Abstract
Relapse of high-risk neuroblastoma (HR-NB) is deemed invariably fatal yet increasing numbers of HR-NB patients achieve a second complete/very good partial remission (CR/VGPR), hence the urgency to find a successful consolidative therapy. Identifying efficacy in patients without assessable disease, however, is problematic. We report the first study providing outcome data for this group of patients with poor prognosis. To prevent another relapse, HR-NB patients in second or later CR/VGPR received the anti-GD2 murine antibody 3F8 plus granulocyte-macrophage colony-stimulating factor plus isotretinoin in a Phase II trial. Upon meeting the target aim for progression-free survival (PFS) in the initial cohort of 33 patients, the trial was amended to allow patients who developed human anti-mouse antibody (HAMA) to receive rituximab to ablate HAMA with or without low-dose maintenance chemotherapy until immunotherapy could resume. For the total of 101 study patients, 5-year PFS and overall survival (OS) rates were 33% ± 5% and 48% ± 5%, respectively. Among the 33 long-term progression-free survivors, 19 had MYCN amplification, 19 had previously received anti-GD2 immunotherapy plus isotretinoin (as first-line therapy), and 15 never received maintenance chemotherapy. In a multivariate analysis of prognostic factors, only absence of minimal residual disease in bone marrow after 2 cycles of immunotherapy and before initiation of isotretinoin or anti-HAMA therapy was significantly favorable for both PFS and OS. Therefore, long-term PFS is possible for HR-NB patients who achieve at least a second CR/VGPR and receive consolidation that includes anti-GD2 immunotherapy plus isotretinoin, even if the patients received these biological treatments before relapse. Results from this prospective study will aid in the development of future Phase II studies for this growing ultra high-risk patient population.
Abbreviations:
- ASCT, autologous stem-cell transplantation
- BM, bone marrow
- CNS, central nervous system
- CR, complete remission
- GM-CSF, granulocyte-macrophage colony-stimulating factor
- HAMA, human anti-mouse antibody
- HR-NB: high-risk neuroblastoma
- INRC, International Neuroblastoma Response Criteria
- INRG, International Neuroblastoma Risk Group
- mAb, monoclonal antibody
- MIBG, metaiodobenzylguanidine
- MRD, minimal residual disease
- OS, overall survival
- PD, progressive disease
- PFS, progression-free survival
- VGPR, very good partial remission
Introduction
Standard treatment for high-risk neuroblastoma (HR-NB) includes myeloablative therapy with autologous stem-cell transplantation (ASCT), isotretinoin, and anti-GD2 monoclonal antibody (mAb).Citation1 Before the routine use of immunotherapy, 3-5 year progression-free survival (PFS) rates in national studies were 20-40%,Citation2-6 even with inclusion of patients with what is now known to be intermediate-risk NB.Citation7 The randomized study of the Children's Oncology Group showed significantly better outcome in patients treated post-ASCT with the anti-GD2 chimeric mAb ch14.18 plus granulocyte-macrophage colony-stimulating factor (GM-CSF) plus interleukin-2.Citation8 Nevertheless, relapse remains common and carries a dismal prognosis,Citation9-17 and has been deemed to be “invariably fatal” in a recent review.Citation1 Adverse prognostic factors for duration of survival post relapse include short time to first relapse and MYCN amplification.Citation11,13-17
The murine IgG3 anti-GD2 mAb 3F8 localizes selectively to NB.Citation18 In Phase II studiesCitation19-21 and in the adjuvant setting,Citation22,23 3F8 caused pain and urticaria without delayed toxicities. Immunosuppressive chemotherapy prevented early emergence of human anti-mouse antibody (HAMA).Citation24 As with other anti-GD2 mAbs,Citation25,26 activity was noted against NB in bone marrow (BM), but not against soft tissue tumor or progressive disease (PD). The ability to mount a HAMA response was consistently correlated with long-term survival, possibly related to the anti-idiotype network or induction of a host antitumor response.Citation27,28
Osteomedullary NB is an attractive target for mAb-mediated immunotherapy because the BM compartment contains tumoricidal macrophages and is immersed in blood. These conditions optimize the accessibility of NB cells to mAb and leukocyte effector cells, bypassing the common limitation of immunotherapy, namely poor trafficking into a bulky tumor. Early response of minimal residual disease (MRD) in BM was a significantly favorable prognostic factor for 3F8 used to consolidate first complete/very good partial remission (CR/VGPR).Citation23 In successive trials, 5-year PFS improved from 44% with 3F8 alone to 62% with 3F8 plus GM-CSF, results that underscore the antineoplastic advantage of GM-CSF activation of myeloid effectors.Citation21,23,29
We now report the first study centered on anti-GD2mAb plus GM-CSF for consolidation of second or later CR/VGPR. In fact, to our knowledge no prior report has presented outcome data with any kind of therapy for HR-NB patients in second or later CR/VGPR. This group of patients is now increasing in numbers because of better salvage treatments, hence the urgency to find successful consolidative treatments. However, identifying efficacy in patients without assessable disease can be difficult. Results from the prospective study reported herein will be useful in the development of future Phase II studies of this ultra high-risk patient population.
Results
Clinical characteristics
The 101 patients had a median age of 6.1 (range, 1.5–20.8) years at study entry, 44/97 (45%) had MYCN-amplified NB, 100 had Stage 4 disease at diagnosis or relapse, and one had MYCN-amplified Stage 3 disease at diagnosis and relapse. The time from diagnosis to first relapse was <12 months in 14 (14%) patients, 12–24 months in 55 (54%) patients, and >24 months in 32 (32%) patients. All patients received HR-NB induction regimens.Citation5,6,30,31 Therapy before relapse included isotretinoin in 85 (84%) patients and anti-GD2 mAb in 51 (50%) patients. Sites of relapse were osteomedullary (BM and/or bone) (n=34), osteomedullary plus soft tissue (n = 31), or soft tissue (n = 36). Salvage therapy for relapse before enrollment in this study included alkylators (temozolomide, cyclophosphamide) in 100 (99%) patients, topoisomerase II inhibitors (irinotecan, topotecan) in 99 (98%) patients, and investigative therapies in 40 (40%) patients (). At study entry, disease status was 15 CR, 82 VGPR, and 4 patients with abnormal skeletal uptake in 123I-metaiodobenzylguanidine (MIBG) scan (in retrospect, these 4 patients should not have been enrolled, but nevertheless were included in the analysis; all four had rapid progressive disease [PD]).
Table 1. Salvage therapy for relapse before enrollment of 101 study patients
Outcome
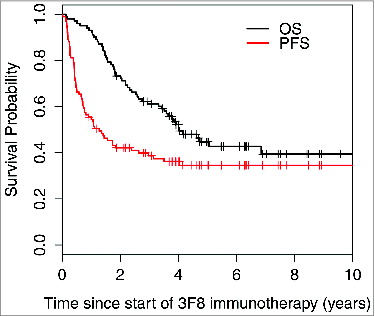
At 5 years, progression-free survival (PFS) was 33% ± 5% and overall survival (OS) was 48% ± 5% (). Median PFS was 1.28 years (95% confidence interval [CI] 0.77, 2.97), with 37/101 patients censored for progression. Among the 33 progression-free survivors, 19 had MYCN-amplified NB, 19 were previously treated with anti-GD2 MAb plus isotretinoin (as first-line therapy), 15 never received temozolomide maintenance, and 5 were enrolled in the third or later CR/VGPR. Three of these 33 patients were censored: 1 was enrolled on chimeric antigen-receptor T-cell therapy at 26 months and 2 started therapy for secondary leukemia at 34 and 69 months, respectively. All 3 remained disease-free and off all oncologic therapy at 115, 114, and 51 months from study entry, respectively.
Figure 1. Progression-free survival and overall survival of all 101 patients enrolled on the 03–077 protocol for consolidation of second or later complete/very good partial remission.
Four patients died while in continuous second CR/VGPR at 14 months (pneumonia), 26 months (an accident), 28 months (complications of treatment for secondary malignancy), and 49 months (pulmonary fibrosis).
Univariate and multivariate analyses of prognostic factors
Risk factors for survival were tested in univariate analyses (). Variables at diagnosis included age, MYCN, serum lactate dehydrogenase, bone metastases, and BM involvement by histology. Variables at study entry included time to first relapse, isolated relapse in the central nervous system (CNS), prior anti-GD2 mAb treatment, second versus third or later CR/VGPR, and pre-MRD. Post-enrollment variables included HAMA, rituximab treatment, temozolamide maintenance, and post-MRD. Isolated CNS relapse and negative post-MRD were significantly favorable for both PFS and OS. HAMA-positivity was significantly favorable for OS.
Table 2. Univariate analyses of patient and tumor characteristics for survival
As HAMA-positivity was associated with rituximab and temozolomide maintenance, the effect of HAMA was highly confounded by these treatments. Therefore, to estimate their independent effects in a multivariate model, rituximab and maintenance were tested as time-dependent variables (). In the subset of 76 patients who were HAMA-positive, when PFS and OS were recalculated from time of HAMA, rituximab was not significantly associated with PFS (P = 0.92) and was marginally significant for OS (HR = 0.47, 95% CI 0.22, 1.02, P = 0.055), whereas maintenance was significantly associated with improved PFS (HR=0.3, 95% CI 0.14, 0.73, P = 0.007) and marginally with OS (HR = 0.5, 95% CI 0.22, 1.03, P = 0.059).
Variables with univariate P < 0.05 were included in the multivariate model (). Since rituximab treatment and temozolomide maintenance were confounded by the HAMA effect, they were not included in the multivariate model. Variables that were no longer significant in the multivariate setting were considered for exclusion and stepwise regression was used to determine the final model. In the final multivariate model, negative post-MRD () and isolated CNS relapse correlated with significantly better PFS. Negative post-MRD () and HAMA-positivity were independent significant predictors of better OS.
Figure 2. Strong association between minimal residual disease status after 2 cycles of 3F8 therapy (post-MRD) and progression-free survival probability (P < 0.001).
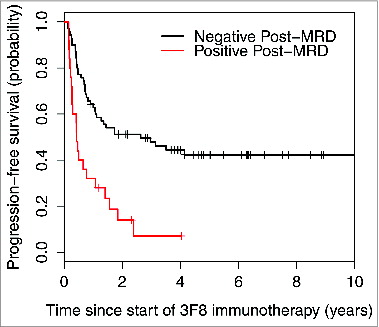
Figure 3. Strong association between minimal residual disease status after 2 cycles of 3F8 therapy (post-MRD) and overall survival probability (P < 0.001).
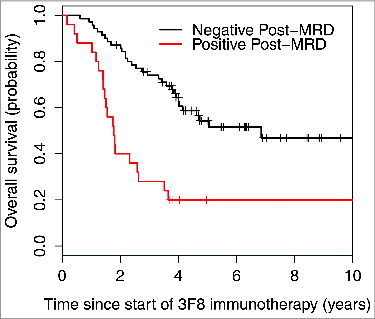
Table 3: Multivariate analysis of prognostic factors for survival
Toxicity
As previously described in detail,Citation20,21,23 3F8/GM-CSF had manageable toxicities, therefore treatment was administered outpatient and 3F8 dosage was never reduced. Common side effects associated with 3F8 in the 451 cycles administered in this study were grade 2 generalized pain and urticaria. Five patients came off the study and were hospitalized because of adverse events: 2 with grade 2 anaphylactoid reactions to 3F8 (in cycles 3 and 4); 2 with grade 3 hypertensive episodes (in cycles 11 and 12); and one with grade 3 posterior reversible encephalopathy syndrome without sequelae (one day after cycle 11). Thirty-one patients completed the protocol therapy without PD or adverse events. Two patients developed grade 2 hives following GM-CSF injections (in cycles 3 and 10). Common side effects of isotretinoin were grade 1–2 dry skin and cheilitis.
Discussion
This study suggests that up to one-third of HR-NB patients who achieve a second or later CR/VGPR can become long-term progression-free survivors with consolidation that includes anti-GD2 mAb and isotretinoin with or without low-dose maintenance chemotherapy. This observation applies even to patients whose prior therapy included an anti-GD2 mAb and isotretinoin, a noteworthy point given that both of these biological agents are now standard treatment for newly-diagnosed HR-NB.Citation1
This trial was devised after it became clear that 3F8 was safe, with acceptable acute toxicities (which allowed outpatient treatment) and without delayed toxicities (a serious concern in heavily prior-treated patients), and active against chemoresistant NB in BM.Citation19,20,22 These features meant that this immunotherapy would have limited impact on quality of life and might help reverse the dismal prognosis of relapsed HR-NB seen with the traditional treatment modalities of chemotherapy, local radiotherapy, and surgery.
For this prospective trial, the gold standard randomized design was deemed impractical because of concerns that, in the grave setting of relapse, families might not agree to forego a safe outpatient treatment that had showed encouraging anti-NB activity in prior studies. Also, with such a poor outcome in past experience, historical controls were considered valid for a single-arm study designed with the aim of improving prognosis. Thus, the trial was implemented with the reasonable expectations that (1) these ultra high-risk patients might benefit from the protocol therapy, and (2) clinical and biological data would prove useful for prognostication.
When the study was designed in 2003, we based statistics on long-term PFS rate <5% after relapse. This figure was derived from the widely held view that relapse of HR-NB was “uniformly fatal,”Citation10 although only a single large study with supportive data was available: The European NB Study Group found a 2-year OS (which is always better than PFS) of 8% from the date of relapse for patients aged >1 year with Stage 3–4 NB diagnosed from 1982–1992.Citation9 Subsequent reports presented confirmatory data about the dismal prognosis post-relapse: 5-year OS rates after relapse were 0% and 11%, respectively, in 2 single institutional studies of HR-NB patients treated in the 1990s to early 2000s;Citation11,12 5% and 8%, respectively, for Stage 4 disease (no age cut-off) in the Italian experience (1979-2004)Citation13 and the International NB Risk Group (INRG) database (1990–2002);Citation14 <10% for HR-NB in German trials (1990–2007);Citation15 and 12.6% in the United Kingdom (1990–2010).Citation17 In the large INRG experience,Citation14 patients with MYCN-amplified stage 4 at any age and MYCN-non-amplified stage 4 at age >18 months were not considered to be “salvageable after relapse.”
None of the previous reports, including those cited above, include data on PFS or OS of patients who achieved second or later CR/VGPR. The current report covering the 03–077 study provides clean outcome data that can be used as controls in clinical trials of this growing patient population. Most study patients were referred from other hospitals in the United States and beyond because no clinical trials were available elsewhere for patients without assessable disease after salvage therapy for relapse. The study population was not only large, but was typical of relapsed patients with respect to the following characteristics: (1) 45% had amplified MYCN, similar to the 44% incidence in a recent report on newly-diagnosed HR-NB;Citation6 (2) patients had been monitored at centers with expertise in pediatric oncology so the relapses were as expected, with the large majority occurring ≤ 24 months from initial diagnosis and involving osteomedullary and/or soft tissue disease;Citation9-13,15-17 (3) although patients were referred after starting salvage therapy, which limited our input regarding the latter, post-relapse treatment before study entry in all but 2 patients included standard anti-NB alkylators (cyclophosphamide, temozolomide) and topoisomerase II inhibitors (topotecan, irinotecan); and (4) investigative therapies, which are often used for relapse, were received by 40% of the 03–077 patients before enrollment on this trial ().
Although the ability to mount HAMA has been correlated with favorable outcome,Citation23 the presence of HAMA could accelerate blood clearance and limit the 3F8 drug effect, therefore 3F8 treatments were deferred until the HAMA titer became negative. After meeting the study's target aim for 2-year PFS using only 3F8/GM-CSF plus isotretinoin, the protocol was amended such that HAMA-positive patients could receive rituximab with or without outpatient maintenance chemotherapy. Anti-NB activity of the latter could have contributed to the duration of PFS, as suggested by analysis of the subset of patients who were HAMA-positive, but several observations made it unlikely that this minimal therapy accounted for the cure: (1) 15/33 long-term progression-free survivors never received maintenance; (2) MRD-negativity after 2 cycles of 3F8/GM-CSF (before maintenance) significantly correlated with PFS and OS, supporting a key role for this immunotherapy in these patients, similar to findings in patients in first CR/VGPR;Citation23 and (3) abundant past experience, including inferior results with maintenance chemotherapy in 2 national randomized trials,Citation2,4 strongly suggests that conventional chemotherapy is not curative for HR-NB.
In patients with no evidence of disease by standard evaluations, MRD assays can serve as a measure of NB sensitivity to this immunotherapy. Indeed, given the significantly prognostic MRD findings after 2 cycles (), early MRD response may allow rapid recognition of treatment efficacy in future trials and provide guidance about whether to continue immunotherapy in a given patient.
HR-NB relapse has long been viewed as tantamount to eventual death from PD or toxicity of salvage therapy.Citation1 Developments in the past decade, however, as evidenced by data in the current report, suggest that the equivalence between relapse and disease-related death may no longer hold true. A critical step toward cure of relapse is achieving second CR/VGPR. Tumor burden might be significantly reduced by surgery and high-dose salvage conventional chemotherapy regimens,Citation33,36 supplemented by local radiotherapy and relatively non-toxic systemic therapies that are non-cross-resistant with prior treatments and have anti-NB activity. Examples include investigative agentsCitation1 (e.g., fenretinideCitation37 and crizotinibCitation38), and targeted radiotherapy using 131I-MIBG or radiolabeled mAbs.Citation39,40 Immunotherapy with anti-GD2mAbs might eradicate remaining MRD, as has been documented in patients in first CR/VGPRCitation23 and as shown now in the current report on second or later CR/VGPR. Success against isolated CNS relapse (), which historically heralded a rapid demise,Citation41 supports the curative potential of the above strategy. Other interventions, including vaccines, are expected to bolster immunotherapeutic attack against chemoresistant NB.Citation42,43
In conclusion, long-term PFS occurred in patients who achieved second or later CR/VGPR of HR-NB and received consolidation with anti-GD2 mAb and isotretinoin with or without low-dose maintenance. This 03–077 trial had limitations as noted above, namely non-randomized design, paucity of historical data for controls, and variability in clinical characteristics and salvage therapies of study patients. Nonetheless, we suggest that the 03–077 experience supports a change in mind-set: no longer should relapse of HR-NB be considered an invariably lethal event, but rather a curative strategy should be applied. Achieving a minimal disease state (CR/VGPR) after relapse remains a challenge but may be facilitated through close monitoring of disease status: asymptomatic, limited relapse may be more amenable to control than symptomatic, widespread, and bulky relapsed disease.Citation44 Greater anti-NB activity is a possibility with new generations of antibody-based immunotherapyCitation26,45,46 and other targeted therapies.Citation40
Patients and Methods
Patients
This report covers all 101 patients enrolled (2003–2010) on the Memorial Sloan Kettering Cancer Center protocol 03–077 [ClinicalTrials.gov NCT00072358] for the treatment of HR-NB in second or later CR/VGPR by the International NB Response Criteria (INRC).Citation47 Major organ toxicity had to be grade ≤2 by Common Terminology Criteria for Adverse Events Version 2.0 (CTCAEv2.0), although neutrophil count ≥500/µl and platelet count ≥10,000/µl were acceptable. Informed written consents for this trial were obtained following institutional review board rules.
Study design
This prospective Phase II trial used 3F8 (prepared as describedCitation18), GM-CSF (Leukine, Immunex), and isotretinoin with the aim of preventing another relapse. When this trial was devised in 2003, the long-term PFS rate after relapse was <5%,Citation9,10 therefore the null hypothesis was that the 2-year PFS rate is 1% and the alternative hypothesis was that it is 15%. Simon's two-stage optimal design was used with a power of 90% and α of 5%. For a total of 33 subjects, 18 were to be accrued during stage 1 and 15 during stage 2. The trial would stop early if none of the first 18 patients survived 2 y without relapse. Otherwise, the trial would continue until 33 patients were accrued. Treatment would be considered promising if ≥ 2 patients experienced 2-year relapse-free survival. When this target aim was successfully achieved, the study was amended to continue enrolling patients, with the rationale that (1) the protocol treatment promised to benefit this ultra high-risk group for whom no other Phase II clinical trials were available; (2) adding patients would generate a large experience appropriate for a robust multivariate analysis of prognostic factors; and (3) results would be more compelling with a large cohort. Patient accrual continued until a successor study was opened.
Protocol treatment
Immunotherapy cycles comprised GM-CSF alone for 5 days, followed by 3F8 plus GM-CSF, with 3F8 at 100 mg/m2/cycle. GM-CSF was administered subcutaneously and 3F8 was infused intravenously over 30 minutes, as described.Citation21,23 These cycles were separated by 2- to 4-week intervals through cycle 4; subsequent cycles were separated by 6- to 8-week intervals through 24 months from study entry. Immunotherapy was deferred only if the patients formed elevated HAMA titers (measured as described previouslyCitation27). Isotretinoin at 160 mg/m2/day × 14 days for 6 coursesCitation2,23 was administered between cycles of 3F8/GM-CSF, beginning after cycle 2 (or after cycle 1 if HAMA-positivity delayed initiation of cycle 2). Toxicity was graded by CTCAEv2.0.
After achievement of the target aim involving the initial cohort of 33 patients treated solely with 3F8/GM-CSF plus isotretinoin (see above), the trial was amended. To avoid treatment delays, patients who became HAMA positive before cycle 3 of 3F8 could receive a cycle of rituximab (375 mg/m2 on days 1 and 14) with or without cyclophosphamide (750 mg/m2 on day 15) to ablate HAMA.Citation21 Pending a return to HAMA negativity, these patients could also receive chemotherapy that entailed relatively modest hematologic and non-hematologic toxicity, either temozolomide alone (75 mg/m2/day × 42 days)Citation48 or irinotecan (50 mg/m2/day)/temozolomide (150 mg/m2/day) × 5 days.Citation32
Extent-of-disease evaluations
Disease status was assessed every 3 months for ≥24 months by histology of BM aspirates and biopsies obtained from bilateral posterior and bilateral anterior iliac crests, 123I-MIBG scan, and computed tomography or magnetic resonance imaging of chest/abdomen/pelvis and head.Citation44 Disease status was defined by INRC,Citation47 modified to incorporate 123I-MIBG findings as follows: CR: no evidence of NB, including normal 123I-MIBG scan; VGPR: volume of primary mass reduced >90%, normal 123I-MIBG scan, BM(−) by histology, normal catecholamine levels; PD: new lesion or >25% increase in an existing lesion.
Detection of MRD
Quantitative reverse transcription-polymerase chain reaction (sensitivity: one NB cell/10Citation6 normal cells) was used to assess MRD, as described previously.Citation23 The MRD marker panel included cyclin D1 (CCND1), ISL LIM homeobox 1 (ISL1), paired-like homeobox 2b (PHOX2B), and GD2 synthase (B4GALNT1). MRD was measured in BM aspirates pooled from bilateral posterior and anterior iliac crests (i.e., 4 sites with 2–2.5 mL/site) before treatment (pre-MRD) and after 2 cycles of 3F8 (post-MRD).
Statistical analysis
PFS and overall survival (OS) from the first dose of 3F8 to PD, death, or last day of follow up were calculated by the Kaplan-Meier method. Four patients who died from unrelated causes were censored for progression, but were still considered events. Log-rank test was used to determine the univariate association between prognostic variables and PFS or OS. Multivariate Cox regression model was fitted with variables that had a univariate P value < 0.05. Three variables—HAMA, rituximab, and temozolomide chemotherapy—were analyzed as time-dependent covariates. Stepwise regression was used to select a final multivariate model. All analysis was performed using R version 3.0.2 (http://cran.us.r-project.org/) together with the survival package.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Supported in part by grants from National Institutes of Health (CA106450, CA118845, P30 CA008748), Bethesda, MD; Robert Steel Foundation, New York, NY; and Katie's Find-A-Cure Fund, New York, NY.
References
- Cole KA, Maris JM. New strategies in refractory and recurrent neuroblastoma: translational opportunities to impact patient outcome. Clin Cancer Res 2012; 18:2423-8; PMID:22427348; http://dx.doi.org/10.1158/1078-0432.CCR-11-1409
- Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsey NK, Swift P, Shimada H, Black CT, Brodeur GM, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Eng J Med 1999; 341:1165-73; PMID:10519894; http://dx.doi.org/10.1056/NEJM199910143411601
- De Bernardi B, Nicolas B, Boni L, Indolfi P, Carli M, Cordero Di Montezemolo L, Donfrancesco A, Pession A, Provenzi M, di Cataldo A, et al. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol 2003; 21:1592-1601; PMID:12697885; http://dx.doi.org/10.1200/JCO.2003.05.191
- Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomised controlled trial. Lancet Oncol 2005; 6:649-58; PMID:16129365; http://dx.doi.org/10.1016/S1470-2045(05)70291-6
- Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol 2008; 9:247-56; PMID:18308250; http://dx.doi.org/10.1016/S1470-2045(08)70069-X
- Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, Haas-Kogan DA, Laquaglia MP, Yu AL, Diller L, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol 2013; 14:999-1008; PMID:23890779; http://dx.doi.org/10.1016/S1470-2045(13)70309-7
- London WB, Castleberry RP, Matthay KK, Look AT, Seeger RC, Shimada H, Thorner P, Brodeur G, Maris JM, Reynolds CP, Cohn SL. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol 2005; 23:6459-65; PMID:16116153; http://dx.doi.org/10.1200/JCO.2005.05.571
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Eng J Med 2010; 363:1324-34; PMID:20879881; http://dx.doi.org/10.1056/NEJMoa0911123
- Cotterill SJ, Pearson AD, Pritchard J, Foot AB, Roald B, Kohler JA, Imeson J. Clinical prognostic factors in 1277 patients with neuroblastoma: results of The European Neuroblastoma Study Group ‘Survey’ 1982–1992. Eur J Cancer 2000; 36:901-8; PMID:10785596; http://dx.doi.org/10.1016/S0959-8049(00)00058-7
- Castel V, Canete A, Melero C, Acha T, Navajas A, Garvia-Miguel P, Contra T, Molina J, Galaron P, Cruz O. Results of the cooperative protocol (N-III-95) for metastatic relapses and refractory neuroblastoma. Med Pediatr Oncol 2000; 35:724-6; PMID:11107156; http://dx.doi.org/10.1002/1096-911X(20001201)35:6%3c724::AID-MPO53%3e3.0.CO;2-U
- Lau L, Tai D, Weitzman S, Grant R, Baruchel S, Malkin D. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol/Oncol 2004; 26:227-32; PMID:15087949; http://dx.doi.org/10.1097/00043426-200404000-00003
- Santana VM, Furman WL, McGregor LM, Billups CA. Disease control intervals in high-risk neuroblastoma. Cancer 2008; 112:2796-801; PMID:18429000; http://dx.doi.org/10.1002/cncr.23507
- Garaventa A, Parodi S, De Bernardi B, Dau D, Manzitti C, Conte M, Casale F, Viscardi E, Bianchi M, D'Angelo P, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian Neuroblastoma Registry. Eur J Cancer 2009; 45:2835-42; PMID:19616426; http://dx.doi.org/10.1016/j.ejca.2009.06.010
- London WB, Castel V, Monclair T, Ambros PF, Pearson AD, Cohn SL, Berthold F, Nakagawara A, Ladenstein RL, Lehara T, Matthay KK. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J Clin Oncol 2011; 29:3286-92; PMID:21768459; http://dx.doi.org/10.1200/JCO.2010.34.3392
- Simon T, Berthold F, Borkhardt A, Kremens B, De Carolis B, Hero B. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: Results of German trials. Pediatr Blood Cancer 2011; 56:578-83; PMID:21298742; http://dx.doi.org/10.1002/pbc.22693
- Morgenstern DA, Baruchel S, Irwin MS. Current and future strategies for relapsed neuroblastoma: Challenges on the road to precision therapy. J Pediatr Hematol Oncol 2013;35:337-347; PMID:23703550; http://dx.doi.org/10.1097/MPH.0b013e318299d637
- Basta N, Makin G, Feltbower RG, Birch JM, Brown N, Elliott M, Ingham D, Moreno L, Baronne G, Pearson A, et al. Factors associated with recurrences and length of survival following relapse in UK patients with high risk neuroblastoma. Advances in Neuroblastoma Research Information Book, 2014; page 134
- Yeh SDJ, Larson SM, Burch L, Kushner BH, LaQuaglia M, Finn R, Cheung N-KV. Radioimmunodetection of neuroblastoma with iodine-131-3F8: Correlation with biopsy, iodine-131-metaiodobenzylguanidine and standard diagnostic modalities. J Nucl Med 1991; 32:769-76; PMID:1902508
- Cheung N-KV, Kushner BH, Yeh SD, Larson SM. 3F8 monoclonal antibody treatment of patients with stage 4 neuroblastoma: A phase II study. Int J Oncol 1998; 12:1299-1306; PMID:9592190
- Kushner BH, Kramer K, Cheung N-KV. Phase II trial of the anti-GD2 monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol 2001; 19:4189-94; PMID:11709561
- Cheung N-KV, Cheung IY, Kramer K, Modak S, Ostrovnaya I, Kuk D, Pandit-Taskar N, Chamberlain E, Kushner BH. Key role for myeloid cells: Phase II results of anti-GD2 antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int J Cancer 2014; 135:2199-205; PMID:24644014; http://dx.doi.org/10.1002/ijc.28851
- Cheung N-KV, Kushner BH, Cheung IY, Canete A, Gerald W, Liu C, Finn R, Yeh SJ, Larson SM. Anti-GD2 antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol 1998; 16:3053-60; PMID:9738575
- Cheung N-KV, Cheung IY, Kushner BH, Ostrovnaya I, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol 2012; 30:3264-270; PMID:22869886; http://dx.doi.org/10.1200/JCO.2011.41.3807
- Kushner BH, Kramer K, Modak S, Cheung N-KV. Camptothecin analogs (irinotecan or topotecan) plus high-dose cyclophosphamide as preparative regimens for antibody-based immunotherapy in resistant neuroblastoma. Clin Cancer Res 2004; 10:84-7; PMID:14734455; http://dx.doi.org/10.1158/1078-0432.CCR-1147-3
- Yu A, Uttenreuther-Fischer MM, Huang C-S, Tsui CC, Gillies SD, Reisfeld RA, Kung FH. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14.18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol 1998; 16:2169-80; PMID:9626218
- Shusterman S, London WB, Gillies SD, Hank JA, Voss SD, Seeger RC, Reynolds CP, Kimball J, Albertini MR, Wagner B, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: A Children's Oncology Group (COG) phase II study. J Clin Oncol 2010; 28:4969-75; PMID:20921469; http://dx.doi.org/10.1200/JCO.2009.27.8861
- Cheung N-KV, Cheung IY, Canete A, Yeh SJ, Kushner BH, Bonilla MA, Heller G, Larson SM: Antibody response to murine anti-GD2 monoclonal antibodies: correlation with patient survival. Cancer Res 1994; 54:2228-33; PMID:8174131
- Cheung NK, Guo HF, Heller G, Cheung IY: Induction of Ab3 and Ab3′ antibody was associated with long-term survival after anti-GD2 antibody therapy of stage 4 neuroblastoma. Clin Cancer Res 2000; 6:2653-60; PMID:10914706
- Cheung IY, Hsu K, Cheung N-KV. Activation of peripheral-blood granulocytes is strongly correlated with patient outcome after immunotherapy with anti-GD2 monoclonal antibody and granulocyte-macrophage colony-stimulating factor. J Clin Oncol 2012; 30:426-32; PMID:22203761; http://dx.doi.org/10.1200/JCO.2011.37.6236
- George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, Pulsipher M, Grupp SA, Diller L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: Long-term survival update. J Clin Oncol 2006; 24:2891-6; PMID:16782928; http://dx.doi.org/10.1200/JCO.2006.05.6986
- Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, Shaw PJ, Cohn SL, Matthay KK. Pilot induction regimen incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high risk neuroblastoma: A Children's Oncology Group study. J Clin Oncol 2011; 29:4351-7; PMID:22010014; http://dx.doi.org/10.1200/JCO.2010.34.3293
- Kushner BH, Kramer K, Modak S, Cheung N-KV. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol 2006; 24:5271-6; PMID:17114661; http://dx.doi.org/10.1200/JCO.2006.06.7272
- Kushner BH, Kramer K, Modak S, Cheung N-KV. High-dose carboplatin-irinotecan-temozolomide: Treatment option for neuroblastoma resistant to topotecan. Pediatr Blood Cancer 2011; 56:403-8; PMID:21049517; http://dx.doi.org/10.1002/pbc.22855
- Saylors, RL, Stine KC, Sullivan J, Kepner JL, Wall DA, Bernstein ML, Harris MB, Hayashi R, Vietti TJ; Pediatric Oncology Group: Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: A Pediatric Oncology Group phase II study. J Clin Oncol 2001; 19:3463-9; PMID:11481351
- Garavanta A, Luksch R, Biasotti S, Severi G, Pizzitola MR, Viscardi E, Prete A, Mastrangelo S, Podda M, Haupt R et al, : A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer 2003; 98:2488-94; PMID:14635085; http://dx.doi.org/10.1002/cncr.11797
- Kushner BH, Modak S, Kramer K, Basu EM, Roberts SS, Cheung N-KV. Ifosfamide, carboplatin, and etoposide for neuroblastoma: A high-dose salvage regimen and review of the literature. Cancer 2013; 119:665-71; PMID:22951749; http://dx.doi.org/10.1002/cncr.27783
- Villablanca JG, Krailo MD, Ames MM, Reid JM, Reaman GH, Reynolds CP. Phase I trial of oral fenretinide in children with high-risk solid tumors: A report from the Children's Oncology Group (CCG 09709). J Clin Oncol 2006; 24:3423-30; PMID:16849757; http://dx.doi.org/10.1200/JCO.2005.03.9271
- Mosse YP, Lim MS, Voss SD, Wilner K, Ruffner K, Laliberte J, Rolland D, Balis FM, Maris JM, Weigel BJ, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children's Oncology Group phase 1 consortium study. Lancet Oncol 2013; 14:472-80; PMID:23598171; http://dx.doi.org/10.1016/S1470-2045(13)70095-0
- Kramer K, Kushner BH, Modak S, Pandit-Taskar N, Smith-Jones P, Zanzonico P, Humm JL, Xu H, Wolden SL, Souweidane MM, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neuro-oncol 2010; 97:409-18; http://dx.doi.org/10.1007/s11060-009-0038-7
- Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res 2012; 18:2740-53; PMID:22589483; http://dx.doi.org/10.1158/1078-0432.CCR-11-1939
- Matthay KK, Brisse H, Couanet D, Couturier J, Benard J, Mosseri V, Edeline V, Lumbroso J, Valteau-Couanet D, Michon J. Central nervous system metastases in neuroblastoma: Radiologic, clinical, and biological features in 23 patients. Cancer 2003; 98:156-65; http://dx.doi.org/10.1002/cncr.11448
- Cheung N-KV, Dyer MA. Neuroblastoma: Developmental biology, cancer genomics and immunotherapy. Nature Rev Cancer 2013; 13:397-411; http://dx.doi.org/10.1038/nrc3526
- Kushner BH, Cheung IY, Modak S, Kramer K, Ragupathi G, Cheung N-KV. Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res 2014; 20:1375-82; PMID:24520094; http://dx.doi.org/10.1158/1078-0432.CCR-13-1012
- Kushner BH, Kramer K, Modak S, Cheung N-KV. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol 2009; 27:1041-6; PMID:19171710; http://dx.doi.org/10.1200/JCO.2008.17.6107
- Navid F, Sondel PM, Barfield R, Shulkin BI, Kaufman RA, Allay JA, Gan J, Hutson P, Seo S, Kim KM, et al. Phase I trial of a novel anti-GD2 monoclonal antibody, hu14.18K322A, designed to decrease toxicity in children with refractory or recurrent neuroblastoma. J Clin Oncol 2014; 32:1445-52; PMID:24711551; http://dx.doi.org/10.1200/JCO.2013.50.4423
- Ahmed M, Cheung N-KV. Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett 2014; 588:288-97; PMID:24295643; http://dx.doi.org/10.1016/j.febslet.2013.11.030
- Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993; 11:1466-77; PMID:8336186
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Eng J Med 2005; 352:987-96; PMID:15758009; http://dx.doi.org/10.1056/NEJMoa043330
