist is likely to lead to immune exhaustion. Hence, a dosing regimen including intermittent administration of a CD40 agonistic antibody and continuous exposure to a checkpoint inhibitor may be advantageous.
ADC-1013 is being developed for intratumoral or subcutaneous administration in patients with advanced solid tumor disease, and recruitment in the first-in-human dose escalating trial started in early 2015. Another CD40 trial, a combination trial with the CD40 agonist RO7009789 and a PD-L1 blocker, is being initiated by Roche almost in parallel. We foresee that the clinical data generated in such trials over the coming years will provide key information on how CD40 stimulation can be used to enhance the cancer-immunity cycle.
Disclosure of Potential Conflicts of Interest
Christina Furebring, Per Norlén, and Peter Ellmark are current employees of Alligator Bioscience AB, and hold stock options and stocks in Alligator Bioscience AB. Thomas Tötterman has received research funding from and is a consultant to Alligator Bioscience AB. Sara Mangsbo is a consultant to Alligator Bioscience AB.
References
- Naidoo J, Page DB, Wolchok JD. Immune modulation for cancer therapy. Br J Cancer 2014; 111:2214-9; PMID:25211661; http://dx.doi.org/10.1038/bjc.2014.348.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1-10; PMID:23890059; http://dx.doi.org/10.1016/j.immuni.2013.07.012.
- Mangsbo SM, Broos S, Fletcher E, Veitonmaki N, Furebring C, Dahlen E, Norlen P, Lindstedt M, Totterman TH, Ellmark P. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T cell dependent tumor immunity. Clin Cancer Res 2015; 21:1115-26. pii: clincanres.0913.2014.
- Vonderheide RH1, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res 2013;19 :1035-43; PMID:23460534; http://dx.doi.org/10.1158/1078-0432.CCR-12-2064.
- Jackaman C, Lew AM, Zhan Y, Allan JE, Koloska B, Graham PT, Robinson BW, Nelson DJ. Deliberately provoking local inflammation drives tumors to become their own protective vaccine site. Int Immunol 2008;20:1467-79; PMID:18824504; http://dx.doi.org/10.1093/intimm/dxn104.
- Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody. Clin Cancer Res 2011; 17:2270-80; PMID:21389097; http://dx.doi.org/10.1158/1078-0432.CCR-10-2888.
- Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Tötterman TH, Mangsbo SM. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res 2014; 2:80-90; PMID:24778163; http://dx.doi.org/10.1158/2326-6066.CIR-13-0067.
- Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res 2014; 20: 1747-56; PMID:24691639; http://dx.doi.org/10.1158/1078-0432.CCR-13-2116.
- Furness AJ, Vargas FA, Peggs KS, Quezada SA. Impact of tumour microenvironment and Fc receptors on the activity of immunomodulatory antibodies. Trends Immunol 2014; 35:290-8; PMID:24953012; http://dx.doi.org/10.1016/j.it.2014.05.002.
- Zippelius A, Schreiner J, Herzig P2, Müller P. Induced PD-L1 expression mediates acquired resistance to agonistic anti-CD40 treatment. Cancer Immunol Res. 2015; 3: 236-44.
