Abstract
Myeloid-derived suppressor cells (MDSCs) play an important role in immune suppression and accumulate under pathologic conditions such as cancer and chronic inflammation. They comprise a heterogeneous population of immature myeloid cells that exert their immunosuppressive function via a variety of mechanisms. Immunoglobulin-like transcript 3 (ILT3) is a receptor containing immunoreceptor tyrosine-based inhibition motifs (ITIMs) that can be expressed on antigen-presenting cells and is an important regulator of dendritic cell tolerance. ILT3 exists in a membrane-bound and a soluble form and can interact with a yet unidentified ligand on T cells and thereby induce T-cell anergy, regulatory T cells, or T suppressor cells. In this study, we analyzed freshly isolated peripheral blood mononuclear cells (PBMCs) of 105 patients with non-small cell lung cancer and 20 healthy controls and demonstrated for the first time that ILT3 is expressed on MDSCs. We show that increased levels of circulating MDSCs correlate with reduced survival. On the basis of ILT3 cell surface expression, an ILT3low and ILT3high population of polymorphonuclear (PMN)-MDSCs could be distinguished. Interestingly, in line with the immunosuppressive function of ILT3 on dendritic cells, patients with an increased proportion of PMN-MDSCs and an increased fraction of the ILT3high subset had a shorter median survival than patients with elevated PMN-MDSC and a smaller ILT3high fraction. No correlation between the ILT3high subset and other immune variables was found. ILT3 expressed on MDSCs might reflect a previously unknown mechanism by which this cell population induces immune suppression and could therefore be an attractive target for immune intervention.
Abbreviations:
- APC, antigen-presenting cell
- DC, dendritic cell
- ELISA, enzyme-linked immunosorbent assay
- HC, healthy control
- ILT3, immunoglobulin-like transcript 3
- MDSC, myeloid-derived suppressor cell
- MFI, mean fluorescence intensity
- MO-MDSC, monocytic MDSC
- NFκB, nuclear factor κB
- NSCLC, non-small cell lung carcinoma
- PBMC, peripheral blood mononuclear cell
- PMN-MDSC, polymorphonuclear MDSC
- sILT3, soluble ILT3
- Treg, regulatory T cell
- Ts, T suppressor cell
Introduction
The immune system influences lung cancer pathogenesis, progression, and response to therapy and thereby strongly contributes to the prognosis of the disease.Citation1 The influence of the immune system is, however, paradoxical, as the different components can either inhibit tumor growth or promote immune evasion and cancer progression.Citation2 Myeloid-derived suppressor cells (MDSCs) are an important contributor to the latter process.Citation3
MDSCs are a heterogeneous population of immature myeloid cells that accumulate in blood, lymphoid organs, and tumor tissue under several pathologic conditions, including cancer.Citation3,4 MDSCs are generally characterized by being positive for CD33 and CD11b and low or negative for HLA-DR.Citation5 This population can be divided in 2 subgroups with different morphology. Monocytic (MO)-MDSCs are mononuclear and CD14 positive, whereas granulocytic or polymorphonuclear (PMN)-MDSCs are CD14 negative.Citation3,5 Both populations contribute to tumor immune escape by inducing immune suppression and tolerance through a variety of mechanisms, such as production of nitric oxide and reactive oxygen species, depletion of arginine and cysteine, and induction of regulatory T cells (Tregs).Citation6-9 However, their regulation and dynamics are poorly understood, especially in humans.Citation10
An important mediator of the induction of immune tolerance is immunoglobulin-like transcript (ILT) 3 (also known as LILRB4, CD85k, or LIR-5), which is expressed on monocytes and antigen-presenting cells (APCs) such as macrophages and dendritic cells (DCs).Citation11,12 ILT3 expression marks tolerogenic DCs and has been reported to be elevated in APCs of cancer patientsCitation13,14 and decreased in autoimmune diseases.Citation15,16 ILT3 is believed to signal via its immunoreceptor tyrosine-based inhibitory motifs (ITIMs). These motifs are known to inhibit NF-κB activation and transcription of costimulatory molecules and thereby render the cell tolerogenic.Citation17 The 2 extracellular Ig-like domains of ILT3 most likely contain ligand-binding sites; however, the nature of the ligand is still unknown.Citation12,17-19 Membrane-bound ILT3 interacts with T cells in a cell-to-cell contact-dependent manner and induces immune suppression or tolerance by inducing anergy in CD4+ T cells, suppressing the differentiation of IFN-γ–producing CD8+ T cells and inhibiting T-cell proliferation and the induction of Tregs and alloantigen-specific CD8+ T suppressor (Ts) cells.Citation17,20 Moreover, a soluble form of ILT3 (sILT3) resulting from alternative splicing has been described to interact with T cells and induce anergy.Citation21 CD68+ tumor-associated macrophages are the major source of sILT3.Citation21,22
We have previously have shown increased levels of MDSCs in patients with treatment-naïve Stage IV non-small cell lung carcinoma (NSCLC).Citation23 Because MDSCs have the ability to induce immune suppression, we hypothesized that ILT3 expression could be part of a yet unidentified mechanism by which MDSCs mediate immune escape. Therefore, we aimed to assess the expression of ILT3 on MDSCs of lung cancer patients with advanced disease before the start of chemotherapy and evaluate its effect on clinical outcome.
Results
Characteristics of study subjects
This study included 118 patients with Stage IV NSCLC who were participating in the NVALT12 study. Of these, 13 patients were excluded from further analysis because blood samples were not available for processing within 6 hours after the samples were taken. shows the characteristics of the 105 NSCLC study participants and 20 healthy controls (HC) that were included in our analyses. The patient characteristics of this study cohort were similar to those of the total NVALT12 population,Citation23 therefore no selection bias was introduced.
Table 1. Characteristics of study subjects
Levels of MO-MDSCs and PMN-MDSCs are elevated in Stage IV NSCLC patients
The two MDSC subsets, monocytic MDSCs (MO-MDSCs) and peripheral blood mononuclear MDSCs (PMN-MDSCs), were assessed in the peripheral blood of patients with Stage IV NSCLC and healthy controls by flow cytometric analysis of freshly obtained PBMCs. The gating strategy for the 2 populations is presented in and was performed as previously described.Citation23 First, mature granulocytes were excluded based on their CD16++ expression.Citation5,24 Next, MO-MDSCs were characterized as CD11b+CD14+HLA-DR−CD33+CD15+, whereas PMN-MDSCs were characterized as CD11b+CD14−HLA-DR−CD33+CD15+.
Figure 1. Levels of circulating MO-MDSCs and PMN-MDSCs are increased in patients with non-small cell lung cancer. (A) Flow cytometry was performed on freshly isolated PBMCs from NSCLC patients and healthy controls. The gating strategy for determination of circulating MO-MDSCs and PMN-MDSCs is depicted. After gating the live cells, CD16high cells were excluded. MO-MDSCs were characterized as CD14+CD11b+HLA-DRlowCD15+ and PMN-MDSCs were characterized as CD14−CD11b+HLA-DRlowCD15+. MDSC levels were determined as a proportion of live cells. (B). Frequency of MO-MDSCs and PMN-MDSCs in PBMCs was significantly higher in NSCLC patients than in healthy controls. **P < 0.01; ***P < 0.001; Mann-Whitney U test. MDSC, myeloid-derived suppressor cell; MO-MDSC, monocytic MDSC; NSCLC, non-small cell lung carcinoma; PBMC, peripheral blood mononuclear cell; PMN-MDSC, polymorphonuclear MDSC.
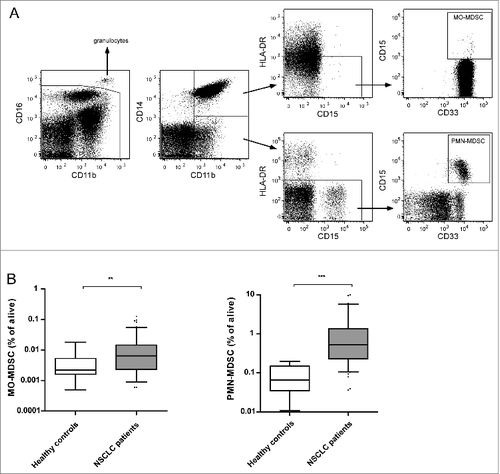
As reported earlier by our group, levels of MDSCs are elevated in NSCLC patients.Citation23 Accordingly, in the cohort used in this study the frequencies of both MO-MDSCs and PMN-MDSCs in peripheral blood were significantly higher in patients than in healthy controls, as shown in .
ILT3 is expressed by subpopulations of MDSCs
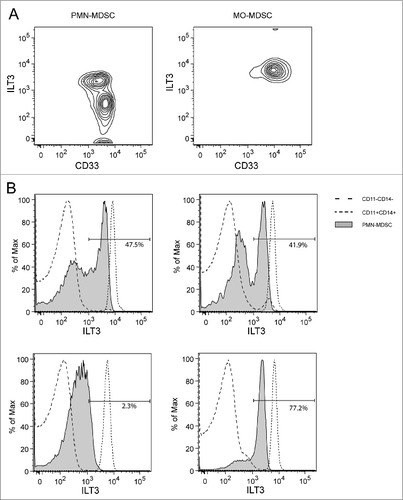
ILT3 expression could be detected on both PMN-MDSCs and MO-MDSCs (). In contrast to MO-MDSCs, which showed homogeneous high expression of ILT3 (right panel), PMN-MDSCs contained 2 subsets of high and low ILT3 expression (left panel). shows ILT3 expression on PMN-MDSCs of 4 different patients, compared to CD11b−CD14− cells (mainly lymphocytes) and CD11b+CD14+ cells (mainly monocytes). Whereas the lymphocytes were consistently negative for ILT3 (mean fluorescence intensity [MFI] = 53), monocytes showed high and homogeneous ILT3 expression (median MFI = 7,399). The expression of ILT3 on PMN-MDSCs was intermediate and showed 2 peaks in most patients, although the distribution over ILT3high (MFI > 10Citation3) and ILT3low (MFI < 103) fractions varied extensively between patients (the percentage of ILT3high cells ranged between 0.2% and 92.9%). In contrast to PMN-MDSCs, virtually all MO-MDSCs were positive for ILT3 with homogeneous expression of the marker, which was slightly, but significantly, lower than expression in the monocyte population (MFI = 6,275, P < 0.001).
Figure 2. ILT3 expression on myeloid-derived suppressor cells. (A) Flow cytometric data of a representative patient, displayed as density plot based on ILT3 and CD33 expression. Left panel: PMN-MDSCs, right panel: MO-MDSCs. (B) Histograms of 4 different patients with ILT3 expression of PMN-MDSCs (shaded) compared to the expression within the CD11b−CD14− population (dashed line, mainly lymphocytes) and CD11b+CD14+ population (dotted line, mainly monocytes). Proportions of the ILT3high fraction are displayed as percentage of PMN-MDSCs. ILT3, immunoglobulin-like transcript 3; MDSC, myeloid-derived suppressor cell; MO-MDSC, monocytic MDSC; PMN-MDSC, polymorphonuclear MDSC.
The ILT3high fraction of PMN-MDSCs is increased in lung cancer patients and is not correlated with frequency of T and B cells or monocytes
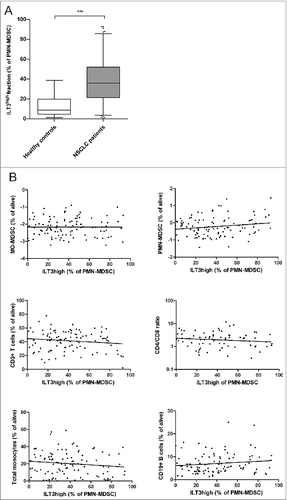
The proportions of ILT3high PMN-MDSCs within the total PMN-MDSC population varied considerably between patients. As shown in , the ILT3high fraction of PMN-MDSCs was significantly higher in NSCLC patients (39 ± 24% [mean ± SD]) compared to healthy controls (12 ± 10%; P < 0.0001). The proportion of ILT3high PMN-MDSCs did not correlate with the proportion of ILT3high PMN-MDSCs (). To investigate whether the ILT3high fraction of PMN-MDSCs had an effect on, or was affected by, other immunologic cell populations, we analyzed T cells, the CD4+/CD8+ T-cell ratio, B cells, and monocytes. No statistically significant correlations were found between the ILT3high fraction of PMN-MDSC and the proportions of B cells, T cells, the CD4+/CD8+ ratio and levels of monocytes in NSCLC patients. Furthermore, no correlation with MO-MDSCs existed (). Analyses of absolute numbers of these cell populations gave similar results (data not shown).
Figure 3. ILT3high proportion of PMN-MDSCs in patients with non-small cell lung cancer. (A) ILT3high proportions of PMN-MDSCs were significantly higher in NSCLC patients than in healthy controls. ***P < 0.001, Student t test. (B) Correlations between the proportion of ILT3high PMN-MDSC and various immune subsets in NSCLC patients were analyzed with the Spearman rho test. None of the tests revealed a significant correlation (P > 0.05 for all analyses). ILT3, immunoglobulin-like transcript 3; MDSC, myeloid-derived suppressor cell; MO-MDSC, monocytic MDSC; PMN-MDSC, polymorphonuclear MDSC.
Soluble ILT3 is elevated in serum of NSCLC patients and does not correlate with immunologic cell populations
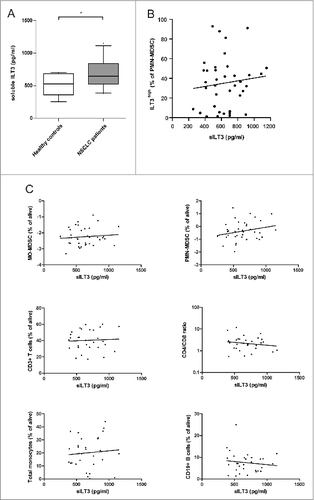
It has been described that, in addition to membrane-bound ILT3, soluble ILT3 (sILT3) can also have immunosuppressive effects.Citation21 In multiple types of cancer, sILT3 is present in the serum of patients and is able to strongly abolish T-cell responses against tumor antigens.Citation21,22 To test whether sILT3 was present in the serum of the NSCLC patients, sILT3 levels were quantified by enzyme-linked immunosorbent assay (ELISA) in a pilot study of 30 randomly chosen NSCLC patients and 8 healthy controls. As shown in , sILT3 was present in the serum of NSCLC patients at significantly higher levels (P = 0.03) than in healthy controls. We hypothesized that soluble ILT3 might be produced by ILT3-expressing MDSCs; however, no correlation was found between the serum levels of sILT3 and the proportions of ILT3high cells in the PMN-MDSC population (). Furthermore, sILT3 was not correlated with MFI values of surface ILT3 on monocytes or MDSC populations (data not shown). To check whether sILT3 levels were related to the peripheral immune profile of the patients, we assessed the correlation between sILT3 serum levels and peripheral immune cell proportions in the patient cohort. No significant correlations were found between the levels of sILT3 and the frequency of PMN-MDSCs and MO-MDSCs, T cells, the CD4+/CD8+ ratio, B cells, and monocytes ().
Figure 4. Serum sILT3 in patients with non-small cell lung cancer. (A) Soluble ILT3 was measured by ELISA in serum samples of healthy controls (n=8) and patients with Stage IV NSCLC (n=30). Levels of sILT3 were significantly higher in NSCLC patients compared to healthy controls. * P < 0.05, Student t test. (B) sILT3 levels of NSCLC patients did not correlate with the fraction of ILT3high cells of PMN-MDSC (Spearman rho test). (C) Correlations between level of sILT3 in serum and various immune subsets in NSCLC patients were analyzed with the Spearman rho test. None of the tests revealed a significant correlation (P > 0.05 in all analyses). ELISA, enzyme-linked immunosorbent assay; ILT3, immunoglobulin-like transcript 3; MDSC, myeloid-derived suppressor cell; MO-MDSC, monocytic MDSC; NSCLC, non-small cell lung carcinoma; PMN-MDSC, polymorphonuclear MDSC; sILT3, soluble ILT3.
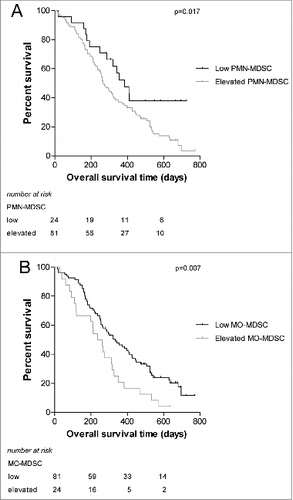
Increased proportions of circulating MDSCs correlate with a poorer outcome in NSCLC patients
For various types of cancer it has been shown that higher levels of MDSCs correlate with reduced survival of patients.Citation25,26 To validate this effect in our patient cohort, patients were divided into 2 groups based on the proportion of MDSCs. Values that were higher than the mean + 2SD of healthy controls were considered to be elevated. In this way, we identified patients with elevated PMN-MDSC and patients with elevated MO-MDSC. In accordance with reported findings,Citation25,26 patients with elevated proportions of PMN-MDSCs had a significantly shorter survival than patients with low proportions of PMN-MDSC (P = 0.017). Likewise, patients with elevated proportions of MO-MDSC had a significantly shorter survival than patients with low MO-MDSC values (P = 0.007). The survival curves are shown in . Of note, proportions of PMN-MDSC and MO-MDSC were significantly correlated (P < 0.001; not shown).
Figure 5. Survival curves of NSCLC patient groups according to the proportion of MDSCs. NSCLC patients were divided into 2 groups based on the mean value + 2SD of healthy controls. (A) Survival of patients with elevated versus low PMN-MDSC levels (B) Survival of patients with elevated versus low MO-MDSC levels. MDSC, myeloid-derived suppressor cell; MO-MDSC, monocytic MDSC; NSCLC, non-small cell lung carcinoma; PMN-MDSC, polymorphonuclear MDSC.
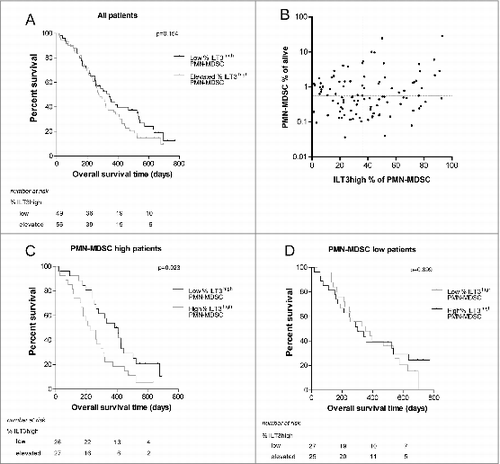
The ILT3high fraction of PMN-MDSC correlates with a poorer outcome in NSCLC patients
To assess whether ILT3 expression on PMN-MDSCs influenced clinical outcome, NSCLC patients were divided into 2 groups based on the percentage of ILT3high cells among PMN-MDSCs, as for the proportions of MDSCs. shows a slightly shorter overall survival for patients with a higher percentage of ILT3high PMN-MDSCs, although this did not reach statistical significance (P = 0.15). However, it is conceivable that the effect of high ILT3 expression on PMN-MDSCs is limited in patients with low proportions of these cells. Therefore, we performed sub-analysis on the group of patients with the highest levels of PMN-MDSC (above median; and found a significant negative correlation with overall survival (P = 0.023, ). In contrast, in patients with low proportions of MDSC, the percentage of ILT3high cells did not influence overall survival (). Analysis of progression-free survival gave similar results, but only the effect of MO-MDSC level reached statistical significance (data not shown). Serum levels of sILT3 were not correlated with survival (data not shown).
Figure 6. NSCLC patient survival based on ILT3 fractions of MDSC. (A) Survival of patients with an elevated percentage of ILT3high PMN-MDSC versus patients with a low percentage of ILT3high PMN-MDSCs. The survival curves were not significantly different. (B) No correlation was found between the level of total PMN-MDSCs and the percentage of ILT3high PMN-MDSCs (Spearman rho; P = 0.38). For the curves in panels C and D, patients were divided based on the frequency of PMN-MDSC and the percentage of ILT3high PMN-MDSCs. Cutoff values were the median value of all patients to create equally sized groups. (C) In patients with high levels of PMN-MDSC, the fraction of ILT3high cells correlated significantly with overall survival. (D) In patients with low levels of PMN-MDSC, the percentage of ILT3high PMN-MDSCs did not significantly contribute to overall survival. The curves were compared with a log-rank test, stratified for treatment arm. ILT3, immunoglobulin-like transcript 3; PBMC, peripheral blood mononuclear cells; MO-MDSC, monocytic myeloid-derived suppressor cell; PMN-MDSC, polymorphonuclear myeloid-derived suppressor cell.
Discussion
This is the first study showing that the immune suppressive molecule ILT3 is expressed by MDSCs. In recent years, MDSCs have received great attention for their immunosuppressive role in cancer, which is executed through a diversity of mechanisms including arginase-1 and iNOS expression and oxidative stress.Citation27 Given that MDSCs are a heterogeneous population of immature cells, these mechanisms are likely to be differentially employed by the different subsets of MDSC and dependent on the context of the microenvironment. With the demonstration of ILT3 expression on the cell membrane of MDSCs, we describe a pathway by which MDSCs might execute their immunosuppressive function that was previously unknown for these cells. The heterogeneity of MDSCs is further demonstrated by our finding that ILT3 is not expressed on all circulating MDSC of lung cancer patients, but only on MO-MDSCs and a subset of PMN-MDSCs.
Little is known about MDSCs under physiological conditions. In healthy individuals, immature myeloid cells with the same phenotype as MDSCs are continuously generated in the bone marrow, where they differentiate into mature myeloid cells before entering the circulation. Under pathologic conditions they can be released from the bone marrow before maturation. However, in mice, MDSCs are also present in the liver during physiological conditions and are thought to play a pivotal role in maintaining homeostasis.Citation28 Therefore, the function of MDSCs is probably different in healthy controls and cancer patients. It has been reported that MDSCs of diseased mice have an increased capacity to suppress T-cell proliferation compared with MDSCs of normal mice.Citation28 This is supported by the finding that MDSCs from healthy controls show decreased expression of immunosuppressive molecules compared with MDSCs from cancer patients.Citation29 Likewise, in a previous study we showed that arginase-1 is expressed in PBMCs in much larger amounts in lung cancer patients than in healthy controls.Citation23,30 Our finding that ILT3 is upregulated on MDSCs of NSCLC patients is in agreement with functional differences with regard to immunosuppression by MDSCs in NSCLC patients and healthy controls.
ILT3 expression on DCs is of critical importance in the induction of tolerance.Citation31,32 ILT3 can be induced on APCs by cytokines such as IL-10, interferon (IFN)-α, and IFN-β and by interaction with CD8+ Ts cells.Citation33,34 Other inducers of ILT3 on APCs are vitamin D3 analogs, COX-1/2 inhibitors, and tryptophan depletion in the environment, resulting in T-cell non-responsiveness and tolerance.Citation18,33,35 Intracellular signaling via the ITIMs of ILT3 induces tolerance in DCs via downregulation of the NFκB pathway and plays an inhibitory role in antigen presentation.Citation36 However, these signaling pathways are not likely to play an important role in ILT3-expressing MDSCs, since MDSCs are not classic APCs and are defined by low HLA-DR expression. Therefore, ILT3-induced immune suppression by MDSCs is not likely to exert its effectiveness via co-stimulation or antigen presentation, as shown for DCs. However, extracellular signaling by membrane-bound ILT3 and sILT3 has been shown to induce immunosuppressive CD8+ Ts cells and CD4+ Tregs.Citation18,19 MDSCs might therefore use this extracellular signaling pathway to exert their immunosuppressive function, as this cell population is known to induce Tregs and T cell anergy.
In this study we did not find a correlation between ILT3 expression on MDSCs and the proportion of T cells in PBMCs. However, the functionality of these T cells was not assessed in this study. Given that MDSCs might induce anergy in T cells or induce Tregs from naïve T cells, the functionality rather than the number of T cells could be decreased by ILT3+ MDSCs and should be investigated in further studies. Unfortunately, the amount of blood collected from each patient, in combination with the low levels and phenotypic instability of the MDSC populations, did not allow for functional studies to compare the ILT3high and ILT3low PMN-MDSC subsets or the suppressive capacity of MO-MDSCs.
The clinical relevance of both membrane-bound and soluble ILT3 has been demonstrated in studies of different cancer types. Membrane-bound ILT3 is expressed on tumor cells in B-cell chronic lymphocytic leukemia (B-CLL), myeloid leukemia, and pancreatic and gastric cancer, and in B-CLL its expression on tumor cells is associated with aggressive growth.Citation13,37,38 Elevated expression on DCs was found in colorectal cancer patients compared to healthy controls.Citation14 Moreover, strong infiltration of CD68+ILT3high macrophages has been demonstrated in lymph nodes containing metastatic carcinoma cells.Citation22 sILT3 is also elevated in serum of patients with melanoma, colorectal cancer, and pancreatic carcinomaCitation21,22 In contrast, ILT3 expression is decreased on DCs of patients with systemic lupus erythematosus (SLE) and autoimmune thyroid disease, where, in contrast to cancer, the immune system is overactivatedCitation15,39 and ILT3 was also reported to be decreased on circulating monocytes of patients with multiple sclerosis.Citation16 In line with these results, we found elevated sILT3 levels and a higher percentage of ILT3+ PMN-MDSCs in NSCLC patients, which indicates a role for this molecule in tumor pathogenesis or progression, most likely by stimulating tumor immune escape. In this cohort of Stage IV NSCLC patients, the detrimental effect of the presence of elevated proportions of circulating MO-MDSC and PMN-MDSC was confirmed as elevated proportions of MDSC were correlated with a decreased overall survival. We were unable to demonstrate a significant effect of the percentage of ILT3high PMN-MDSC on clinical outcome for the whole patient population, although the mean survival time was shorter in patients with a higher proportion of ILT3high PMN-MDSCs (). However, when patients with PMN-MDSC proportions above the median were selected, an elevated percentage of ILT3high PMN-MDSC was correlated with reduced survival. This finding supports our hypothesis that ILT3 expression on MDSCs plays a role in immune suppression, but its influence is only large enough to be detected in survival analyses in patients with higher proportions of PMN-MDSCs. In contrast to PMN-MDSCs, all MO-MDSCs expressed ILT3 on their membrane. Given that the frequency of MO-MDSCs in peripheral blood was very low but still yielded a significant correlation with overall survival, MO-MDSCs might be stronger immune suppressors than PMN-MDSCs in patients with Stage IV NSCLC. Although we did not provide evidence, one might speculate that this could be due to the constitutive expression of ILT3 on MO-MDSCs. Moreover, the proportion of ILT3high PMN-MDSCs did not correlate with the proportions of other immunologic cell types, indicating that its clinical value is not a reflection of an altered balance in the rest of the immune system but instead might represent a tumor-promoting function, through either altering the functionality of the immune cells rather than cell numbers, or by acting locally on tumor progression or immune escape. The absence of evidence supporting any clinical relevance of the level of sILT3 in serum in this study might be due to the small number of serum samples measured, although the lack of any correlation with circulating immune cell populations provided no further indication of its role in peripheral blood. sILT3 might, however, function more locally, since it has been shown to be produced by tumor-associated CD68+ macrophages.Citation21 Given that sILT3 levels did not correlate with the expression level on circulating MDSCs or monocytes, it is not likely that sILT3 is produced in the periphery in high amounts, but rather might reflect the local immune composition and a suppressive microenvironment.
Taken together, our results show that ILT3 is expressed on MDSCs and indicate that this affects the clinical outcome. The relevance of ILT3 in cancer patients is supported by results from the literatureCitation14,37,38 and therefore further investigation of its mode of action should be performed. Nonetheless, it is debatable whether ILT3 on its own would determine the immunosuppressive status of MDSCs; rather, the sum of all immunosuppressive mechanisms and their relative contribution to the activity of MDSC will probably determine the extent of its unfavorable effects in cancer patients.
MDSCs play an important role in mediating immunosuppression and therefore represent a significant hurdle to successful immunotherapy in NSCLC.Citation2 Therefore, combining immunotherapeutic approaches with MDSC-inhibiting drugs like gemcitabine or VEGF blockers to elicit more potent anticancer effects is a promising approach.
To our knowledge this is the first study that demonstrates the expression of ILT3 on human MDSCs. Future studies will underscore the importance of this molecule on MDSC in other experimental or clinical settings.
Materials and Methods
Study population
The patients in this study were participating in the NVALT12 study (trial number NCT01171170), a randomized Phase II multicenter study on the effect of a nitroglycerin patch or placebo in patients with stage IV NSCLC treated with carboplatin, paclitaxel, and bevacizumab. Patients in the NVALT12 study were diagnosed with Stage IV non-squamous NSCLC and were not eligible for treatment with curative intent. Disease stage was determined in accordance with the American Joint Committee on Cancer (AJCC). Blood samples were collected before the start of treatment and analyzed by flow cytometry. In this study, 118 patients were included for analysis of ILT3 expression.
Twenty age-matched healthy controls (HC; mean age 54 years) with no history of malignancies and/or autoimmune diseases were also enrolled in the study.
Written consent was obtained from all individuals before blood sampling and the study was approved by the ethical committee of the Erasmus Medical Center (MEC-2012-048 [HC] and CCMO: NL33442.042.10 [NSCLC patients]).
Isolation of PBMCs
PBMCs were isolated using a Ficoll-Hypaque (GE Healthcare, Diegem, Belgium) density gradient. Blood was supplemented to a volume of 50 mL with phosphate-buffered saline (PBS, Gibco, Breda, the Netherlands) before layering onto Ficoll-Hypaque. After centrifugation for 20 min at 1200 × g, PBMCs were collected from the plasma-Ficoll interphase. Cells were washed twice with 50 mL PBS and counted prior to further analysis. In previous research, we demonstrated that MDSC levels remain constant for the first 6 h, but decrease significantly when stored for a longer time.Citation23 Therefore, flow cytometry on PBMCs was performed immediately or within 6 h after blood was collected.
Flow cytometry
PBMCs were stained with the following conjugated monoclonal antibodies for analysis of MDSCs: anti-CD15–PE, anti-CD16–PERCP-Cy5.5, anti-CD33–PE-Cy7, anti-CD11b–APC, anti-HLA-DR–APC-Cy7 (all from BD Biosciences), anti-CD14–PE-Texas-Red (Invitrogen, Breda, the Netherlands), anti-ILT3–FITC (R&D Systems) and a live/dead marker 4’,6-diamidino-2-phenylindole (DAPI, Molecular Probes). Staining with anti-CD4–FITC, anti-CD8–APC, (BD Biosciences), anti-CD3–APC-eFluor 780 (eBioscience) antibodies and DAPI was performed for the analysis of T cells.
Cells were washed with FACS buffer (PBS, 0.25% BSA, 5 mM EDTA, 0.05% NaN3) and stained for 30 min at 4 °C with the abovementioned antibodies, appropriately diluted in FACS buffer supplemented with 2% normal human serum. Flow cytometry was performed on a LSRII flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (Tree Star).
MDSCs were characterized as previously described.Citation23 For MDSC staining, the CD16 marker was used to exclude mature granulocytes based on their CD16++ expression, as previously described to be important for MDSC purity.Citation5,24 PMN-MDSC were characterized as CD11b+CD14−HLA-DR−CD33+ CD15+ and MO-MDSCs were characterized as CD11b+ CD14+HLA-DR−CD33+CD15+. Total monocytes were characterized as CD11b+CD14+. For lymphocyte staining, T cells were characterized as CD3+ and divided into CD4+CD8− (CD4+ T cells) and CD4−CD8+ (CD8+ T cells) subsets. B cells were characterized as CD19+ cells.
Measurement of soluble ILT3 in serum
For quantitative detection of ILT3 in serum, a commercially available enzyme-linked immunosorbent assay (ELISA) was used according to the manufacturer's specifications (Cusabio Biotech.). All samples were assayed in duplicate and quantified using a standard curve. The detection range was from 31.2–2,000 pg/mL.
Statistical analysis
Differences between healthy controls and lung cancer patients were evaluated by the Mann–Whitney U test. Correlations were assessed using the Spearman rho correlation test. The effects of size of immunologic cell populations and expression levels of ILT3 on survival were assessed with a log-rank (Mantel-Cox) test. In these analyses, patients were stratified for treatment group. Statistical analysis was performed using the statistical program SPSS (version 21.0, SPSS Inc.). All P values were 2-sided and P values below the conventional level of significance (P < 0.05) were considered statistically significant. Figures were generated in GraphPad Prism (version 5.0, GraphPad Software).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Bremnes RM, Al-Shibli K, Donnem T, Sirera R, Al-Saad S, Andersen S, Stenvold H, Camps C, Busund LT. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: emphasis on non-small cell lung cancer. J Thorac Oncol 2011; 6:824-33; PMID:21173711; http://dx.doi.org/10.1097/JTO.0b013e3182037b76
- Heuvers ME, Aerts JG, Cornelissen R, Groen H, Hoogsteden HC, Hegmans JP. Patient-tailored modulation of the immune system may revolutionize future lung cancer treatment. BMC Cancer 2012; 12:580; PMID:23217146; http://dx.doi.org/10.1186/1471-2407-12-580
- Jiang JW, Guo WJ, Liang XH. Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Human Immunol 2014; 75:1128-37; PMID:25305034; http://dx.doi.org/10.1016/j.humimm.2014.09.025
- Ortiz ML, Lu L, Ramachandran I, Gabrilovich DI. Myeloid-derived suppressor cells in the development of lung cancer. Cancer Immunol Res 2014; 2:50-8; PMID:24778162; http://dx.doi.org/10.1158/2326-6066.CIR-13-0129
- Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin Cytom 2014; PMID:25504825; http://dx.doi.org/10.1002/cyto.b.21206
- Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010; 40:2969-75; PMID:21061430; http://dx.doi.org/10.1002/eji.201040895
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/10.1038/nri3175
- Filipazzi P, Huber V, Rivoltini L. Phenotype, function and clinical implications of myeloid-derived suppressor cells in cancer patients. Cancer Immunol Immunother 2012; 61:255-63; PMID:22120756; http://dx.doi.org/10.1007/s00262-011-1161-9
- Buijs N, Luttikhold J, Houdijk APJ, van Leeuwen PAM. The Role of a Disturbed Arginine/NO Metabolism in the Onset of Cancer Cachexia: A Working Hypothesis. Curr Med Chem 2012; 19:5278-86; PMID:22963622; http://dx.doi.org/10.2174/092986712803833290
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol 2011; 32:19-25; PMID:21067974; http://dx.doi.org/10.1016/j.it.2010.10.002
- Colonna M, Nakajima H, Navarro F, Lopez-Botet M. A novel family of Ig-like receptors for HLA class I molecules that modulate function of lymphoid and myeloid cells. J Leukoc Biol 1999; 66:375-81; PMID:10496306
- Cheng H, Mohammed F, Nam G, Chen Y, Qi J, Garner LI, Allen RL, Yan J, Willcox BE, Gao GF. Crystal structure of leukocyte Ig-like receptor LILRB4 (ILT3/LIR-5/CD85k): a myeloid inhibitory receptor involved in immune tolerance. J Biol Chem 2011; 286:18013-25; PMID:21454581; http://dx.doi.org/10.1074/jbc.M111.221028
- Colovai AI, Tsao L, Wang S, Lin H, Wang C, Seki T, Fisher JG, Menes M, Bhagat G, Alobeid B, et al. Expression of inhibitory receptor ILT3 on neoplastic B cells is associated with lymphoid tissue involvement in chronic lymphocytic leukemia. Cytometry B Clin Cytom 2007; 72:354-62; PMID:17266150; http://dx.doi.org/10.1002/cyto.b.20164
- Orsini G, Legitimo A, Failli A, Ferrari P, Nicolini A, Spisni R, Miccoli P, Consolini R. Quantification of Blood Dendritic Cells in Colorectal Cancer Patients During the Course of Disease. Pathol Oncol Res 2013; 20:267-76; PMID:24022399; http://dx.doi.org/10.1007/s12253-013-9691-4
- Leskela S, Rodriguez-Munoz A, de la Fuente H, Figueroa-Vega N, Bonay P, Martin P, Serrano A, Sánchez-Madrid F, González-Amaro R, Marazuela M. Plasmacytoid dendritic cells in patients with autoimmune thyroid disease. J Clin Endocrinol Metab 2013; 98:2822-33; PMID:23666960; http://dx.doi.org/10.1210/jc.2013-1273
- Jensen MA, Yanowitch RN, Reder AT, White DM, Arnason BG. Immunoglobulin-like transcript 3, an inhibitor of T cell activation, is reduced on blood monocytes during multiple sclerosis relapses and is induced by interferon beta-1b. Mult Scler 2010; 16:30-8; PMID:20007427; http://dx.doi.org/10.1177/1352458509352794
- Suciu-Foca N, Cortesini R. Central role of ILT3 in the T suppressor cell cascade. Cell Immunol 2007; 248:59-67; PMID:17923119; http://dx.doi.org/10.1016/j.cellimm.2007.01.013
- Kim-Schulze S, Scotto L, Vlad G, Piazza F, Lin H, Liu Z, Cortesini R, Suciu-Foca N. Recombinant Ig-like transcript 3-Fc modulates T cell responses via induction of Th anergy and differentiation of CD8+ T suppressor cells. J Immunol 2006; 176:2790-8; PMID:16493035; http://dx.doi.org/10.4049/jimmunol.176.5.2790
- Vlad G, Suciu-Foca N. Induction of antigen-specific human T suppressor cells by membrane and soluble ILT3. Exp Mol Pathol 2012; 93:294-301; PMID:23018130; http://dx.doi.org/10.1016/j.yexmp.2012.09.011
- Vlad G, Chang CC, Colovai AI, Vasilescu ER, Cortesini R, Suciu-Foca N. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int Rev Immunol 2010; 29:119-32; PMID:20132030; http://dx.doi.org/10.3109/08830180903281185
- Cortesini R. Pancreas cancer and the role of soluble immunoglobulin-like transcript 3 (ILT3). JOP 2007; 8:697-703; PMID:17993722
- Suciu-Foca N, Feirt N, Zhang QY, Vlad G, Liu Z, Lin H, Chang CC, Ho EK, Colovai AI, Kaufman H, et al. Soluble Ig-like transcript 3 inhibits tumor allograft rejection in humanized SCID mice and T cell responses in cancer patients. J Immunol 2007; 178:7432-41; PMID:17513794; http://dx.doi.org/10.4049/jimmunol.178.11.7432
- Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AM, Groen HJ, Smit EF, Hoogsteden HC, Hegmans JP, Aerts JG. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer 2013; 81:468-74; PMID:23850196; http://dx.doi.org/10.1016/j.lungcan.2013.06.005
- Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009; 69:1553-60; PMID:19201693; http://dx.doi.org/10.1158/0008-5472.CAN-08-1921
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419-30; PMID:21644036; http://dx.doi.org/10.1007/s00262-011-1028-0
- Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother 2013; 62:1711-22; PMID:24072401; http://dx.doi.org/10.1007/s00262-013-1475-x
- Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol 2012; 144:250-68; PMID:22858650; http://dx.doi.org/10.1016/j.clim.2012.06.003
- Chen S, Akbar SM, Abe M, Hiasa Y, Onji M. Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 2011; 166:134-42; PMID:21762128; http://dx.doi.org/10.1111/j.1365-2249.2011.04445.x
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res 2007; 13:721s-6s; PMID:17255300; http://dx.doi.org/10.1158/1078-0432.CCR-06-2197
- Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14(−)/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol 2010; 136:35-45; PMID:19572148; http://dx.doi.org/10.1007/s00432-009-0634-0
- Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol 2002; 3:237-43; PMID:11875462; http://dx.doi.org/10.1038/ni760
- Ge G, Tian P, Liu H, Zheng J, Fan X, Ding C, Jin Z, Luo X, Xue W. Induction of CD4+ CD25+ Foxp3+ T regulatory cells by dendritic cells derived from ILT3 lentivirus-transduced human CD34+ cells. Transpl Immunol 2012; 26:19-26; PMID:22005288; http://dx.doi.org/10.1016/j.trim.2011.10.001
- Vlad G, Suciu-Foca N. Induction of antigen-specific human T suppressor cells by membrane and soluble ILT3. Exp Mol Pathol 2012; PMID:23018130
- Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Hum Immunol 2008; 69:681-6; PMID:18817834; http://dx.doi.org/10.1016/j.humimm.2008.08.286
- Waschbisch A, Sanderson N, Krumbholz M, Vlad G, Theil D, Schwab S, Mäurer M, Derfuss T. Interferon Beta and vitamin d synergize to induce immunoregulatory receptors on peripheral blood monocytes of multiple sclerosis patients. PLoS One 2014; 9:e115488; PMID:25551576; http://dx.doi.org/10.1371/journal.pone.0115488
- Cella M, Dohring C, Samaridis J, Dessing M, Brockhaus M, Lanzavecchia A, Colonna M. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med 1997; 185:1743-51; PMID:9151699; http://dx.doi.org/10.1084/jem.185.10.1743
- Zhang Y, Lu N, Xue Y, Zhang M, Li Y, Si Y, Bian X, Jia Y, Wang Y. Expression of immunoglobulin-like transcript (ILT)2 and ILT3 in human gastric cancer and its clinical significance. Mol Med Rep 2012; 5:910-6; PMID:22246571
- Dobrowolska H, Gill KZ, Serban G, Ivan E, Li Q, Qiao P, Suciu-Foca N, Savage D, Alobeid B, Bhagat G, et al. Expression of immune inhibitory receptor ILT3 in acute myeloid leukemia with monocytic differentiation. Cytometry B Clin Cytom 2013; 84:21-9; PMID:23027709; http://dx.doi.org/10.1002/cyto.b.21050
- Jensen MA, Patterson KC, Kumar AA, Kumabe M, Franek BS, Niewold TB. Functional genetic polymorphisms in ILT3 are associated with decreased surface expression on dendritic cells and increased serum cytokines in lupus patients. Ann Rheum Dis 2013; 72:596-601; PMID:22904259; http://dx.doi.org/10.1136/annrheumdis-2012-202024
