 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The multifaceted immunomodulatory activity of DNA hypomethylating agents improves immunogenicity and immune recognition of neoplastic cells; thus, we predicted they could be utilized to design new immunotherapeutic combinations in cancer. Testing this hypothesis, the antitumor efficacy of the DNA hypomethylating agent 5-aza-2′-deoxycytidine (5-AZA-CdR) combined with the anti-CTLA-4 monoclonal antibody (mAb) 9H10 in syngeneic transplantable murine models was investigated. Murine mammary carcinoma TS/A or mesothelioma AB1 cells were injected in BALB/c, athymic nude, and SCID/Beige mice that were treated with 5-AZA-CdR, mAb 9H10, or their combination. Tumor volumes were captured at different time-points; molecular and immunohistochemical assays investigated changes in neoplastic and normal tissues. A significant antitumor effect of 5-AZA-CdR combined with mAb 9H10 was found: compared to controls, a 77% (p < 0.01), 54% (p < 0.01) and 33% (p = 0.2) decrease in TS/A tumor growth was induced by 5-AZA-CdR combined with mAb 9H10, 5-AZA-CdR or mAb 9H10, respectively. These antitumor activities were confirmed utilizing the AB1 model. 5-AZA-CdR-based regimens induced a promoter-demethylation-sustained tumor expression of cancer testis antigens. MHC class I expression was up-regulated by 5-AZA-CdR. Antitumor efficacy of 5-AZA-CdR in athymic nude and SCID/Beige mice was not increased by mAb 9H10. In BALB/c mice, combined treatment induced the highest tumor infiltration by CD3+ lymphocytes, which included both CD8+ and CD4+ T cells; no such infiltrates were observed in normal tissues. This significant immune-related antitumor activity of 5-AZA-CdR combined with CTLA-4 blockade, demonstrated in highly aggressive mouse tumor models, provides a strong scientific rationale to implement epigenetically-based immunotherapies in cancer patients.
Introduction
Among the pleiotropic activities of epigenetic drugs,Citation1 we have extensively characterized the immunomodulatory properties of DNA hypomethylating agents in human malignances of different histotype.Citation2,3 Exposure of neoplastic cells to these agents effectively improved T cell recognition of melanoma and renal carcinoma cells in vitro.Citation3-5 This functional effect was found to be mediated, at least in part, by the upregulation of the expression of tumor antigens (e.g., Cancer Testis Antigens), HLA class I and accessory/co-stimulatory molecules by neoplastic cells.Citation3,4,6,7 To further explore the immunomodulatory potential of DNA hypomethylating agents, we also demonstrated that changes in genome-wide expression profiles induced by 5-AZA-CdR in BALB/c mice grafted with the murine mammary adenocarcinoma TS/A cells were preferentially observed in neoplastic tissues as compared to normal counterparts, and that they affected mainly immunologic pathways.Citation6 Supporting the immunomodulatory activity of DNA hypomethylating agents, we also showed that their second-generation agent designated SGI-110, induced the expression of different tumor-associated antigens (e.g., NY-ESO-1, MAGE-A1 and -A3) in PBMC from patients affected by myelodysplastic syndrome or acute myelogenous leukemia.Citation8 Overall these findings further demonstrate that DNA hypomethylating agents may be of potential clinical use also as immunomodulatory compounds capable of combining with a variety of new immunotherapeutic agents.
Among the latters, mAb targeting different immune-checkpoints are emerging as powerful therapeutic tools in cancer.Citation9-11 The anti-cytotoxic T lymphocyte associated antigen 4 (CTLA-4) mAb ipilimumab represents the prototype of this new category of molecules. Ipilimumab has received regulatory approval since it significantly prolonged the survival of metastatic melanoma patients.Citation12-14 In spite being ipilimumab monotherapy presently the mainstay for first-line immunotherapy in melanoma, only 20% of the patients experience long term survival.Citation12,13 Thus, ongoing clinical trials are exploring novel therapeutic combinations to improve its clinical efficacy.
Based on the comprehensive evidence above, we reasoned that DNA hypomethylating agents could represent potential pharmacologic partners to improve the therapeutic activity of CTLA-4 blocking mAb by taking advantage of the functional immunomodulatory activity of these compounds on neoplastic and immune cells, respectively. To provide experimental support to this hypothesis, in this study we investigated the therapeutic and immunologic aspects of 5-AZA-CdR in combination with CTLA-4 blockade utilizing two syngeneic murine transplantable cancer models. Our results show a significant antitumor activity of this combination that warrants being explored in the clinical setting.
Results
Effect of 5-AZA-CdR combined with mAb 9H10 on tumor growth
The antitumor activity of 5-AZA-CdR combined with the anti-CTLA-4 mAb 9H10, as compared to monotherapy, was investigated in BALB/c mice grafted with the poorly immunogenic TS/A breast carcinoma cells (#5 mice/group). Representative data from three independent experiments are shown in .
Figure 1. Antitumor activity of 5-AZA-CdR combined with mAb 9H10 in the syngeneic TS/A mouse tumor model. BALB/c mice were inoculated sc with 2 × 105 TS/A cells. Groups of five mice were injected ip with 5-AZA-CdR on days 0, 7 (1st cycle) and 42, 49 (2nd cycle); with mAb 9H10 on days 2, 5, 8 (1st cycle) and 44, 47, 50 (2nd cycle); with combined administration of 5-AZA-CdR and mAb 9H10 according to the above reported schedules, or with saline solution for control. Tumor volumes from mice were measured periodically, all along the treatment. Mean tumor volume for each group are reported. Vertical arrows indicate days of different treatments. *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001 vs. tumor volume of control group. †, p ≤ 0.05; ††, p ≤ 0.01 vs. tumor volume of 5-AZA-CdR group. Representative data are shown from three experiments.
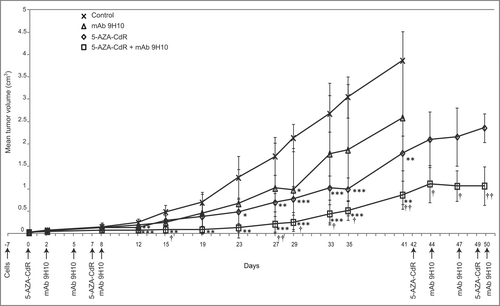
At day 12 from the beginning of treatment a 67% (p < 0.001), 44% (p < 0.01) and 24% (p = 0.41) reduction in tumor volumes was induced by 5-AZA-CdR combined with mAb 9H10, 5-AZA-CdR, and mAb 9H10, respectively, as compared to control mice (). The inhibition in tumor growth observed early in the course of treatment with both 5-AZA-CdR-based therapies persisted at day 41, being 77% (mean tumor volume = 0.86 ± 0.31 cm3) (p < 0.01) and 54% (mean tumor volume = 1.8 ± 0.38 cm3) (p < 0.01) for the combination and for 5-AZA-CdR alone, respectively (). On the other hand, the reduction (33%) in tumor volumes, obtained at day 41, from mice treated with mAb 9H10 alone (mean tumor volume = 2.59 ± 1.93 cm3) as compared to control mice (mean tumor volume = 3.87 ± 0.74 cm3) remained not significant; furthermore, these two sets of mice had to be euthanized as the tumor volumes exceeded the maximum allowed standards (). Control hamster IgG administered alone or combined with 5-AZA-CdR did not affect tumor growth over the whole treatment course (data not shown).
To evaluate the cumulative antitumor activity of repeated administrations of combination therapy, surviving mice received a 2nd cycle of 5-AZA-CdR combined with mAb 9H10 or 5-AZA-CdR alone at day 42 (). At day 50 the tumor volume was significantly (p < 0.01) lower in mice receiving the combination (5 out of 5) (mean tumor volume = 1.07 ± 0.43 cm3) as compared to 5-AZA-CdR alone (4 out of 5) (mean tumor volume = 2.36 ± 0.32 cm3) (); this difference persisted until day 57 when animals from the 5-AZA-CdR monotherapy-treated group (3 out of 5) had to be euthanized due to tumor volume (data not shown).
The strong antitumor activity of this combination regimen was further validated in a pilot study using mice grafted with syngeneic AB1 mesothelioma cells (#3 mice/group). In detail, a 81% (p < 0.05) and 33% (p = 0.10) reduction in tumor volumes was observed at day 20 of treatment with 5-AZA-CdR in combination with mAb 9H10 and 5-AZA-CdR alone, respectively, as compared to control mice. No reduction in tumor volumes was observed in mice treated with mAb 9H10 alone, as compared to control mice (Fig. S1).
Immunomodulatory activity of 5-AZA-CdR combined with mAb 9H10 on TS/A tumors
The immunomodulatory activity of 5-AZA-CdR combined with mAb 9H10 was investigated in TS/A tumors excised one week after the end of treatment from three randomly selected treated and control mice; changes in the expression of different murine Cancer Testis Antigen (i.e., tumor rejection antigen P1A (P1A) and Melanoma Antigen A (Mage-a) family members) and of MHC class I antigens were utilized as readouts.
RT-PCR unveiled a de novo expression of P1A and Mage-a members in neoplastic tissues from animals treated with 5-AZA-CdR alone or combined with mAb 9H10; in contrast, no effect was observed following treatment with the anti-CTLA-4 mAb alone ().
Figure 2. Regulation of Cancer Testis Antigen expression by 5-AZA-CdR combined with mAb 9H10 in the syngeneic TS/A mouse tumor model. (A) Total RNA was extracted from tumors excised from TS/A grafted mice treated with: saline solution, as control group (CTRL), mAb 9H10, 5-AZA-CdR, or the combination of 5-AZA-CdR with mAb 9H10. RT-PCR analysis was performed using P1A-, Mage-a- or β-actin-specific primer pairs. PCR products were then separated on a 2% agarose gel and visualized by ethidium bromide staining. Total RNA from mouse testis and splenocytes was utilized as positive (ctrl +) or negative (ctrl −) controls, respectively. Figure shows data from three representative mice out of five for control and treated groups. (B) genomic DNA was extracted from TS/A tumors excised from control mice (black) and mice treated with mAb 9H10 (white), 5-AZA-CdR (vertical line) or the combination of 5-AZA-CdR with mAb 9H10 (horizontal line). Real-time quantitative Methylation-Specific PCR analyses of P1A promoter were performed on bisulfite-modified genomic DNA, extracted from three out of five mice per group, using methylated- or unmethylated-specific primer pairs. Data are reported as percentage of average methylation that was defined as the ratio between methylated molecules and the sum of methylated and unmethylated molecules. Bars, SD; *, p ≤ 0.05 vs. control group.
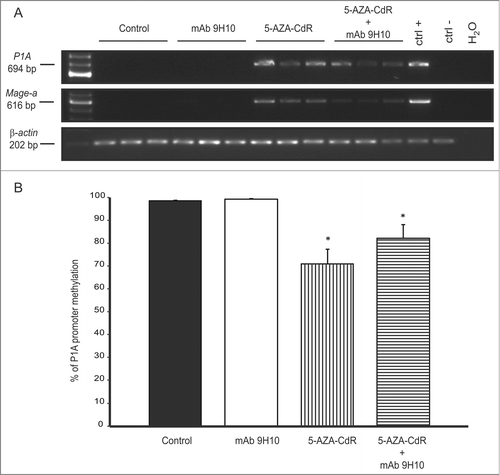
Consistent with the direct involvement of DNA methylation in the regulation of Cancer Testis Antigen expression, quantitative Methylation-Specific PCR analysis identified a significant (p < 0.05) reduction of P1A promoter methylation in tumor tissues from mice treated with 5-AZA-CdR alone or combined with mAb 9H10, as compared to control mice (). No reduction in the methylation of P1A promoter was observed in tumors from mice treated with the mAb 9H10 alone ().
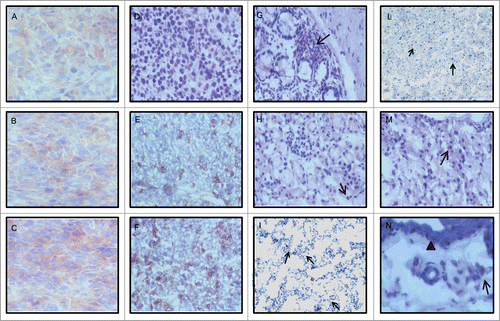
Representative results of the immunohistochemical analysis for the expression of MHC class I antigens reported in demonstrate a weak and heterogeneous expression, with intermingled negative and weakly positive areas, of MHC class I molecules in control (), mAb 9H10 (), and hamster IgG (data not shown) treated mice; in contrast, a stronger and more homogeneous expression of MHC class I antigens was detected in tumors from mice treated with 5-AZA-CdR alone () or combined with hamster IgG (data not shown). The extensive necrosis of tumors from mice treated with 5-AZA-CdR combined with mAb 9H10 allowed a conclusive interpretation on the changes of MHC class I antigens expression only in two animals (data not shown).
Figure 3. Modulation of MHC class I antigen expression and T cell infiltration by 5-AZA-CdR combined with mAb 9H10 in the syngeneic TS/A mouse tumor model. BALB/c mice were inoculated sc with 2 × 105 TS/A cells. Groups of 5 mice were injected ip with 5-AZA-CdR; with mAb 9H10; with combined administration of 5-AZA-CdR and mAb 9H10 according to the above reported schedules, or with saline solution for control. A week after the end of treatment, neoplastic and normal tissues were excised and snap frozen in liquid nitrogen. Four micron acetone-fixed cryostat sections were processed for IHC assays. Representative results from tumors (A–F) and normal tissues (G–N) are reported. (A– C): MHC class I staining of tumors from mice treated with saline solution, with mAb 9H10 or 5-AZA-CdR, respectively; (D– F): CD3 staining of tumors from mice treated with saline solution, with mAb 9H10 or the combination of 5-AZA-CdR and with mAb 9H10, respectively; (G–I and L–N): CD3 staining of glandular epithelium of large intestine, liver, lung, myocardium renal parenchyma and dermis from mice treated with mAb 9H10 or 5-AZA-CdR, respectively. (A–F), 200× magnification, (G–M), 160× magnification; (N), 250× magnification. The arrowed marks the dermal-epidermal junction; black arrows, CD3+ lymphocytes.
Antitumor activity of 5-AZA-CdR combined with mAb 9H10 in immunodeficient mice
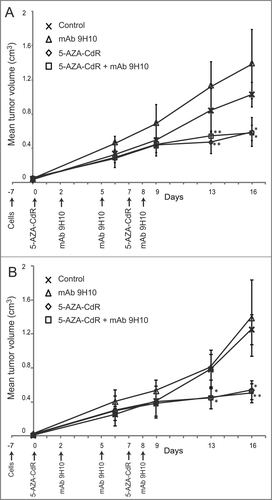
To investigate the contribution of host's immune response in mediating the antitumor effect observed in BALB/c mice, the therapeutic combination of 5-AZA-CdR and mAb 9H10 was also explored in T cell-deficient athymic nude mice and in T-cell-, B-cell- and NK cell-deficient SCID/Beige mice grafted with TS/A cells. Groups of 4 mice for each strain, were injected ip with 5-AZA-CdR, mAb 9H10, the combined administration of 5-AZA-CdR and mAb 9H10, or with saline solution for control. Treatment with mAb 9H10 did not affect tumor growth in either immunodeficient models investigated (). 5-AZA-CdR reduced tumor growth in both animal models (); noteworthy, no further reduction in tumor growth was detected when mAb 9H10 was added to 5-AZA-CdR monotherapy in both athymic nude mice () and SCID/Beige immunocompromised mice (). Treatment of immunocompetent BALB/c mice, utilized as internal controls, led to results similar to those previously obtained (data not shown).
Figure 4. Antitumor activity of 5-AZA-CdR combined with mAb 9H10 in immunocompromised mice. Athymic nude mice (A) and SCID/Beige (B) mice were inoculated sc with 2 × 105 TS/A cells. Groups of 4 mice, for each strains, were injected ip with 5-AZA-CdR on days 0 and 7; with mAb 9H10 on days 2, 5, 8; with combined administration of 5-AZA-CdR and mAb 9H10 according to the above reported schedules, or with saline solution for control. Tumor volumes from mice were measured periodically, all along the treatment. Mean tumor volumes for each group are reported. Vertical arrows indicate days of different treatments. *, p ≤ 0.05; **, p ≤ 0.01 vs. tumor volume of control group.
Analysis of immune cell infiltrates in neoplastic and normal tissues
To characterize the relative contribution of the T cell compartment in mediating the antitumor activity of 5-AZA-CdR combined with mAb 9H10, TS/A tumor tissues, randomly selected from three out of five treated and control mice, were evaluated for T cells infiltration.
Tumors from control animals displayed no necrosis and no infiltration by CD3+ lymphocytes (). In contrast, treatment with 5-AZA-CdR or with mAb 9H10 resulted in tumors with an average of 30% of necrosis and a CD3+ infiltrate of 15.2 (+/− 0.5) (data not shown), or with variable areas of necrosis and CD3+ lymphocytes infiltrate of 27 (+/− 1.7) () with a balanced presence of CD4+ and CD8+ cells, respectively. Treatment with 5-AZA-CdR combined with mAb 9H10 generated extensive areas of necrosis, loss of tissue architecture and the highest number of tumor infiltrating CD3+ lymphocytes (more than 40 CD3+ cells) (). Also in this instance a balanced presence of CD4+ and CD8+ cells was observed (Fig. S2). Number of T-cell infiltrates are summarized in .
Table 1. Count of T cell infiltrates in neoplastic tissues from control and treated mice grafted with TS/A cells
In contrast to tumor tissues, staining for CD3+ lymphocytes showed only isolated T cells in normal colon, ileum, skin, liver, kidney, brain, hearth, muscle and lung of two randomly selected mice with no differences between control and treated mice in terms of number and localization ( and data not shown).
Discussion
The notion that epigenetically-driven events can downregulate the immunogenicity and immune recognition of neoplastic cells, and that DNA hypomethylating agents can efficiently revert this phenomenon,Citation2 led us to hypothesize that combining such compounds with emerging immunotherapeutic agents, such as immune check-point blocking mAb, could result in potentially more effective anticancer strategies. This working hypothesis is now supported by the experimental data of the present study that demonstrated an immune-mediated, antitumor activity of 5-AZA-CdR combined with the anti-CTLA-4 mAb 9H10.
The antitumor efficacy of DNA hypomethylating agents combined with CTLA-4 blockade that we observed is highly intriguing and likely relevant from the translational standpoint also in view of the well-known limited activity of anti-CTLA-4 mAb utilized as single agents in poorly immunogenic mouse models,Citation15-18 which was confirmed also with the TS/A model utilized in this study. From a prospective clinical development these findings seem to imply that DNA hypomethylating agents in combination with CTLA-4 blockade could be efficiently utilized to improve the effectiveness of anti-CTLA-4 mAb also in poorly immunogenic human malignancies. Furthermore, the findings of the present study demonstrate that the efficacy of this novel combination becomes appreciable at early treatment time-points. Translating this finding into the clinics of anti-CTLA-4 therapy could be of particular relevance to counteract the late-in-onset antitumor activity of CTLA-4 blocking mAb in cancer patients,Citation19,20 though, this hypothesis remains to be fully validated in the clinical setting.
We have recently demonstrated that epigenetic remodeling of TS/A tumors by 5-AZA-CdR preferentially modulated gene expression profiles belonging to immune-related pathways,Citation6 suggesting for a broad spectrum of immune genes and mechanisms that could contribute to improve the immunogenicity and immune-recognition of DNA hypomethylating agents -treated cancer cells. Confirming this activity of 5-AZA-CdR, in this study the expression of the methylation-regulated P1A gene was upregulated exclusively in 5-AZA-CdR-containing regimens. Additional support to the broad functional immunomodulation of neoplastic cells by DNA hypomethylating agents derives from the immunohistochemical finding that the expression of MHC class I molecules was up-regulated in 5-AZA-CdR-treated tumors. This observation is particularly appealing in view of the demonstration that the upregulation of HLA class I antigens induced by 5-AZA-CdR was per se sufficient to improve gp100-specific cytotoxic T cell recognition of melanoma cells.Citation5 In addition, loss of expression of HLA class I molecules by tumor cells has been recently suggested to represent a mechanism of tumor resistance that can develop during CTLA-4 therapy with ipilimumab.Citation21 Therefore, the upregulation of HLA class I molecules induced by DNA hypomethylating agents in vivo could contribute to: (i) improve immune-recognition of neoplastic cells; (ii) recover the efficacy of CTLA-4 blockade in patients progressing to treatment due to down-regulation of HLA class I molecules on tumor cells. Even though these evidence do not allow to restrict the antitumor activity of the combined regimen to the upregulated MHC class I and tumor antigen expression on neoplastic cells, the involvement of immune effector mechanism(s) in the antitumor activity of the combination regimen observed in immunocompetent mice is strongly underlined by the overlapping patterns of tumor growth observed in immune-compromised mice treated with 5-AZA-CdR alone or combined with mAb 9H10. The potential contribution of T cell immunity in the therapeutic effectiveness of the combination regimen investigated in this study is further supported by the highest degree of CD3+ infiltrating cells identified in TS/A tumors from mice treated with 5-AZA-CdR and mAb 9H10. Consistent with previous observations demonstrating that tumor-specific immune responses induced by immune checkpoint blockade depend on both CD4+ and CD8+ T cells,Citation22,23 the lymphocyte tumor infiltration comprised both CD4+ and CD8+ T cells.
Opposite to tumor tissues, only isolated T cells were detected in different organs from treated and control mice. This finding might bear a significant practical relevance for the clinical use of the combined regimen. Potentially fatal immune-related adverse effects (irAEs), associated with heavy lymphocytic infiltration in normal organs, have been extensively documented in patients treated with CTLA-4-blocking mAb.Citation24,25 Therefore, although the weaknesses of the preclinical model at detecting irAEs for the resistance of the mice strain used in developing irAEs,Citation26 it can be envisaged that these auto-reactive phenomena will be likely not be worsened by the combination therapy. Along this very same line are our previous data reporting comprehensively limited effect(s) of DNA hypomethylating agents on gene expression profiles of normal tissues in mice.Citation6
Of note, the antitumor activity achieved with DNA hypomethylating agents combined with CTLA-4-blockade utilizing as pilot confirmatory study the AB1 mesothelioma was obtained in spite of its high in vivo aggressiveness, that required control and mAb 9H10-treated mice to be euthanized at day 21. The efficacy of the combined treatment also in this model demonstrates that its antitumor activity is not limited to the TS/A model but it rather represents a general phenomenon occurring regardless of tumor histotype.
Overall, the findings of this study provide a sound scientific rationale to translate the immunomodulatory activities of epigenetic drugs into the clinic, for novel and potentially more effective combinatorial immunotherapeutic strategies with anti-CTLA-4 blocking mAb. Both 5-AZA-CdR and ipilimumab have been extensively utilized in cancer patients and are available in the daily practice to be rapidly utilized to test such novel combination approach. Along this line, the phase I and II Italian Network for Tumor Biotherapy NIBIT-M4 study will first test this combination in metastatic cutaneous melanoma patients.
Methods
Cells and animals
The murine mammary carcinoma TS/A cell line, established from a spontaneously originating malignancyCitation27 and displaying no significant transplant immunogenicity in syngeneic host, was grown in DMEM Medium (Biochrom AG, Cat # FG 0445) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Lonza, Cat # DE14–802), 2 mM L-glutamine (Biochrom AG, Cat # K 0282) and 50 μM β-mercaptoethanol. The commercially available murine malignant mesothelioma AB1 cellCitation28 line was purchased from CellBank Australia (Cat # CBA-0144) and grown in RPMI Medium (Biochrom AG, Cat # FG 1215) supplemented with 5% heat-inactivated FBS, and 2 mM L-glutamine.
Six-week-old female BALB/c, athymic nude and SCID/Beige mice were purchased from Harlan Laboratories. Animal care and experiments were in accordance with Institutional guidelines and the indications of Workman et al.Citation29
Monoclonal antibodies and demethylating agents for in vivo treatment
The hamster mAb 9H10 against murine CTLA-4 was purchased from BioXCell (cat. # BE0131). The isotype control ChromePure Syrian hamster IgG were purchased from Jackson Immuno Research (cat. # 007-000-003). 5-AZA-CdR was purchased from Sigma Chemical Co. (cat. # A3656).
Mice treatment
Mice were injected subcutaneously (sc) in the flank region with TS/A (2 × 105) (BALB/c, athymic nude and SCID/Beige) or AB1 (3 × 106) (BALB/c) cells resuspended in 0.1 mL of physiologic saline. Animals were examined daily and after a latency period of 1 week for TS/A and of 10 d for AB1, mice bearing clearly palpable and visible tumor grafts (diameter ≥0.2 cm) were randomly grouped and treated intraperitoneally (ip) with 0.2 mL/injection of: (i) 15 mg/kg of 5-AZA-CdR (fractionated in three injections a day, every 3 h) on days 0 and 7 (1st cycle of treatment) and on days 42 and 49 (2nd cycle of treatment); (ii) 100μg of hamster mAb 9H10, on days 2, 5 and 8 (1st cycle of treatment) and on days 44, 47 and 50 (2nd cycle of treatment); (iii) combined administration of 5-AZA-CdR and mAb 9H10 according to the above reported schedules; (iv) 100 μg of isotype control hamster IgG on days 2, 5 and 8; or (v) combined administration of 5-AZA-CdR and hamster IgG according to the above reported schedules. Control mice were injected ip with 0.2 mL of saline solution. The rational choice of the dose/schedule of 5-AZA-CdR utilized for these experiments derived from preliminary experiments we had performed in immunocompetent and immunocompromised mice (data not shown). The 15 mg/kg/d regimen had the best tumor immunomodulation with no/limited mice toxicity and was therefore chosen for the in vivo experiments.
Animals were monitored weekly for changes in tumor size and sacrificed by CO2 overdose. Tumor and normal tissues were surgically removed, and each specimen, divided under sterile conditions, was snap-frozen in liquid nitrogen and stored at −80°C until used for RNA and DNA extraction or IHC assays.
In vivo antitumor activity and tolerability
Tumor size, evaluated by caliper measurements, and body weight were recorded periodically all along the treatment. Tumor volume was calculated as follows: tumor volume = LD2/2 (in which L and D are the longest and the shortest diameters, respectively). % of tumor growth inhibition was calculated using the formula: .
In vivo tolerability was evaluated by measurements of body weight and mortality rate, as well as by periodic veterinary control. For the duration of treatment, veterinary inspection showed a good tolerability of the experimental therapies which was not associated with relevant changes in body weight (data not shown).
RNA and DNA extraction
RNA and DNA were extracted from tissues sections, removed from TS/A tumor of control and treated mice and homogenized with the aid of Tissue Lyser II (QIAGEN) in Trizol reagent (Invitrogen, cat # 15596–026) or lysis buffer, respectively. Total RNA was extracted following the manufacturer's instructions and stored at −80°C. Total genomic DNA was extracted by digestion with 100 μg/mL proteinase K in the presence of 0.5% SDS at 50°C overnight, followed by phenol/chloroform extraction and ethanol precipitation. Genomic DNA was dissolved in TE buffer and stored at −20°C.
RT-PCR analysis
RT-PCR reactions were performed using oligonucleotide primer sequences and PCR amplification programs specific for P1A and Mage-a.Citation30,31 The integrity of RNA and random primers-synthesized cDNA was confirmed by the amplification of all cDNA samples with mouse β-actin-specific primers, as previously described.Citation6 Five μL of each RT-PCR sample were run on a 2% agarose gel, stained with ethidium bromide and visualized by Gel doc XR (BioRad Laboratories).
Quantitative Methylation-Specific PCR analyses
Bisulfite conversion was carried out on 500 ng genomic DNA using EZ DNA Methylation-Gold™ Kit (Zymo Research, cat # D5005), according to the manufacturer's protocol. Primers for the analysis of the methylation status of P1A, designed using the free on-line software MethPrimer,Citation32 were: P1A (Methylated), forward 5′-TTAAGTGCGTTATTACGTTTGGTTTTTAC-3′, reverse 5′-ATAACCGATTATTTAATACAAAAATCGACG-3′; P1A (Unmethylated), forward 5′-GATTAAGTGTGTTATTATGTTTGGTTTTTAT-3′, reverse 5′- ACATAACCAATTATTTAATACAAAAATCAACA-3′. SYBR green quantitative Methylation-Specific PCR reactions were performed on 2 μL of bisulfite-modified genomic DNA in a final volume of 25 μL 1X Power SYBR green mastermix (Applied Biosystems, cat # 4367659) at 95°C for 10 min, followed by 45 cycles of 15 s at 95°C and 1 min at 60°C, using methylated- or unmethylated-specific primer pairs. The copy number of methylated or unmethylated sequences for the target gene was established in each sample by extrapolation from the standard curves. The percentage of methylation was defined as the ratio between methylated molecules and the sum of methylated and unmethylated molecules.
Immunohistochemical studies
One week after the end of treatment, TS/A tumors from three animals randomly selected from each experimental and control group, and normal tissues (lung, skeletal muscle, kidney, colon, ileum, hearth, liver, skin, brain cortex) from two animals from the same groups, were collected, immediately snap frozen in liquid nitrogen and kept at −80°C before further processing. Four micron non-consecutive cryostat sections were collected upon single tissue slides using the Leica CM 1850 cryostat. After air drying, sections were fixed 10 min in absolute acetone and immediately assayed immunohistochemically or stored at −20°C with no loss of immune reactivity. The following antibodies were employed: polyclonal anti-human CD3+ cross reacting with murine T lymphocytes (Dako, cat # A045201), anti-mouse MHC class I mAb 28-14-8S anti-H2Db (ATCC HB-27)Citation33 and AF6–88.5.3 anti H2Kb (ATCC HB-158).Citation34 Rat anti-mouse CD4+ (Clone H129.19 cat # 550278) and CD8+a (Clone 53-6-7 cat # 563332) lymphocytes were purchased from BD Bioscience. Primary antibodies dilutions were established using normal mouse spleen sections. Samples incubated with isotype matched immunoglobulins were used as negative controls. Reactivity of murine antibodies was assessed using the Vector Labor immunoenzymatic MOM Kit employing AEC as chromogenic substrate. The reactivity of non-murine primary antibodies was established using an biotin labeled secondary antibodies and Vectastain immunoenzymatic kit and AEC as enzymatic substrate. Nuclear counterstain was done with Mayer's hematoxylin. To assure a comprehensive analysis, number of CD3+, CD4+ and CD8+ lymphocytes was counted in at least three non-consecutive sections of the same tumor, on at least five randomly selected microscopic fields at 160×, 200× and 250× magnification. Tumor areas with extensive necrosis were excluded from this analysis. The counts were independently done blindly by two investigators and average values are reported.
Statistical analysis
Data analyzed by Student's unpaired t-test with p < 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
MM has served on Advisory Boards from Bristol-Myers Squibb, MedImmune, Roche and GlaxoSmithKline. AC, SC and MM have a patent-pending application (WO2014/128245) partially based on findings included in this manuscript. AMDG has served as speaker to Bristol-Myers Squibb and Roche-Genentech. All other authors declare no conflicts of interest.
Acknowledgments
The authors thank Maria Rita Nicotra for the technical help with immunohistochemical studies.
Funding
This work was supported in part by grants from the Associazione Italiana per la Ricerca sul Cancro (IG 11746 to MM and 06/30/C/9 to PGN) and the Italian Ministry of Health (MM).
References
- Sigalotti L, Covre A, Fratta E, Parisi G, Colizzi F, Rizzo A, Danielli R, Nicolay H J, Coral S, Maio M. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med 2010; 8:56; PMID:20540720; http://dx.doi.org/10.1186/1479-5876-8-56
- Sigalotti L, Fratta E, Coral S, Maio M. Epigenetic drugs as immunomodulators for combination therapies in solid tumors. Pharmacol Ther 2014; 142(3):339-50; PMID:24384533; http://dx.doi.org/10.1016/j.pharmthera.2013.12.015
- Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I, Maraskovsky E, Jager E, Seliger B, Maio M. 5-aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin Cancer Res 2002; 8(8):2690-95; PMID:12171902
- Sigalotti L, Fratta E, Coral S, Tanzarella S, Danielli R, Colizzi F, Fonsatti E, Traversari C, Altomonte M, Maio M. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2′-deoxycytidine. Cancer Research. 2004; 64(24):9167-71; PMID:15604288; http://dx.doi.org/10.1158/0008-5472.CAN-04-1442
- Fonsatti E, Nicolay HJ, Sigalotti L, Calabro L, Pezzani L, Colizzi, F, Altomonte M, Guidoboni M, Marincola F M, Maio M. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2′-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res 2007; 13(11):3333-38; PMID:17545540; http://dx.doi.org/10.1158/1078-0432.CCR-06-3091
- Coral S, Covre A, Nicolay HJ, Parisi G, Rizzo A, Colizzi F, Dalla Santa S, Fonsatti E, Fratta E, Sigalotti L et al. Epigenetic remodelling of gene expression profiles of neoplastic and normal tissues: immunotherapeutic implications. Br J Cancer 2012; 107(7):1116-24; PMID:22910318; http://dx.doi.org/10.1038/bjc.2012.361
- Coral S, Sigalotti L, Gasparollo A, Cattarossi I, Visintin A, Cattelan A, Altomonte M, Maio M. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2′-deoxycytidine (5-AZA-CdR). J Immunother 1999; 22(1):16-24; PMID:9924695; http://dx.doi.org/10.1097/00002371-199901000-00003
- Covre A, Coral S, Di Giacomo AM, Taverna P, Azab M, Maio M. Epigenetics meets immune checkpoints. Semin Oncol. Cancer Res 2015; 42(3): 506-513;http://dx.doi.org/10.1053/j.seminoncol2015.02.00315
- Calabro L, Danielli R, Sigalotti L, Maio M. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol 2010; 37(5):460-67; PMID:21074061; http://dx.doi.org/10.1053/j.seminoncol.2010.09.006
- Calabro L, Maio M. Immune checkpoint blockade in malignant mesothelioma: A novel therapeutic strategy against a deadly disease? Oncoimmunology 2014; 3(1):e27482; PMID:24734215; http://dx.doi.org/10.4161/onci.27482
- Maio M, Di Giacomo AM, Robert C, Eggermont AM. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol 2013; 25(2):166-72; PMID:23299197; http://dx.doi.org/10.1097/CCO.0b013e32835dae4f
- Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski, L, de Pril V, Humphrey R, Lebbe C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol 2013; 24(8):2174-80; PMID:23666915; http://dx.doi.org/10.1093/annonc/mdt161
- Di Giacomo AM, Calabro L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C et al. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother 2013; 62(6):1021-28; PMID:23591982; http://dx.doi.org/10.1007/s00262-013-1418-6
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23; PMID:20525992; http://dx.doi.org/10.1056/NEJMoa1003466
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12(4):252-64; PMID:22437870; http://dx.doi.org/10.1038/nrc3239
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer-preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010; 37(5):430-39; PMID:21074057; http://dx.doi.org/10.1053/j.seminoncol.2010.09.005
- Curran MA, Kim M, Montalvo W, Al-Shamkhani A, Allison JP. Combination CTLA-4 blockade and 4-1BB activation enhances tumor rejection by increasing T-cell infiltration, proliferation, and cytokine production. PLoS One 2011; 6(4):e19499; PMID:21559358; http://dx.doi.org/10.1371/journal.pone.0019499
- Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 1999; 190(3):355-66; PMID:10430624; http://dx.doi.org/10.1084/jem.190.3.355
- Pennock GK, Waterfield W, Wolchok JD. Patient responses to ipilimumab, a novel immunopotentiator for metastatic melanoma: how different are these from conventional treatment responses? Am J Clin Oncol 2012; 35(6):606-11; PMID:21336089; http://dx.doi.org/10.1097/COC.0b013e318209cda9
- Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009; 15(23):7412-20; PMID:19934295; http://dx.doi.org/10.1158/1078-0432.CCR-09-1624
- Anichini A, de Braud FG, Montarini R, Bersani I, Tragni G, de Cecco L, Canevari S, Di guardo L, Pilla L, Del Vecchio M. Loss of HLA molecules as melanoma resistance mechanism in immune checkpoint blockade therapy. Mol Cancer Ther 2013; 12:A89; http://dx.doi.org/10.1158/1535-7163.TARG-13-A89
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107(9):4275-80; PMID:20160101; http://dx.doi.org/10.1073/pnas.0915174107
- Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 1999; 11(4):483-93; PMID:10549630; http://dx.doi.org/10.1016/S1074-7613(00)80123-5
- Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Scientifica (Cairo) 2013:857519; PMID:24278787; http://dx.doi.org/10.1155/2013/857519
- Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012; 30(21):2691-97; PMID:22614989; http://dx.doi.org/10.1200/JCO.2012.41.6750
- Liu J, Blake SJ, Smyth MJ, Teng MWL. Improved mouse models to assess tumour immunity and irAEs after combination cancer immunotherapies. Clin Transl Immunol 2014; 3:e22; PMID:25505970; http://dx.doi.org/10.1038/cti.2014.18
- Nanni P, De Giovanni C, Lollini PL, Nicoletti G, Prodi G. TS/A: a new metastasizing cell line from a BALB/c spontaneous mammary adenocarcinoma. Clin Exp Metastasis 1983; 1(4):373-80; PMID:6546207; http://dx.doi.org/10.1007/BF00121199
- Davis MR, Manning LS, Whitaker D, Garlepp MJ, Robinson BW. Establishment of a murine model of malignant mesothelioma. Int J Cancer 1992; 52(6):881-86; PMID:1459729; http://dx.doi.org/10.1002/ijc.2910520609
- Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer 2010; 102(11):1555-77; PMID:20502460; http://dx.doi.org/10.1038/sj.bjc.6605642
- Guo ZS, Hong JA, Irvine KR, Chen GA, Spiess PJ, Liu Y, Zeng G, Wunderlich JR, Nguyen DM, Restifo NP et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res 2006; 66(2):1105-13; PMID:16424047; http://dx.doi.org/10.1158/0008-5472.CAN-05-3020
- De Plaen E, De Backer O, Arnaud D, Bonjean B, Chomez P, Martelange V, Avner P, Baldacci P, Babinet C, Hwang SY, et al. A new family of mouse genes homologous to the human MAGE genes. Genomics 1999; 55(2):176-84; PMID:9933564; http://dx.doi.org/10.1006/geno.1998.5638
- Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics 2002; 18(11):1427-31; PMID:12424112; http://dx.doi.org/10.1093/bioinformatics/18.11.1427
- Ozato K, Sachs DH. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol 1981; 126(1):317-21; PMID:6935293
- Kuhns ST, Pease LR. A region of conformational variability outside the peptide-binding site of a class I MHC molecule. J Immunol 1998; 161(12):6745-50; PMID:9862704
