Abstract
The CC chemokine receptor 4 (CCR4) is highly expressed on type 2 helper T cells and regulatory T (Treg) cells. Mogamulizumab, an anti-CCR4 antibody, reduces the numbers of CCR4+ malignant T cells and CCR4+ Treg cells in cutaneous T-cell lymphoma. Depleting Treg cells by targeting CCR4 has great potential in cancer immunotherapies.
The CC chemokine receptor type 4 (CCR4) is a 7-transmembrane, G-protein–coupled receptor that is highly expressed in type 2 helper T (Th2) cells and regulatory T (Treg) cells involved in T-cell migration and skin homing. Cutaneous T-cell lymphomas (CTCLs) are a group of lymphoproliferative neoplasia with clonal proliferation of skin-homing T cells. Mycosis fungoides (MF) and Sézary syndrome (SS) are the 2 most common CTCL variants. Mogamulizumab (KW-671) is a new defucosylated anti-CCR4 monoclonal antibody that binds to the extracellular N-terminus of CCR4 and induces enhanced antibody-dependent cellular cytotoxicity (ADCC).Citation1 Mogamulizumab was launched in Japan in 2012 for the treatment of patients with CCR4+ adult T-cell leukemia-lymphoma (ATLL). Clinical trials with mogamulizumab in ATLL, peripheral T-cell lymphoma (PTCL), and CTCL in the US are ongoing. We conducted a translational study accompanying a Phase I/II trial of mogamulizumab in patients with aggressive/refractory CTCL.Citation2-4 The purpose of this study was to evaluate CCR4 expression on malignant T cells, Treg cells, and CD8+ T cells in MF and SS patients, the effects of mogamulizumab on T-cell subsets, and their clinical relevance.
We found that 20.8%-100% of CD4+CD26− or/and CD7− malignant T cells in the peripheral blood of MF/SS patients were positive for CCR4, whereas 58.6%-100% of CD3+CD4+CD25+CD127− Treg cells were positive for CCR4. Following 4-week treatment with mogamulizumab, dramatic decreases in cell numbers and CCR4 expression were seen in both malignant T cells and Treg cells in MF/SS patients. In contrast, only 3.2%-23.2% of CD8+T cells was CCR4+ at baseline. CCR4 expression on CD8+ T cells was decreased after 4 weeks of treatment, but the percentage of CD8+ T cells was increased. The number of CCR4+ infiltrating lymphocytes in skin lesions varied, but was reduced by different degrees in most lesions after 4 weeks of therapy. Moreover, we observed a post-treatment increase in NK cell percentages and NK cell cytotoxicity in approximately two-thirds of patients.Citation2 Therefore, multiple beneficial effects of mogamulizumab were achieved in MF/SS patients. This antibody not only reduces the level of CCR4+ malignant T cells locally and systemically but also eliminates CCR4+ Treg cells (), which are instrumental for restoring NK cell antitumor function in patients with MF/SS.
Figure 1. Antibody-dependent cellular cytotoxicity (ADCC) during mogamulizumab treatment in patients with mycosis fungoides (MF) and Sézary syndrome (SS). (A) Mogamulizumab induces ADCC between NK cells and CCR4+ malignant T cells. (B) Mogamulizumab induces ADCC between NK cells and CCR4+ Treg cells.
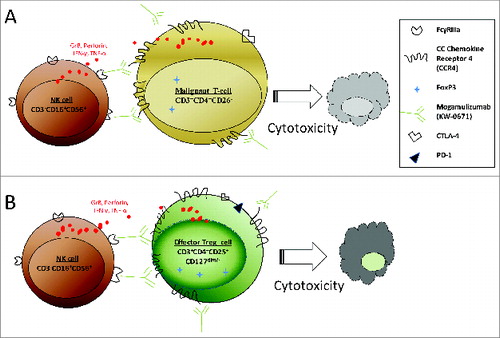
Our findings have important clinical significance. Our study demonstrates differences in CCR4 expression on malignant T cells, Treg cells, and CD8+ T cells of MF/SS patients, resulting in different responses to mogamulizumab. It is therefore important to tailor anti-CCR4 treatment for MF/SS patients. Our study also provides evidence for broad applications of mogamulizumab therapy for other types of CTCL. Moreover, the depletion of Treg cell populations by mogamulizumab has great potential for the treatment of many other tumors with immunosuppressive mechanisms involving Treg cells.
CCR4+ malignant T cells are found not only in MF/SS, but also in many other types of CTCL, including primary cutaneous anaplastic large cell lymphoma and peripheral T-cell lymphomas not otherwise specified.Citation5 Thus, mogamulizumab or anti-CCR4 therapy can be used to treat a broad spectrum of patients with CTCL. In addition, CCR4 is a superior therapeutic target in CTCL compared with the broad effects of targeting CD3, CD4, or CD52. Targeting CD3 or CD4 removes both normal and malignant T cells, whereas targeting CD52 removes not only mature T lymphocytes but also B lymphocytes, NK cells, and dendritic cells, which increases the risk of infection and other adverse events. Because CCR4 is highly expressed on skin-homing malignant T cells in CTCL but weakly expressed on CD8+ T cells, B cells, NK cells, and monocytes, it is a highly specific target and may protect innate immune cells from destruction. Studies have reported that CCR4 may be expressed on platelets and is important for platelet activation, but we saw only a minor transitory effect of mogamulizumab on platelets in our study.Citation2
The depletion of Treg cells by mogamulizumab has great potential as an immunotherapy in anticancer strategies. Treg cells accumulate in many tumor tissues, allowing the tumors to escape from immune surveillance. Two CCR4 ligands, macrophage-derived chemokine (MDC, also known as CCL22) and thymus and activation-regulated chemokine (TARC, as known as CCL17), are found at high levels in the tumor environment and track CCR4+ Treg cells into tumor tissues. Eliminating and inhibiting Treg cells to boost host antitumor immunity has long been attempted in studies targeting CD25, CTLA-4, and/or PD-1/PD-L1.Citation6,7 However, successful elimination of Treg cells may unleash undesirable severe autoimmunity. Studies have found that CCR4 is expressed mainly on effector Treg cells, but not on naïve Treg cells which may differentiate into effector Treg cells.Citation8 Specific removal of effector Treg cells by targeting CCR4 should reduce the degree of autoimmunity. Recently, AstraZeneca and Kyowa Kakko Kirin partnered on a Phase I/IB immuno-oncology clinical study to evaluate anti-PD-L1 antibody (MED14736), anti-CTLA antibody (tremelimumab), and mogamulizumab in cancer patients. We expect to see more effective depletion of Treg cells but lower autoimmunity in this study.
The number and activity of Treg cells are often found to be increased during antitumor vaccine treatment. This indicates that Treg cells may interfere with the antitumor immune responses generated as a result of the vaccine. Thus, mogamulizumab or similar therapy to remove Treg cells could be added or combined with antitumor vaccines to enhance their effectiveness. We also see a rationale for using mogamulizumab to deplete Treg cells in vaccination trials of patients with CTCL.Citation9,10
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Ishii T. [Development of an anti-CCR4 antibody, mogamulizumab, the first approved antibody made by POTELLIGENT((R)) technology]. Nihon Yakurigaku Zasshi 2013; 142:167-71; PMID:24107520; http://dx.doi.org/10.1254/fpj.142.167
- Ni X, Jorgensen JL, Goswami M, Challagundla P, Decker WK, Kim YH, Duvic MA. Reduction of Regulatory T Cells by Mogamulizumab, a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients with Aggressive/Refractory Mycosis Fungoides and Sezary Syndrome. Clin Cancer Res 2015; 21:274-85; PMID:25376389
- Duvic M, Pinter-Brown LC, Foss FM, Sokol L, Jorgensen JL, Challagundla P, et al. Phase 1/2 study of mogamulizumab, a defucosylated anti-CCR4 antibody, in previously treated patients with cutaneous T-cell lymphoma. Blood 2015; 125:1883-9.
- Kim YH, Pinter-Brown L, Foss F, Sokol L, Jorgensen JL, Spitalny GL, et al. CO26. Results of a phase 1/2 study for KW-0761, a monoclonal antibody directed against CC chemokine receptor type 4 (CCR4), in CTCL patients. Melanoma Res 2011; 21:e15-e6 0; http://dx.doi.org/10.1097/01.cmr.0000399460.48119.fe
- Jones D, O'Hara C, Kraus MD, Perez-Atayde AR, Shahsafaei A, Wu L, Dorfman DM. Expression pattern of T-cell-associated chemokine receptors and their chemokines correlates with specific subtypes of T-cell non-Hodgkin lymphoma. Blood 2000; 96:685-90; PMID:10887135
- de Rezende LC, Silva IV, Rangel LB, Guimaraes MC. Regulatory T cell as a target for cancer therapy. Arch Immunol Ther Exp 2010; 58:179-90; PMID:20373146; http://dx.doi.org/10.1007/s00005-010-0075-0
- Andersen MH. The targeting of immunosuppressive mechanisms in hematological malignancies. Leukemia 2014; 28:1784-92; PMID:24691076; http://dx.doi.org/10.1038/leu.2014.108
- Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, Ezoe S, Kanakura Y, Sato E, Fukumori Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci U S A 2013; 14:14; PMID:24127572
- Ni X, Richmond HM, Liao XM, Decker WK, Shiue LH, Shpall EJ, Duvic M. Induction of T-cell responses against cutaneous T-cell lymphomas ex vivo by autologous dendritic cells transfected with amplified tumor mRNA. J Invest Dermatol 2008; 128:2631-9; PMID:18480841; http://dx.doi.org/10.1038/jid.2008.125
- Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WYZ, Morales A, Abdulla F, Xing L, Navi D, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 2012; 119:355-63; PMID:22045986; http://dx.doi.org/10.1182/blood-2011-05-355222
