Abstract
Therapeutic vaccines for follicular lymphoma have had limited success. A novel in situ immunotherapeutic strategy combining 3 different treatment modalities induced regression of disseminated follicular lymphoma, which correlated with systemic antitumor T-cell immunity. These results should renew interest in the development of local combined radio- and immunotherapies to achieve abscopal effects.
Rare cases have been reported in which regression of tumors distant from the radiation field was achieved after ionizing radiotherapy for metastatic cancers—a phenomenon referred to as the abscopal effect.Citation1 The proposed mechanism involves local release of tumor antigens that induce systemic immune effects acting at distant sites. However, the anecdotal occurrence of abscopal effects suggests that radiotherapy by itself is usually inadequate to break tolerance in the tumor microenvironment. To date, local immunotherapies administered intratumorally have not received much attention relative to systemic immunotherapies. Here, we discuss the possible concerted actions of local radiation and in situ immunostimulatory strategies to induce T cell-mediated immune responses that result in clinical effects and the potential implications for the design of future trials.
In our recent study, 14 patients with Stage III/IV follicular lymphoma (FL) Grade I-IIIA, the majority of whom were previously untreated, received sequential intranodal immunotherapy targeting single lymph nodes ().Citation2 Three different treatment modalities were combined: the lymphoma nodes received 8 Gy of radiotherapy (RT) accompanied by ultrasound-guided injections of low-dose (5 mg) rituximab and immature autologous dendritic cells (DCs). Clinical responses were observed in 5 out of 14 patients (36%), and 2 patients had durable complete remissions. Notably, vaccination-induced systemic CD8 and CD4 T cell-mediated responses against autologous tumor cells were detected in peripheral blood after treatment in responding patients. Reduction in total tumor volume was closely correlated with the magnitude of the vaccination-induced systemic CD8 T cell-mediated responses, and immune responders showed a prolonged time to next treatment compared to non-responders. Thus, this in situ vaccine strategy could override local suppression of T-cell responses targeting the tumor. Moreover, activated tumor-reactive T cells were rendered resistant to further suppression. In some patients this resulted in the elimination of bulky tumor masses, probably as a result of T-cell migration to distant tumor sites.
Figure 1. Schedule for intranodal immunotherapy and proposed mechanism of action. Rituximab (5 mg) is injected into a solitary lymphoma node or lesion on days 1 and 3 (1). Rituximab induces apoptosis in CD20-expressing lymphoma cells, sensitizes them to RT of 8 Gy targeting the same site and administered on day 2 (2), and mediates FcR-binding and uptake of lymphoma cells by immature DCs (5–10 × 107) injected into the irradiated lesion on days 4 and 5 (3). The irradiation induces the expression and release of damage-associated molecular patterns (DAMPs) by the lymphoma cells, which in turn mediates maturation of the DCs. DCs present tumor antigens from the lymphoma cells to tumor-reactive CD4 and CD8 T cells, resulting in activation and migration of the T cells to distant tumor nodes. The treatment schedule was performed 3 times, at weeks 1, 3 and 5, targeting a different lymphoma lesion each week.
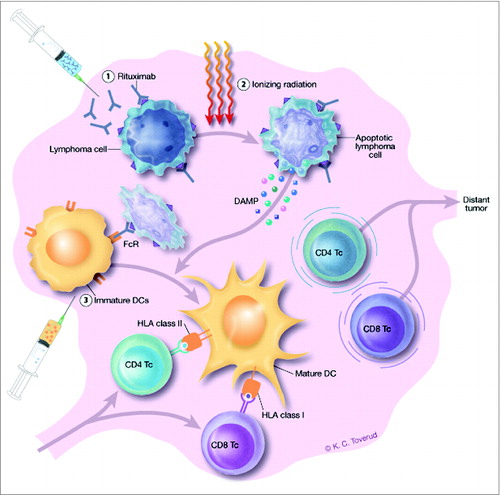
Which therapeutic mechanisms could overcome suppression and activate tumor-reactive T cells? Although low-dose irradiation of only 4 Gy has been shown to eradicate lymphoma locally due to direct cytotoxic effects, systemic effects are rare. However, radiation can set the stage for antitumor immunity by inducing inflammation and immunogenic cell death (ICD) of tumor cells.Citation3 Thus, radiotherapy has been shown to induce membrane expression of a variety of damage-associated molecular patterns (DAMPs) crucial for ICD, including calreticulin (CRT) and heat-shock protein 70, as well as the release of high mobility group box 1. Such irradiated tumor cells can induce maturation of DCs and IFN-γ–producing T cells. The RT would, however, be expected to eliminate local DCs alongside tumor cells. To facilitate uptake and presentation of tumor antigens to T cells, DCs could either be attracted by intratumoral injection of Toll-like receptor (TLR) ligands, as elegantly demonstrated by Brody and colleagues,Citation4 or injected following ex vivo generation. As only 5% of intradermally injected DCs reach the draining lymph nodes,Citation5 direct intranodal injection is favored. Immature DCs have the highest potential for uptake of antigen. Preconditioning of the lymphoma node by RT provides an ample source of “danger signals” to facilitate DC maturation and presentation of tumor antigens to T cells. Memory T cells migrating into the irradiated lymph nodes can then make contact with, and become primed by, the injected and matured DCs. Moreover, intranodally administrered DCs can migrate to distal lymph nodes,Citation5 opening the possibility that DCs might bring tumor antigen to distant sites for further T-cell priming. A third alternative is that the RT does not eradicate all T cell subsets, but permits survival of resident tumor-infiltrating T lymphocytes (TILs). Thus, in both mice and humans, recent stimulation can profoundly modulate the radioresistance of effector memory T cells (J. L. Pugh et al., personal communication). Studies of serial biopsies sampled from the tumor before and after RT could shed light on these questions.
Therapeutic antibodies can mediate indirect antitumor effects through the induction of adaptive immune responses, similar to RT. Binding of rituximab to CD20-expressing lymphoma cells will enhance Fc-receptor (FcR)-mediated phagocytosis by DCs and promote cross-presentation of tumor antigens to CD8 T cells, as well as antigen uptake and fragmentation by other FcR-expressing innate immune cells migrating into the tumor. Furthermore, rituximab has been shown to increase the sensitivity of lymphoma cells to external beam radiation. To our knowledge, RT has not previously been combined with anti-CD20 antibodies in close temporal proximity. However, the addition of local RT for a melanoma patient who was progressing on systemic treatment with monoclonal antibodies targeting the checkpoint regulator CTLA-4 (ipilimumab) successfully induced a clinical response that correlated with immune activation.Citation6 The combination might be equally, or more, efficient with fewer side effects if local anti–CTLA-4 is utilized, as suggested by studies in animal models.Citation7 This concept is currently being tested in a Phase I clinical trial including patients with melanoma, lymphoma, or colorectal cancer (NCT01769222).
Following the in situ immunostimulatory approach, we detected systemic T-cell responses in peripheral blood of patients with tumor reduction after treatment.Citation2 In keeping with some other studies applying local immunotherapy, CD8 responses were the strongest. What do these T cells recognize in the tumor? This remains to be investigated, but it is interesting to look to recent lessons learned from the anti-tumor T-cell responses identified among TILs or following treatment with checkpoint inhibitors. In those therapies, neoantigens generated from DNA mutations seem to be important drivers of the antitumoral T-cell responses.Citation8,9 Although the mutational load of lymphoma is lower than that of melanoma,Citation10 one might speculate that follicular lymphoma constitutes a particularly immunogenic disease given the high response rate to checkpoint inhibition and occasional spontaneous remissions.
The checkpoint inhibitor anti-PD1 has shown impressive clinical effects in multiple cancers including follicular lymphoma, but with less toxicity than anti-CTLA4 therapy. Thus, a logical next step to further unmask inhibited antitumor immunity and enhance vaccination responses might be to add anti-PD1 as a fourth modality to the three-strike combination of RT, rituximab and DCs. A major advantage of local therapy is the low toxicity, which allows mixes of therapeutic modalities in different sequential order and temporal relations to be assessed. However, to move forward in a logical manner with a rational clinical design, there is a need for further detailed investigation into the therapeutic mechanisms. We predict that local treatment involving combined immunotherapies to generate systemic antitumor immunity and clinical responses will become important in the future development of novel therapies for lymphoma and other cancers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
1014773_Supplementary_Materials.zip
Download Zip (189.4 KB)References
- Tang C, Wang X, Soh H, Seyedin S, Cortez MA, Krishnan S, Massarelli E, Hong D, Naing A, Diab A, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014; 2:831-8; PMID:25187273; http://dx.doi.org/10.1158/2326-6066.CIR-14-0069
- Kolstad A, Kumari S, Walczak M, Madsbu U, Hagtvedt T, Bogsrud TV, Kvalheim G, Holte H, Aurlien E, Delabie J, et al. Sequential intranodal immunotherapy induces antitumor immunity and correlated regression of disseminated follicular lymphoma. Blood 2015; 125:82-89; PMID:25293773; http://dx.doi.org/10.1182/blood-2014-07-592162
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691-701; PMID:16365148; http://dx.doi.org/10.1084/jem.20050915
- Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, et al. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010; 28:4324-32; PMID:20697067; http://dx.doi.org/10.1200/JCO.2010.28.9793
- Adema GJ, de Vries IJ, Punt CJ, Figdor CG. Migration of dendritic cell based cancer vaccines: in vivo veritas? Curr Opin Immunol 2005; 17:170-4; PMID:15766677; http://dx.doi.org/10.1016/j.coi.2005.01.004
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366:925-31; PMID:22397654; http://dx.doi.org/10.1056/NEJMoa1112824
- Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 2013; 123:2447-63; PMID:23728179; http://dx.doi.org/10.1172/JCI64859
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31:e439-42; PMID:24043743; http://dx.doi.org/10.1200/JCO.2012.47.7521
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19:747-52; PMID:23644516; http://dx.doi.org/10.1038/nm.3161
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, et al. Signatures of mutational processes in human cancer. Nature 2013; 500:415-21; PMID:23945592; http://dx.doi.org/10.1038/nature12477
