Abstract
We recently demonstrated that splenectomy restores an interaction between CD8+ T cells and macrophages necessary for intraocular tumor elimination. Taking into consideration other studies indicating that intraocular tumor growth does not induce tumor-specific CD8+ T-cell tolerance, our data suggest that splenectomy may influence the phenotype of tumor-associated macrophages.
Over 30 years ago Streilein and NiederkornCitation1 revealed that the spleen contributes to immune escape of intraocular tumors by demonstrating that P815 tumors of DBA/2 origin, which grew progressively when transplanted in the anterior chamber of the eye of semi-allogeneic Balb/C mice, were rejected if the mice were splenectomized (SPLNX) prior to intraocular tumor challenge. Recently, we revisited and extended these observations using E.G7-OVA tumors in C57Bl/6 miceCitation2 by characterizing the requirements for elimination of intraocular tumors in SPLNX mice as a first step toward understanding how to restore tumoricidal activity within the eye.
Intraocular tumor growth induces a unique form of systemic tolerance, termed anterior chamber-associated immune deviation (ACAID), in which CD4+ T-cell responses to tumor antigens are inhibited by antigen-specific CD8+ T suppressor cells.Citation3 SPLNX abrogated the generation of T suppressor cells and restored tumor-specific CD4+ T cell responses, suggesting a critical tumoricidal effector.Citation4 However, we demonstrated that CD4+ T cells were not required for rejection of intraocular tumors;Citation2 rather, CD8+ T cells were indispensable.Citation2
The simplest explanation for how the spleen limits immunosurveillance of intraocular tumors is that CD8+ T-cell tolerance to tumor antigens arises in the spleen, which is supported by our previous study indicating that introduction of soluble antigens into the anterior chamber induced expansion but functional inactivation of antigen-specific CD8+ T cells.Citation5 However, we and others have shown that intraocular tumor growth does not induce systemic CD8+ T-cell tolerance.Citation6-8 In fact, mice with intraocular tumors reject a subsequent tumor challenge in the skin or opposite eye via tumor-specific CD8+ T-cell responses.Citation6 Therefore, if T-cell tolerance contributes to intraocular tumor growth it is confined to the primary tumor microenvironment.
In support of regional immune suppression within the eye, we have shown that E.G7-OVA eye tumors are resistant to adoptive cell transfer therapy (ACT) with in vitro generated tumor-specific OT-I CD8+ T-cell effectors whereas E.G7-OVA skin tumors are sensitive.Citation8 Progressive intraocular tumor growth occurred despite CD8+ T-cell infiltration of intraocular tumors at higher T cell: tumor ratios than were observed in regressing E.G7-OVA skin tumors receiving the same treatment. CD8+ T cells infiltrating intraocular or skin tumors demonstrated comparable effector function, as measured by granule exocytosis and cytokine production (IFNγ and TNFα),Citation8 indicating that T-cell tolerance could not explain the impaired immunosurveillance of intraocular tumors and suggesting that the interaction of CD8+ T cells with another immune cell population was critical for intraocular tumor elimination. In support of that notion, elimination of E.G7-OVA skin tumors required intratumoral macrophages that were induced to express tumoricidal concentrations of nitric oxide(NO) by IFNγ produced by CD8+ T cells.Citation8,9 In contrast, macrophages within intraocular tumors expressed low non-tumoricidal concentrations of NO despite their expression of NO-synthase-2, which was also induced by IFNγ expressed by CD8+ T cells.Citation8 Hence, impaired effector function in macrophages, rather than infiltrating CD8+ T cells, contributed to progressive intraocular tumor growth.
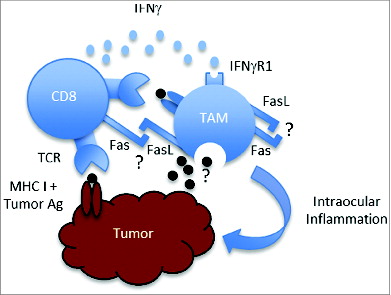
Rejection of intraocular tumors in SPLNX mice required CD8+ T cells, IFNγ, and FasL () but not perforin or TNFα.Citation2 IFNγ and FasL did not target tumor cells directly as SPLNX IFNγR1−/− and Fas-defective lpr mice failed to eliminate intraocular tumors that expressed IFNγR1 and Fas.Citation2 Bone marrow chimeric mice indicated that rejection of intraocular tumors in SPLNX mice required IFNγR1 and Fas expression on immune cells, and SPLNX increased the frequency of activated macrophages within intraocular tumors in an IFNγ and Fas/FasL-dependent manner suggesting a cellular target for IFNγ and FasL. Intratumoral macrophages were necessary at the effector stage as the elimination of intraocular tumors in SPLNX mice deficient for CD8+ T cells given ACT therapy with activated OT-I CD8+ T-cell effectors was abrogated if intratumoral macrophages were eliminated by local administration of clodronate liposomes.Citation2 Hence, SPLNX restored intratumoral macrophage effector function that was associated with tumor rejection. It remains unclear how macrophages eliminate intraocular tumors in SPLNX mice as NO production is dispensable.Citation2
Figure 1. Model of intraocular tumor elimination in splenectomized mice. CD8+ T cells that engage tumor antigens presented by MHC Class I on tumors and potentially cross-presented by tumor-associated macrophages (TAMs) are activated to express IFNγ. IFNγ, together with Fas/FasL interactions that may occur between CD8+ T cells and TAMs or within TAMs, causes TAM activation. This is associated with severe intraocular inflammation that promotes elimination of intraocular tumors by inducing complete destruction of the eye (ocular phthsis).
An adjuvant effect of SPLNX is not limited to intraocular tumors because Ugel and coworkersCitation10 recently demonstrated greater efficacy of ACT therapy with activated OT-I CD8+ T cells in SPLNX mice with established E.G7-OVA skin tumors. As tumor growth was associated with increased numbers of splenic CD11b+ Ly6C+ GR-1int cells that inhibited OT-I CD8+ T-cell responses in vitro, the authors concluded that tumor progression was the result of CD8+ T-cell tolerance induced by these myeloid derived suppressor cells (MDSCs). However, CD11b+ Ly6C+ GR-1int cells are also precursors of tumor-associated macrophages (TAMs).Citation10 As TAMs are required for elimination of E.G7-OVA skin and intraocular tumors,Citation2,8 SPLNX may change the composition of the tumor microenvironment by increasing the frequency of TAMs that are sensitive to polarization by CD8+ T cells toward a tumoricidal phenotype. It is important to note that low-dose chemotherapy also reduced the number of splenic MDSCs and demonstrated a similar adjuvant effect for ACT therapy,Citation10 suggesting a viable alternative to SPLNX in patients with malignancies.
In conclusion, it is clear that SPLNX restores tumoricidal CD8+ T-cell responses; however, the mechanism remains unclear. Our observations of intraocular tumor progression despite induction of tumor-specific CD8+ T-cell responses indicate that T-cell tolerance induced by MDSCs does not explain the impaired immunosurveillance of intraocular tumors. As the interaction between CD8+ T cells and macrophages within the tumor microenvironment is critical for tumor elimination, a careful comparison of macrophages in SPLNX and control mice is warranted to evaluate whether SPLNX alters TAM phenotypes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Streilein JW, Niederkorn JY. Induction of anterior chamber-associated immune deviation requires an intact, functional spleen. J Exp Med 1981; 153:1058-1067; PMID:6788883; http://dx.doi.org/10.1084/jem.153.5.1058
- Miller MR, Mandell JB, Beatty KM, Harvey SAK, Rizzo MJ, Previte DM, Thorne SH, McKenna KC. Splenectomy promotes indirect elimination of intraocular tumors by CD8+ T cells that is associated with IFNγ-and Fas/FasL-dependent activation of intratumoral macrophages. Cancer Immunol Res 2014; 2:1175-1185; PMID:25248763; http://dx.doi.org/10.1158/2326-6066.CIR-14-0093-T
- McKenna KC, Previte DM. Influence of CD8+ T regulatory cells on intraocular tumor development. Fron Immunol 2012; 3:303; PMID:NOT_FOUND
- Streilein JW, Niderkorn JY. Characterization of the suppressor cell(s) responsible for anterior chamber-associated immune deviation (ACAID) induced in BALB/c mice by P815 cells. J Immunol 1985; 184:1381-1387; PMID:3155766
- McKenna KC, Xu Y, Kapp JA. Injection of soluble antigen into the anterior chamber of the eye induces expansion and functional unresponsiveness of antigen-specific CD8+ T cells. J Immunol 2002; 169:5630-5637; PMID:12421942; http://dx.doi.org/10.4049/jimmunol.169.10.5630
- McKenna KC, Kapp JA. Accumulation of immunosuppressive CD11b+ myeloid cells correlates with the failure to prevent tumor growth in the anterior chamber of the eye. J Immunol 2006; 177:1599-1608; PMID:16849468; http://dx.doi.org/10.4049/jimmunol.177.3.1599
- Ksander BR, Streilein JW. Analysis of cytotoxic T cell responses to intracameral allogeneic tumors. Invest Ophthalmol Vis Sci 1989; 30:323-329; PMID:2492486
- Vicetti Miguel RD, Cherpes TL, Watson LJ, McKenna KC. CTL induction of tumoricidal nitric oxide production by intratumoral macrophages is critical for tumor elimination. J Immunol 2010; 185:6706-6718; PMID:21041723; http://dx.doi.org/10.4049/jimmunol.0903411
- Hollenbaugh JA, Dutton RW. IFN-gamma regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol 2006; 177:3004-3011; PMID:16920936; http://dx.doi.org/10.4049/jimmunol.177.5.3004
- Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012; 2:628-639; PMID:22959433; http://dx.doi.org/10.1016/j.celrep.2012.08.006
