Abstract
Autologous dendritic cell (DC) therapy is an experimental cellular immunotherapy that is safe and immunogenic in patients with advanced melanoma. In an attempt to further improve the therapeutic responses, we treated 15 patients with melanoma, with autologous monocyte-derived immature DC electroporated with mRNA encoding CD40 ligand (CD40L), CD70 and a constitutively active TLR4 (caTLR4) together with mRNA encoding a tumor-associated antigen (TAA; respectively gp100 or tyrosinase). In addition, DC were pulsed with keyhole limpet hemocyanin (KLH) that served as a control antigen. Production of this DC vaccine with high cellular viability, high expression of co-stimulatory molecules and MHC class I and II and production of IL-12p70, was feasible in all patients. A vaccination cycle consisting of three vaccinations with up to 15×106 DC per vaccination at a biweekly interval, was repeated after 6 and 12 months in the absence of disease progression. mRNA-optimized DC were injected intranodally, because of low CCR7 expression on the DC, and induced de novo immune responses against control antigen. T cell responses against tyrosinase were detected in the skin-test infiltrating lymphocytes (SKIL) of two patients. One mixed tumor response and two durable tumor stabilizations were observed among 8 patients with evaluable disease at baseline. In conclusion, autologous mRNA-optimized DC can be safely administered intranodally to patients with metastatic melanoma but showed limited immunological responses against tyrosinase and gp100.
Abbreviations:
- caTLR4, constitutively active TLR4
- CD40L, CD40 ligand
- DC, dendritic cell(s)
- DTH, delayed-type hypersensitivity
- GMP, good manufacturing practice
- HS, human serum
- IL, interleukin
- KLH, keyhole limpet hemocyanin
- PBMC, peripheral blood mononuclear cells
- SKIL, skin-test infiltrating lymphocytes
- TAA, tumor-associated antigens
Introduction
DC are the most potent professional antigen-presenting cells of the immune system. At present, DC-based immunotherapy is explored at different centers in therapeutic vaccination trials in cancer patients aiming to induce and augment the anticancer immune response.Citation1,2 Immature DC are considered to be primarily involved in the recognition and uptake of antigens. When stimulated by appropriate “danger signals” they mature and migrate from peripheral tissues to lymphoid organs. Maturation is characterized by the upregulation of cell surface molecules involved in antigen presentation and co-stimulation (e.g., CD80, CD86, CD40, and MHC class II) as well as the release of pro-inflammatory cytokines (e.g., interleukin (IL)-12p70). Within the lymph node, matured DC are able to activate naive T cells which recognize the antigenic epitopes presented on the surface of the DC.Citation3-5 Immunotherapy with ex vivo-generated autologous DC pulsed with tumor peptides has provided proof of concept in clinical trials.Citation6 We and others have demonstrated that tumor-specific immune responses can be induced in regional and distant metastatic melanoma patients and clinical responses have been reported in a small percentage of patients.Citation7-10
One important aspect in DC-based immunotherapy is the ex vivo maturation of DC.Citation11 Studies that have compared the immunogenicity of immature vs. mature DC show that maturation is essential for the induction of immunological responses in cancer patients.Citation11,12 Moreover, the use of mature DC appears to be associated with a better clinical outcome as compared to immature DC.Citation11,13 IL-12p70, a pro-inflammatory cytokine, plays an essential role in the type of immune response that is induced as IL-12p70 promotes Th1 responses necessary for obtaining an effective cytotoxic T cell response. The cytokine cocktails commonly used for obtaining DC maturation however fail to induce such production of IL-12p70 by the DC in vitro.Citation14 The IL-12p70 production and the T cell-stimulatory capacity of DC can be greatly enhanced by providing them with three different molecular adjuvants through electroporation with mRNA encoding CD40L, CD70, and a constitutively active form of TLR4 (caTLR4).Citation15,16 The combination of CD40L and TLR4 mimics CD40 ligationCitation17 and TLR4 signaling of the DCCitation18 and generates phenotypically mature, cytokine-secreting DC. The introduction of CD70 into the DC provides a co-stimulatory signal to naive T cells by inhibiting activated T cell apoptosis and by supporting T cell proliferation.Citation19 In early clinical trials, mRNA-optimized DC were shown to prime CD4+ and CD8+ T cells in melanoma patients.Citation20,21
Another crucial aspect in DC-based immunotherapy is the efficacy of antigen-loading of DC. To date, one of the most widely used techniques to load human DC with TAA for the induction of an antitumor immune response is the incubation of DC with HLA class I-binding peptides. Tumor antigen-derived peptides have the advantage that many peptides are commercially available. On the downside, antigenic peptides are restricted to a given HLA type, restricting the number of patients that can be treated. Furthermore, MHC-peptide complexes have a relative short half-life due to low affinity and MHC turnover,Citation22,23 and peptide loading does not account for posttranscriptional modifications of peptide epitopes.Citation24,25 In addition, the exploitation of MHC class I-restricted peptides targeting cytotoxic CD8+ T cells only, do not activate CD4+ T helper cells which are able to enhance and sustain antitumor responses by cytotoxic T cells.Citation26
For all of these reasons various methods have been designed to enhance tumor antigen presentation by DC, including electroporation with synthetic mRNA encoding full length TAA, or fusion proteins of TAA and an HLA-class II processing signal. Proteolytic processing of the endogenously produced antigen within the DC will result in loading of multiple suitable peptides onto the patient's own MHC molecules expressed by the DC. mRNA-loading obviates the need of specified epitopes and there is no need for restriction of patients based on HLA type.Citation27 Another benefit of this technique is the presentation of multiple epitopes in both MHC class I for the induction of CD8+ T cells and in MHC class II for interaction with CD4+ T helper cells. Although DC loading with tumor cell lysate has overlapping advantages with TAA mRNA electroporation, it not only leads to presentation of all relevant TAA but also of self-antigens, which may lead to immune suppression or auto-immunity. Furthermore, the method is dependent on the availability of tumor tissue. Previously, it has been shown that electroporation of DC with mRNA is effective and safe.Citation23,28,29 DC retain their phenotype and maturation potential upon electroporation, as well as their migratory capacities.Citation28,30 The loading of tumor antigen by mRNA encoding TAA can be simultaneously performed with mRNA electroporation with CD40L, CD70 and caTLR4 to improve the DC maturation and T cell stimulation.Citation16
Previously, so-called TriMix DC vaccination was found to be feasible, safe and immunogenic after intradermal, intravenous or combined intradermal/intravenous administration in metastatic melanoma patients in single center studies.Citation10,21 In addition, intradermally/intravenously injected TriMix DC co-electroporated with MAGE.A3, MAGE.C2, tyrosinase, and gp100 resulted in durable objective clinical tumor responses in 4 out of 15 patients treated in a phase IB clinical trial.Citation21 In this phase I/II study we investigated the feasibility, safety, and immunological responses in metastatic melanoma patients to intranodal vaccination with monocyte-derived mRNA-optimized DC loaded with tyrosinase and gp100 mRNA. Intranodal vaccination was chosen because of low CCR7 expression on mRNA-optimized DC. CCR7 is a chemokine receptor, with CCL19 and CCL21 as its ligands, known to facilitate directed migration of DC from the peripheral tissue to the T cell rich areas in draining lymph nodes.Citation31 A potential advantage of injecting DC directly into a lymph node is that the cells are immediately located inside the network of target lymphoid organs, minimizing the requirements for migration in order to reach the anatomical location where stimulation of T cells occurs and resulting in a more efficient distribution of the vaccine over multiple lymph nodes.
Results
Patient characteristics
A total of 15 melanoma patients, 5 patients with regional metastases (AJCC stage III disease) and 10 patients with distant metastases (AJCC stage IV disease), were included in this study. Ten patients had primary melanoma of the skin, three patients presented with a primary uveal melanoma and one patient had an unknown primary. One patient with distant metastatic disease was non-evaluable for safety and immunogenicity, since he did not receive any vaccinations due to rapid progressive disease (A-07). One additional patient was only evaluable for safety and immunologic response. He had not been documented with progressive disease on prior treatment with dacarbazine at revision of the pre-study CT scan, but completed the first cycle of vaccinations (DE-04). Fourteen patients completed the first cycle of three DC vaccine administrations. Five patients (3 out of 5 stage III patients and 2 out of 9 distant metastatic patients) completed the full three cycles. Patient characteristics are summarized in .
Table 1. Patient characteristics
Vaccine characteristics
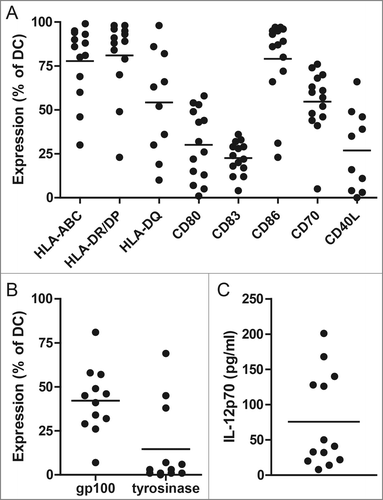
Phenotypic and functional release criteria were predefined for the mature DC used in our clinical vaccination protocol to ensure the DC vaccines comply with the minimal quality criteria.Citation2 The phenotype of the ex vivo-generated DC was determined by flow cytometry prior to injection. Viability of the DC after electroporation was >50% in all DC productions, and >70% in 19 out 21 productions (90%). Electroporation of immature DC with mRNA encoding CD40L, CD70 and caTLR4 induced expression of the co-stimulatory molecules CD80 and CD86 (). The phenotype of cytokine-matured DC (used for DTH testing only) showed a different expression pattern than the mRNA-optimized DC of the same patients. For example, cytokine-matured DC show a higher median CCR7 expression compared to mRNA-optimized DC (median 35% and 1%, respectively; Fig. S1A). In general, MHC class I and class II were highly expressed on mRNA-optimized DC. Intracellular expression of tumor associated antigens gp100 and tyrosinase 4 h after electroporation was highly variable, with in general low expression of tyrosinase (), and decreased within 24 h after electroporation (Fig. S1B). IL-12p70 production by activated DC is desired as it stimulates IFNγ production in naive T cells, thereby promoting Th1 responses. The median IL-12p70 concentration in culture medium after overnight incubation of DC was 41 pg/mL (range; 8–201 pg/mL) (). IL-12p70 production is rarely seen in cytokine-matured DC (median 0 pg/mL: range 0–17 pg/mL; unpublished data). These data demonstrate the feasibility of the production of mRNA-optimized DC and are in line with the previously published results on TriMix DC production.Citation15,16
Figure 1. mRNA-optimized dendritic cell (DC) vaccine characteristics. Expression of HLA-ABC, HLA-DR/DP, HLA-DQ, CD80, CD86, CD70 and CD40L was measured by flow cytometry on DC of all patients (A). Tumor antigen expression of gp100 and tyrosinase by DC 4 h after electroporation with mRNA encoding gp100 and tyrosinase (B). Data are shown as percentage of positive DC of the first DC vaccine of each patient. The production of IL-12p70 by DC was measured in the supernatants 16 h after induction of maturation (C). Each dot represents the first DC vaccine of a patient.
DC-related adverse events
Based on the experience with our cytokine-matured DC and the studies performed at the Vrije Universiteit Brussel exploiting TriMix DC, we expected that the DC vaccines would be well tolerated.Citation10,21,32 Mild flu-like symptoms and local reaction at the injection site, not exceeding CTCAE grade 1 severity, were each seen in four patients (29%; ). Flu-like symptoms were usually of short duration, around 2 d, and did not require any intervention. The injection site reactions consisted of minimal swelling of the lymph node region and redness of the overlying skin.
Table 2. Immunological and clinical responses
Proliferation of PBMC upon stimulation with KLH
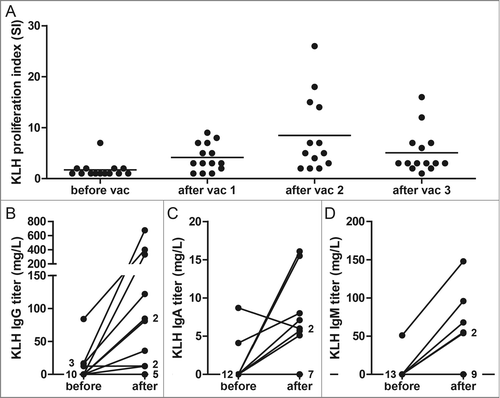
To test the capacity of the patients in this study to generate an immune response upon DC vaccination, we loaded the DC with the control antigen KLH. All patients showed increased T cell proliferation upon stimulation with KLH in peripheral blood mononuclear cells (PBMC) isolated after vaccination, even patients who received a limited amount of DC (). Anti-KLH IgG antibodies were detected in 5 out of 5 stage III patients and 3 out of 9 distant metastatic patients after one cycle of vaccinations. Anti-KLH IgA and IgM antibodies were detected in 7 and 5 patients, respectively (). These data demonstrate that the vaccine induced de novo immune responses.
Figure 2. KLH-specific immune responses before and after dendritic cell (DC) vaccination. KLH-specific T cell proliferation was analyzed after each DC vaccination during the first vaccination cycle in peripheral blood mononuclear cells of melanoma patients. The proliferative response to KLH is given as a proliferation index (proliferation with KLH/proliferation without KLH) (A). KLH-specific IgG (B), IgA (C) and IgM antibodies (D) were quantitatively measured before and after each vaccination cycle in sera of vaccinated patients. The best Ig response was shown for each patient. Each dot represents 1 patient or the number of patients as indicated.
Tumor-associated antigen-specific T cell responses in blood and skin
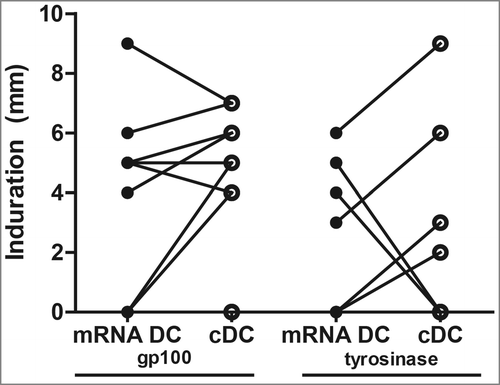
Delayed-type hypersensitivity (DTH) skin tests were performed after each series of vaccinations to monitor TAA-specific T cell responses. Cytokine-matured DC or mRNA-optimized DC electroporated with gp100-encoding or tyrosinase-encoding mRNA were injected intradermally in the skin of the back of the patients at four different sites. Previously we showed that the presence of TAA-specific T cells in SKIL cultures positively correlates with survival in distant metastatic melanoma patients.Citation8 The maximum diameter of induration was measured after 48 h and was on average 3 mm (range 0–20 mm), with no differences in induration between stimulation with mRNA-optimized DC or cytokine-matured DC after the first cycle of vaccination (p = 0.24; ). To investigate if TAA-specific immune responses were induced by vaccination, PBMC and SKIL, cultured from punch biopsies of the DTH sites, of HLA-A*02:01-positive patients were screened with tetrameric-MHC complexes. Additionally, in both HLA-A*02:01-postive and -negative patients we determined the presence of TAA-specific immune responses in SKIL using autologous EBV-transformed B (EBV-B) cells, as described previously.Citation33 Tetramer positive CD8+ T cells in SKIL were detected in 1 out of 8 HLA-A*02:01-positive patients, recognizing tyrosinase (DE-05; Fig. S2A). No tetramer positive CD8+ T cells were detected in PBMC after vaccination. We did not find any positive responses in the six HLA-A*02:01-negative patients with the EBV-assay. In one of the HLA-A*02:01-positive patients CD137 was evidently upregulated, but without concomitant upregulation of CD69 or CD107a (A-05; Fig. S2B). Whereas, the combination of CD137 upregulation together with IFNγ or TNF-α secretion, without CD69 or CD107a, can be considered as a functional tumor-specific immune response.Citation33
Figure 3. Induration at the delayed type hypersensitivity (DTH) skin tests after cycle 1. DTH skin tests were performed after each series of vaccinations. Cytokine-matured dendritic cells (cDC) or mRNA-optimized DC (mRNA DC) electroporated with gp100-encoding mRNA or tyrosinase-encoding were injected intradermally in the skin of the back of the patients at four different sites. The maximum diameter of induration was measured after 48 h. Induration after the first vaccination cycle is shown. Dots representing the same patient are connected with a line.
Clinical outcome
Two stage III patients had progressive disease within 3 months after start of vaccinations and died shortly thereafter. In both patients the absence of distant metastasis had been confirmed by a PET scan before radical lymph node dissection. Another patient developed two in transit metastases after the second cycle of vaccinations which were resected and vaccinations were continued; eventually, the patient progressed 16 months later. The other two stage III patients still have no evidence of disease with follow-up of 52 and 56 months.
One patient with distant metastatic disease (A-03) showed a mixed response with progression of pulmonary, renal and subcutaneous metastases, while metastases in liver and lymph nodes decreased in size. The patient developed symptomatic brain metastases 6 months after inclusion. Two patients with advanced melanoma (DE-02 and A-04) had stable disease for approximately 2 years, one of those patients (A-04) lived over 4 years after the start of DC vaccination for metastatic melanoma. The other 6 patients with distant metastases had progressive disease at the first clinical evaluation.
Discussion
In this study we show the feasibility and safety of the intranodal administration of autologous mRNA-optimized DC presenting the tyrosinase and gp100 melanoma associated antigens. Viable DC could be produced in all patients and only grade 1 local injection site reactions and flu-like symptoms occurred after intranodal vaccination. Previously, it was reported that intradermal and intravenous administration of TriMix DC showed similar side effects with slightly higher severity (up to CTCAE grade 2); in addition, intravenous administration produced CTCAE grade 2 acute post-infusion chills.
In this study, intranodal administration of mRNA-optimized DC showed good feasibility and safety but limited TAA-specific immunological responses were observed. In previous TriMix DC vaccination trials, were the vaccine was administered intradermal or intradermal/intravenous, TAA-specific T cells were found in about 50% of patients.Citation10,21 In our trial, TAA-specific T cell responses were detected in only two patients (14%). One response was detected by tetramer analysis (DE-05) and one by the EBV-based antigen presentation assay (A-05). We did not find any positive responses in the six HLA-A*02:01-negative patients. Various factors may be underlying the absence of TAA-specific T cell responses in HLA-A*02:01-negative patients. Previously we have shown that the prevalence of a positive SKIL test following DC vaccination in patients with distant metastatic melanoma is approximately 30%Citation8 and about 70% of the stage III melanoma patients.Citation34 Given the small number of HLA-A*02:01-negative patients and most patients had distant metastatic disease; the odds of detecting a positive SKIL test in this study were anticipated to be low beforehand. However, one HLA-A*02:01-negative distant metastatic patient showed an interestingly long progression-free survival (A-04) of 28 months. As immunological responses are less frequently detected in more advanced disease stages, less frequent immunological responses could be expected in our trial compared to the previous TriMix DC vaccination trial, as we had relatively more patients with distant metastatic melanoma and less stage III melanoma patients. Secondly, in contrast to previous studies where TriMix DC were injected intradermally or intradermally/intravenously, mRNA-optimized DC injected intranodally could be less potent in the activation of the immune system compared to cytokine-matured monocyte-derived DC. In this study anti-KLH IgG antibodies were detected only in 3 out of 9 distant metastatic patients after one cycle of vaccinations. Previously, we could detect anti-KLH IgG antibodies in about 60% of patients receiving mature DC, while we could detect no KLH IgG antibodies in any of the patients receiving immature DC.Citation11,32,35 Furthermore, although KLH-specific T cell responses were induced in the majority of patients in this trial, proliferation indices were generally lower after mRNA-optimized DC vaccination (on average 9; range 2–26) vs. on average 32 (range 2–116) in cytokine-matured DC-vaccinated metastatic melanoma patients in previous trials.Citation32,35
The seemingly lower potency of DC in this study, compared to cytokine-matured DC and intradermal/intravenous administered TriMix DC, could be explained by lower peptide expression on the DC, usage of different tumor antigens (e.g. MAGE), the intranodal route of administration or the minor differences in DC manufacturing protocol (e.g., different culture medium, time of DC administration after electroporation) which might have resulted in lower IL-12p70 secretion. Intranodal vaccination was chosen because of low CCR7 expression on mRNA-optimized DC. Previously, intradermal vaccination of cytokine-matured DC showed superior TAA-specific T cell induction compared to intranodal vaccination.Citation36 This might in part be explained by the partial destruction of the lymph node architecture by injection of DC directly into a lymph node. Additionally, naturally, DC mature on their way toward the lymph node and arrive in a mature state in the lymph node. After intradermal injection, all DC that enter the lymph nodes are viable and have migrated. They may represent the most mature and hence most potent DC. In contrast, as a result of intranodal injection, all DC, including less mature or nonviable DC, are directly delivered into the lymph node and might even activate nonspecific or nonfunctional T cells or regulatory T cells. This may be even more unfavorable for mRNA-optimized DC than for cytokine-matured DC, since mRNA-optimized DC were injected into the lymph node in a semi-immature state and expected to mature further after injection. Upregulation of co-stimulatory molecules was seen after electroporation of immature DC with mRNA encoding CD40L, CD70 and caTLR4, although in a lesser extent compared to cytokine-matured DC, but with higher IL-12p70 production.Citation26,32 On the contrary, the difference in the degree of upregulation of co-stimulatory molecules might be explained by the time of measurement. mRNA-optimized DC are administered to the patient within a few hours after electroporation, while maturation is still ongoing and thus further upregulation will take place in vivo. In vivo maturation of DC, and the subsequent release of immunostimulatory cytokines in vivo, resembles the natural process of DC activation more closely and thus might lead to enhanced T cell activation rather than decreased T cell activation.Citation37 Another factor underlying the absence of TAA-specific T cell responses may be low tyrosinase expression and antigen presentation by the mRNA-optimized DC. This could be caused by the concomitant electroporation with four different types of mRNA, resulting in competition between the types of mRNA to enter the DC and to be translated into protein. However, protein expression might not be an appropriate measure, as the assay measures the endogenously produced full protein within the DC, and not the peptide presented in the MHC molecule on the cell surface. Fast processing of the protein might have led to the low expression levels. Indeed, despite low expression levels, specific T cells against the tyrosinase peptide were detected in both immunological responding patients.
One of the major advantages of the usage of DC loaded with mRNA encoding the whole TAA, compared to peptide loaded DC, is that it obviates HLA restriction and immune responses will not be restricted to a specific HLA-type. The endogenous processing of TAA will result in the presentation of multiple peptides in both MHC class I and II molecules and could lead to the induction of a considerable larger repertoire of TAA-specific T cells. As a consequence, the monitoring of TAA-specific immunological responses will become far more complicated. Of the broad array of possible epitopes, only a few will be recognized by the tetramers available and used in this study. Therefore, the induction of CD8+ T cell responses after mRNA-optimized DC vaccination were analyzed by a recently developed alternative approach.Citation33 The assay combines the use of autologous EBV-immortalized B cells as antigen presenting cells and CD137 upregulation to detect antigen-specific T cells in blood or SKIL. Previously, the combination of CD137 upregulation together with IFNγ or TNF-α secretion was considered as a functional tumor-specific immune response.Citation33 Unfortunately, the cytokine secretion was not measured in the patient with CD137 positive cells (A-05) and did not show a functional profile in the patient with tetramer positive TAA-specific T cells (DE-05).
Finally, high background activation of SKIL by EBV-transfected B cells may have masked TAA-specific T cell activation in our EBV assays. Most people are exposed to EBV early in life and have consequently developed EBV-specific T cells. Since only relative small numbers of TAA-specific T cells are usually induced by DC vaccination, these might be lost in the background scatter caused by EBV-specific T cells. This could explain why we could detect TAA-specific T cells by this method in only one patient, and not in the patient with tetramer positive TAA-specific T cells. To bypass this problem, peripheral blood lymphocytes electroporated with TAA mRNA could be used instead or immortalized autologous B cells generated by retroviral introduction of the oncogene BCL−6 and the anti-apoptotic gene BCL−XL.Citation38
DC-based immunotherapy has proven to be feasible, safe and able to induce or augment tumor-specific immune responses. Nevertheless, only a limited number of clinical responses have been observed. Besides the electroporation of DC with CD40L, CD70, and caTLR4 mRNA to improve immunological and clinical responses of DC vaccination strategies, a number of other variables, e.g., the use of naturally occurring DC subsets, are being tested in clinical trials.Citation39,40 Furthermore, combination treatments to counteract tumor escape mechanisms are being explored. Recently, the treatment of TriMix DC combined with ipilimumab, a monoclonal anti-CTLA4 antibody, showed an encouraging rate of durable tumor responses.Citation41 Further studies should determine the optimal treatment modality that may enhance the efficacy of DC-based immunotherapy.
In conclusion, this study demonstrates that therapeutic vaccination with an autologous mRNA-optimized DC vaccine can be safely administered intranodally to patients with metastatic melanoma. However, few TAA-specific immunological and clinical responses were detected suggesting that for mRNA-optimized DC the intranodal route of administration probably is not preferred over technically less demanding intradermal or intravenous administration.
Patients and Methods
Patient population
In this phase I/II study, melanoma patients with locoregional resectable disease (referred to as stage III) within 2 months after radical dissection of regional lymph node metastases and patients with irresectable locoregional or distant metastatic disease were included. Additional inclusion criteria were melanoma expressing the melanoma-associated antigens gp100 (compulsory) and tyrosinase (non-compulsory), and WHO performance status 0 or 1. Patients with brain metastases, serious concomitant disease or a history of a second malignancy were not eligible. The study was approved by the appropriate Medical Ethical Review Board, and written informed consent was obtained from all patients. Clinical trial registration number is NCT01530698.
Study protocol
Patients received a DC vaccine via intranodal injection. Intranodal vaccination was conducted in a clinically tumor-free lymph node under ultrasound guidance. The mRNA-optimized DC vaccine consisted of autologous immature monocyte-derived DC electroporated with mRNA encoding CD40L, CD70 and caTLR4 combined with mRNA encoding gp100 or tyrosinase protein, and pulsed with KLH protein for an immune control. Patients received a cycle consisting of three vaccinations at a biweekly interval. Prior to each vaccination 80 mL of blood was collected for immunological monitoring. One to two weeks after the last vaccination a skin test was performed. All patients who remained free of recurrence (stage III) or disease progression (distant metastases) after the first vaccination cycle received a maximum of two maintenance cycles at 6-month intervals, each consisting of three biweekly intranodal vaccinations followed by a skin test. All vaccinations were administered between May 2010 and February 2012.
Study endpoints
The main study endpoints were the toxicity of mRNA-optimized DC and immunological responses upon DC vaccination. Clinical efficacy was a secondary endpoint, defined as disease-free survival in stage III melanoma patients and as progression-free survival in stage IV melanoma patients. All distant metastatic patients were evaluated for clinical response at 3-month intervals with CT scan or at extra time points when progressive disease was clinically suspected. A clinical response was defined as stable disease for more than 4 months or any partial or complete response, according to the Response Evaluation Criteria in Solid Tumors (RESIST version 1.1). Toxicity was assessed according to NCI common toxicity criteria version 3.0.
DC preparation and characterization
Monocyte-derived DC were generated from PBMC prepared from leukapheresis products as described previously.Citation11 Briefly, plastic-adherent monocytes or monocytes isolated by counterflow elutriation using Elutra-cell separator (Gambro BCT, Inc.) were cultured for 5–7 d in X-VIVO 15™ medium (Lonza) supplemented with 2% pooled human serum (HS; Sanquin) in the presence of IL-4 (500 units/mL) and granulocyte macrophage colony-stimulating factor (800 units/mL; both from Cellgenix). DC were pulsed with KLH (10 µg/mL; Immucothel, Biosyn Arzneimittel GmbH) and matured via electroporation with mRNA encoding CD40L, CD70, and caTLR4.
For the DTH skin test, DC were matured with a cytokine cocktail consisting of recombinant tumor necrosis factor α (10 ng/mL), IL-1β (5 ng/mL), IL-6 (15 ng/mL) (all CellGenix), and prostaglandin E2 (10 µg/mL, Pharmacia & Upjohn) for 48 h (cytokine-matured DC).Citation7 Harvested DC were analyzed by FACS analysis as described below.
Plasmids and in vitro mRNA transcription
Plasmid pGEM4Z-5′ÚT-hgp100-3′UT-A64, pGEM4Z-5′ÚT-CD40L-3′UT-A64, pGEM4Z-5′ÚT-CD70-3′UT-A64 and pGEM4Z-5′ÚT-caTLR4-3′UT-A64 were provided by Vrije Universiteit Brussel. PGEM4Z-5′ÚT-tyrosinase-3′UT-A64 was constructed by digestion of pGEM4Z-5′UT-tNGFR-3′UT-A64 with BglII and XbaI and digestion of pcDNA1.amp/tyrosinase with BamHI and XbaI and insertion of the tyrosinase into the cut pGEM4Z. Plasmids were linearized with SpeI (pGEM4Z-5′UT-CEA-3′UT-A64) or NotI enzyme (pGEM4Z-5′UT-tNGFR-3′UT-A64, pGEM4Z-5′UT-hgp100-3′UT-A64, and pGEM4Z-5′UT-tyrosinase-3′UT-A64), purified with phenol/chloroform extraction and ethanol precipitation, and used as DNA templates.Citation15 Good manufacturing practice (GMP) grade gp100 and tyrosinase mRNA was produced by CureVac GmbH as described previously.Citation32 Despite the absence of an MHC class II trafficking signal, we showed antigen-specific CD4+ T cell responses in our previous trial with mRNA-electroporated DC, proving the MHC class II expression of the used antigens, gp100 and tyrosinase.Citation32 CD40L-, CD70- and caTLR4-mRNA was manufactured according to GMP by University Hospital Erlangen. The generated mRNAs contain a 5′ cap and 3′ poly A-tail that leads to high RNA stability and increased protein expression in transfected cells. RNA quality was verified by agarose gel electrophoresis; RNA concentration was measured spectrophotometrically; and RNA was stored at −40°C in small aliquots.
mRNA electroporation of DC
DC were washed twice in PBS and once in OptiMEM without phenol red (Invitrogen). mRNA-optimized DC were electroporated in immature state. Twenty micrograms of mRNA encoding gp100 or tyrosinase and 6.6 µg of each mRNA encoding CD40L, CD70 and caTLR4 were transferred to a 4 mm cuvette (Bio-Rad) and 10–12 × 106 cells were added in 200 µL OptiMEM and incubated for 3′ before being pulsed in a Genepulser Xcell (Bio-Rad) by an exponential decay pulse of 300 V, 150 µF, as described before.Citation28 Immediately after electroporation, cells were transferred to warm (37°C) X-VIVO 15™ without phenol red supplemented with 5% HS and left for at least 2 h at 37°C, before further manipulation. Electroporation efficiency was analyzed after 4 h by flow cytometric analysis. After overnight culture, DC were harvested, the phenotype was analyzed by flow cytometry and viability was assessed by Trypan Blue (Invitrogen Life Technologies) exclusion. The first vaccination was given with fresh DC the day after electroporation. DC for subsequent vaccinations were frozen, thawed at the day of vaccination, and incubated for an additional hour at 37°C before injection. Protein expression of the tumor antigens was not affected by freezing and thawing of the DC.
Flow cytometric analysis
Flow cytometry was done using the following monoclonal antibodies (mAb) and appropriate isotype controls: anti-HLA class I (W6/32), anti-HLA DR/DP (Q5/13), anti-HLA DR, anti-CD70, anti-CD80 (all BD Biosciences), anti-CD14, anti-CD83 (both Beckman Coulter), anti-CD86 (BD PharMingen), and anti-CCR7 (R&D systems). For intracellular staining of the TAA NKI/beteb (IgG2b; purified antibody) against gp100, and T311 (IgG2a; Cell Marque Corp., Rocklin, CA) against tyrosinase were used. Cells were also stained intracellular with anti-CD40L (Beckman Coulter). For intracellular staining, cells were fixed for 4′ on ice in 4% (w/v) paraformaldehyde (Merck) in PBS, permeabilized in PBS/2%BSA/0.02% azide/0.5% saponin (Sigma-Aldrich) (PBA/saponin), and stained with mAb diluted in PBA/saponin/2%HS, followed by staining with Alexa488-labeled goat-anti-mouse (BD PharMingen). Flow cytometry was performed with FACSCalibur™ flow cytometer equipped with CellQuest software (BD Biosciences).
IL-12p70 production
Immediately after electroporation, 10–12 × 106 cells were transferred to 5 mL warm (37°C) X-VIVO 15™. The production of IL-12p70 was measured in the supernatants approximately 16 h after electroporation with mRNA using a standard sandwich ELISA (Pierce Biotechnology).
KLH-specific antibody production
KLH-specific antibodies were measured in the sera of patients before and after vaccination by ELISA (www.klhanalysis.com). Briefly, microtiter plates (96 wells) were coated overnight at 4°C with KLH (25 μg/mL in PBS per well). After washing the plates, patient serum was added in duplicate for 1 hour at room temperature. After extensive washing, patient KLH-specific antibodies were detected with mouse anti-human IgG, IgA or IgM antibodies labeled with horseradish peroxidase (Invitrogen). 3,3′ 5,5-tetramethyl-benzidine was used as a substrate and plates were measured in a microtiter plate reader at 450 nm. For quantification an isotype-specific calibration curve for the KLH response was included in each microtiter plate.Citation42
Proliferative response to KLH
Cellular responses against KLH were measured before and after vaccination by proliferation assay. PBMC were isolated from heparinized blood by Ficoll-Paque density centrifugation. PBMC were stimulated with KLH (4 μg/2×105 PBMC) in medium with 2% human AB serum. After 3 days, cells were pulsed with 1 μCi/well3H-thymidine for 8 h, and incorporation was measured with a betacounter. Experiments were performed in sextuplicate. A proliferation index >2 was considered positive.
Skin-test infiltrating lymphocytes cultures
One to two weeks after the last DC vaccination a DTH skin test was performed, as described beforeCitation7,8 and at www.labtube.tvplayvideo.aspx?vid=131825. Briefly, cytokine-matured DC electroporated with gp100-encoding mRNA or tyrosinase-encoding mRNA or mRNA-optimized DC electroporated with gp100-encoding mRNA or tyrosinase-encoding mRNA were injected intradermally in the skin of the back of the patients at four different sites (1×106 DC each). The maximum diameter (in millimeters) of induration was measured after 48 h and punch biopsies (6 mm) were obtained under local anesthesia. Half of the biopsy was cryopreserved for immunohistochemistry and the other half was manually cut and cultured in RPMI1640 containing 7% HS supplemented with IL-2 (100 U/mL; Proleukin®, Chiron). Every 7 d, half of the medium was replaced by fresh medium containing HS and IL-2. After 2 to 4 weeks of culturing, SKIL were tested for antigen recognition and tetramer binding.
Tetramer staining
SKIL and PBMC from HLA-A*02:01 positive patients were stained with tetrameric-MHC complexes containing the HLA-A*02:01 epitopes gp100:154–162, gp100:280–288 or tyrosinase:369–377 (Sanquin) combined with CD8 staining as described previously.Citation7 Tetrameric-MHC complexes recognizing HIV were used as a negative control.
Antigen and tumor recognition
Antigen recognition by SKIL was determined using autologous EBV-B cells as described previously.Citation33 Autologous EBV-B cells were generated from PBMC and either loaded with HLA-A2-binding gp100 (gp100:154–162 or gp100:280–288) or tyrosinase (tyrosinase:369–377) peptides (for HLA-A*02:01-positive patients only) or electroporated with mRNA encoding full-length gp100 or tyrosinase (CureVac GmbH). For mRNA electroporation, EBV-B cells were washed twice in PBS and once in OptiMEM without phenol red. Twenty micrograms of mRNA were transferred to a 4 mm cuvette (Bio-Rad) and 10–20 × 106 cells were added in 200 µL OptiMEM and incubated for 3′ before being pulsed in a Genepulser Xcell (Bio-Rad) by an exponential decay pulse of 450 V, 150 μF. Immediately after electroporation, cells were transferred to warm (37°C) X-VIVO 15 without phenol red supplemented with 6% HS and left for at least 2 h at 37°C, before further manipulation. EBV-B cells electroporated with CEA-mRNA were used as a negative control. mRNA- or peptide-loaded EBV-B cells were co-cultured 1:1 with SKIL for 24 h and after 24 h antigen specificity was analyzed by flow cytometry by expression of the early activation markers CD69 and CD137 on CD8+ T cells. PMA-stimulated (5 μg/mL; Sigma-Aldrich) SKIL were used as a positive control. Cytokine production was measured in supernatants after 24 h of co-culture with a FlowCytomix Multiplex kit (Bender MedSystems GmbH). Cytotoxicity was evaluated through addition of PeCy5-conjugated CD107a (BD PharMingen) and GolgiStop™ (1:2000; monensin, BD Biosciences PharMingen) during co-culture and subsequent analysis of surface binding of CD107a antibodies to CD8+ T cells by flow cytometry. A DTH test response was considered positive when the percentage of CD8+CD137+, CD8+CD69+ or CD8+CD107a+ cells was more than twice that obtained with the negative control.
For HLA-A*02:01-positive patients, antigen recognition was also determined by the production of cytokines of SKIL after co-culture with T2 cells pulsed with HLA-A*02:01-binding peptides gp100:154–162, gp100:280–288 or tyrosinase:369–377 or BLM (a melanoma cell line expressing HLA-A*02:01 but no endogenous expression of gp100 and tyrosinase), transfected with control antigen G250, with gp100 or tyrosinase, or an allogeneic HLA-A*02:01-positive, gp100-positive, and tyrosinase-positive tumor cell line (MEL624). Cytokine production was measured in supernatants after 16 h of co-culture with a FlowCytomix Multiplex kit (Bender MedSystems GmbH).
Statistical analysis
Data were analyzed statistically by means of analysis of variance and Student–Newman–Keuls test. Statistical significance was defined as p < 0.05. Progression-free and overall survival were calculated from the time from apheresis to recurrence (stage III) or progression (distant metastases) or death, respectively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
2015ONCOIMM0038R-f05-z-bw.tif
Download TIFF Image (346.2 KB)2015ONCOIMM0038R-f04-z-bw.tif
Download TIFF Image (225.9 KB)Funding
This work was supported by grants from the Netherlands Organization for Scientific Research (95100106), the Dutch Cancer Society (KUN2010-4722, KUN2009-4580 and KUN2009-4402) and the European Union Projects Cancer Immunotherapy (LSHC-CT-2006-518234) and DC-THERA (LSHB-CT-2004-512074). IJMdV received a NWO VICI award (91814655) and JFMJ received a NWO VENI award (016136101).
References
- Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007; 449:419-26; PMID:17898760; http://dx.doi.org/10.1038/nature06175
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475-80; PMID:15122249; http://dx.doi.org/10.1038/nm1039
- Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T et al. A dendritic-cell-derived C-C chemokine that preferentially attracts naive T cells. Nature 1997; 387:713-7; PMID:9192897; http://dx.doi.org/10.1038/42716
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/10.1038/32588
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999; 5:405-11; PMID:10202929; http://dx.doi.org/10.1038/7403
- Lesterhuis WJ, Aarntzen EH, De Vries IJ, Schuurhuis DH, Figdor CG, Adema GJ, Punt CJ. Dendritic cell vaccines in melanoma: from promise to proof? Crit Rev Oncol Hematol 2008; 66:118-34; PMID:18262431; http://dx.doi.org/10.1016/j.critrevonc.2007.12.007
- de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, Ruiter DJ, Figdor CG, Punt CJ, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol 2005; 23:5779-87; PMID:16110035; http://dx.doi.org/10.1200/JCO.2005.06.478
- Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, van Rossum MM, Adema GJ, Figdor CG, Punt CJ, de Vries IJ. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell based vaccination in metastatic melanoma. Cancer Res 2012:72:6102-10; PMID:23010076; http://dx.doi.org/10.1158/0008-5472.CAN-12-2479
- Schultz ES, Schuler-Thurner B, Stroobant V, Jenne L, Berger TG, Thielemanns K, van der Bruggen P, Schuler G. Functional analysis of tumor-specific Th cell responses detected in melanoma patients after dendritic cell-based immunotherapy. J Immunol 2004; 172:1304-10; PMID:14707109; http://dx.doi.org/10.4049/jimmunol.172.2.1304
- Wilgenhof S, Van Nuffel AM, Corthals J, Heirman C, Tuyaerts S, Benteyn D, De Coninck A, Van Riet I, Verfaillie G, Vandeloo J et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother 2011; 34:448-56; PMID:21577140; http://dx.doi.org/10.1097/CJI.0b013e31821dcb31
- de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 2003; 9:5091-100; PMID:14613986
- Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer 2001; 93:243-51; PMID:11410873; http://dx.doi.org/10.1002/ijc.1323
- McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother 2003; 52:583-91; PMID:12827310; http://dx.doi.org/10.1007/s00262-003-0414-7
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003; 3:133-46; PMID:12563297; http://dx.doi.org/10.1038/nri1001
- Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B, Thielemans K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther 2008; 16:1170-80; PMID:18431362; http://dx.doi.org/10.1038/mt.2008.77
- Bonehill A, Van Nuffel AM, Corthals J, Tuyaerts S, Heirman C, Francois V, Colau D, van der Bruggen P, Neyns B, Thielemans K. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res 2009; 15:3366-75; PMID:19417017; http://dx.doi.org/10.1158/1078-0432.CCR-08-2982
- Kikuchi T, Moore MA, Crystal RG. Dendritic cells modified to express CD40 ligand elicit therapeutic immunity against preexisting murine tumors. Blood 2000; 96:91-9; PMID:10891436
- Cisco RM, Abdel-Wahab Z, Dannull J, Nair S, Tyler DS, Gilboa E, Vieweg J, Daaka Y, Pruitt SK. Induction of human dendritic cell maturation using transfection with RNA encoding a dominant positive toll-like receptor 4. J Immunol 2004; 172:7162-8; PMID:15153540; http://dx.doi.org/10.4049/jimmunol.172.11.7162
- Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol 2005; 17:275-81; PMID:15886117
- Van Nuffel AM, Benteyn D, Wilgenhof S, Pierret L, Corthals J, Heirman C, van der Bruggen P, Coulie PG, Neyns B, Thielemans K et al. Dendritic cells loaded with mRNA encoding full-length tumor antigens prime CD4+ and CD8+ T cells in melanoma patients. Mol Ther 2012; 20:1063-74; PMID:22371843; http://dx.doi.org/10.1038/mt.2012.11
- Wilgenhof S, Van Nuffel AM, Benteyn D, Corthals J, Aerts C, Heirman C, Van Riet I, Bonehill A, Thielemans K, Neyns B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol 2013; 24:2686-93; PMID:23904461; http://dx.doi.org/10.1093/annonc/mdt245
- Laverman P, de Vries IJ, Scharenborg NM, de Boer A, Broekema M, Oyen WJ, Figdor CG, Adema GJ, Boerman OC. Development of 111In-labeled tumor-associated antigen peptides for monitoring dendritic-cell-based vaccination. Nucl Med Biol 2006; 33:453-8; PMID:16720236; http://dx.doi.org/10.1016/j.nucmedbio.2006.02.005
- Mitchell DA, Nair SK. RNA-transfected dendritic cells in cancer immunotherapy. J Clin Invest 2000; 106:1065-9; PMID:11067858; http://dx.doi.org/10.1172/JCI11405
- Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y, Shabanowitz J, Wolfel T, Slingluff CL Jr, Boon T et al. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med 1996; 183:527-34; PMID:8627164; http://dx.doi.org/10.1084/jem.183.2.527
- Zarling AL, Ficarro SB, White FM, Shabanowitz J, Hunt DF, Engelhard VH. Phosphorylated peptides are naturally processed and presented by major histocompatibility complex class I molecules in vivo. J Exp Med 2000; 192:1755-62; PMID:11120772; http://dx.doi.org/10.1084/jem.192.12.1755
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/10.1158/0008-5472.CAN-12-1127
- Van Nuffel AM, Wilgenhof S, Thielemans K, Bonehill A. Overcoming HLA restriction in clinical trials: Immune monitoring of mRNA-loaded DC therapy. Oncoimmunology 2012; 1:1392-s4; PMID:23243604; http://dx.doi.org/10.4161/onci.20926
- Schuurhuis DH, Verdijk P, Schreibelt G, Aarntzen EH, Scharenborg N, de Boer A, van de Rakt MW, Kerkhoff M, Gerritsen MJ, Eijckeler F et al. In situ expression of tumor antigens by messenger RNA-electroporated dendritic cells in lymph nodes of melanoma patients. Cancer Res 2009; 69:2927-34; PMID:19318559; http://dx.doi.org/10.1158/0008-5472.CAN-08-3920
- Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 2001; 98:49-56; PMID:11418462; http://dx.doi.org/10.1182/blood.V98.1.49
- Schuurhuis DH, Lesterhuis WJ, Kramer M, Looman MG, van Hout-Kuijer M, Schreibelt G, Boullart AC, Aarntzen EH, Benitez-Ribas D, Figdor CG et al. Polyinosinic polycytidylic acid prevents efficient antigen expression after mRNA electroporation of clinical grade dendritic cells. Cancer Immunol Immunother 2009; 58:1109-15; PMID:19018531; http://dx.doi.org/10.1007/s00262-008-0626-y
- Sanchez-Sanchez N, Riol-Blanco L, Rodriguez-Fernandez JL. The multiple personalities of the chemokine receptor CCR7 in dendritic cells. J Immunol 2006; 176:5153-9; PMID:16621978; http://dx.doi.org/10.4049/jimmunol.176.9.5153
- Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, van Rossum MM, Blokx WA, Jacobs JF, Duiveman-de Boer T et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res 2012; 18:5460-70; PMID:22896657; http://dx.doi.org/10.1158/1078-0432.CCR-11-3368
- Van Nuffel AM, Tuyaerts S, Benteyn D, Wilgenhof S, Corthals J, Heirman C, Neyns B, Thielemans K, Bonehill A. Epitope and HLA-type independent monitoring of antigen-specific T-cells after treatment with dendritic cells presenting full-length tumor antigens. J Immunol Methods 2012; 377:23-36; PMID:22269772; http://dx.doi.org/10.1016/j.jim.2011.12.010
- Bol K, Aarntzen E, Hout F, Schreibelt G, Creemers J, Lesterhuis W, Gerritsen W, Grunhagen D, Verhoef C, Punt C et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology 2015; in press.
- Lesterhuis WJ, Schreibelt G, Scharenborg NM, Brouwer HM, Gerritsen MJ, Croockewit S, Coulie PG, Torensma R, Adema GJ, Figdor CG et al. Wild-type and modified gp100 peptide-pulsed dendritic cell vaccination of advanced melanoma patients can lead to long-term clinical responses independent of the peptide used. Cancer Immunol Immunother 2011; 60:249-60; PMID:21069321; http://dx.doi.org/10.1007/s00262-010-0942-x
- Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, Scharenborg NM, van de Rakt MW, de Boer AJ, Croockewit S et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res 2011; 17:5725-35; PMID:21771874; http://dx.doi.org/10.1158/1078-0432.CCR-11-1261
- Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, Vieweg J, Gilboa E. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol 2003; 171:6275-82; PMID:14634145; http://dx.doi.org/10.4049/jimmunol.171.11.6275
- Linnemann C, van Buuren MM, Bies L, Verdegaal EM, Schotte R, Calis JJ, Behjati S, Velds A, Hilkmann H, Atmioui DE et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med 2015; 21:81-5; PMID:25531942; http://dx.doi.org/10.1038/nm.3773
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013; 73:1063-75; PMID:23345163; http://dx.doi.org/10.1158/0008-5472.CAN-12-2583
- Bol KF, Tel J, de Vries IJ, Figdor CG. Naturally circulating dendritic cells to vaccinate cancer patients. Oncoimmunology 2013; 2:e23431; PMID:23802086; http://dx.doi.org/10.4161/onci.23431
- Neyns B WS, Corthals J, Heirman C, Thielemans K. Phase II study of autologous mRNA electroporated dendritic cells (TriMixDC-MEL) in combination with ipilimumab in patients with pretreated advanced melanoma. ASCO Meeting Abstracts 32(15_suppl): 3014.
- Aarntzen EH, de Vries IJ, Goertz JH, Beldhuis-Valkis M, Brouwers HM, van de Rakt MW, van der Molen RG, Punt CJ, Adema GJ, Tacken PJ, et al. Humoral anti-KLH responses in cancer patients treated with dendritic cell-based immunotherapy are dictated by different vaccination parameters. Cancer Immunol Immunother 2012; 61:2003-11; PMID:22527252; http://dx.doi.org/10.1007/s00262-012-1263-z
