Abstract
Gamma delta T lymphocytes (γδT cells) have pleiotropic properties including innate cytotoxicity, which make them attractive effectors for cancer immunotherapy. Combination treatment with zoledronic acid and IL-2 can activate and expand the most common subset of blood γδT, which express the Vγ9Vδ2 T cell receptor (TCR) (Vδ2 T cells). Vγ9Vδ2 T cells are equipped for antibody-dependent cell-mediated cytotoxicity (ADCC) through expression of the low-affinity FcγR CD16. GD2 is a highly ranked tumor associated antigen for immunotherapy due to bright expression on the cell surface, absent expression on normal tissues and availability of therapeutic antibodies with known efficacy in neuroblastoma. To explore the hypothesis that zoledronic acid, IL-2 and anti-GD2 antibodies will synergize in a therapeutic combination, we evaluated in vitro cytotoxicity and tumor growth inhibition in the GD2 expressing cancers neuroblastoma and Ewing's sarcoma. Vδ2 T cells exert ADCC against GD2-expressing Ewing's sarcoma and neuroblastoma cell lines, an effect which correlates with the brightness of GD2 expression. In an immunodeficient mouse model of small established GD2-expressing Ewing's sarcoma or neuroblastoma tumors, the combination of adoptively transferred Vδ2+ T cells, expanded in vitro with zoledronic acid and IL-2, with anti-GD2 antibody ch14.18/CHO, and with systemic zoledronic acid, significantly suppressed tumor growth compared to antibody or γδT cell-free controls. Combination treatment using ch14.18/CHO, zoledronic acid and IL-2 is more effective than their use in isolation. The already-established safety profiles of these agents make testing of the combination in GD2 positive cancers such as neuroblastoma or Ewing's sarcoma both rational and feasible.
Abbreviations
| FCS | = | Fetal calf serum |
| IPP | = | isopentenyl-5-pyrophosphate |
| TCR | = | T cell receptor |
| ADCC | = | antibody-dependent cellular cytotoxicity |
| mAb | = | monoclonal antibody |
| γδT cell | = | gamma delta T cell. |
Introduction
γδT cells are T cells that share characteristics of the innate immune system, recognizing markers of cellular stress or altered-self in an MHC-independent manner.Citation1,2 This makes them potentially potent mediators of antitumor immunotherapy. However, they comprise only a small percentage of circulating T cells, and many tumors have developed means of evading γδT cell-mediated killing. There are a number of subsets of γδT cells, defined by their Vγ and Vδ chain usage, of which the most common in human blood expresses a relatively invariant Vγ9Vδ2 TCR. Vγ9Vδ2+ γδT cells have previously been shown to be potent killers of a range of hematological and solid tumor cell linesCitation3-5 but in the majority of cases their cytotoxicity is significantly enhanced by target opsonization; and in diseases such as neuroblastoma is almost entirely antibody dependent,Citation5,6 which is in part a reflection of that cancer's immunoinhibitory environment. For example, neuroblastoma cells secrete immunosuppressive factors such as soluble ligands of NKG2D, down regulate HLA-class I and produce immunosuppressive cytokines.Citation7–9 It is unknown whether neuroblastoma cells are deficient in production of Vγ9Vδ2 γδTCR ligands such as phosphoantigens. From a cellular therapy perspective however, Vγ9Vδ2+ γδT cells are the most immediately feasible subset on account of the relative ease of expanding their numbers in vivo using the combination of zoledronate and IL-2, about which there is pre-existing safety data.Citation6 There is some evidence of clinical efficacy in hematological and solid malignancyCitation10 but results have been variable suggesting that additional combination treatments are required fully to harness the antitumor potential of γδT cells.
Neuroblastoma strongly expresses GD2, a ganglioside antigen, which is only very sparsely expressed on healthy tissue. Gangliosides are molecules composed of glycosphingolipids associated with one or more sialic acid residues. A number of monoclonal antibodies targeting GD2 are already in clinical use with promising results,Citation11–13 although the mechanism underlying their action has not been fully elucidated. Immunotherapy using GD2-targeting antibodies has become a component of standard of care, first line treatment for high risk neuroblastoma, identifying this cancer type as an attractive model for development of further GD2-targeting immunotherapies. GD2 has been found at varying levels of expression on a number of other tumor types including Ewing's sarcoma,Citation14 small cell lung cancer,Citation15 osteosarcomaCitation16 and melanomaCitation17 suggesting that GD2-targeted immunotherapy should be further explored outside the neuroblastoma field. Indeed, its favorable differential expression has led to GD2 being ranked 12th in the National Cancer Institute list of most promising cancer antigens.Citation18
Many immunotherapies that have been evaluated in clinical trials involve combinations of modalities. For example, in neuroblastoma the combination of cytokines (IL-2+/– GM-CSF) with anti-GD2 monoclonal antibodies has been evaluated clinically.Citation11–13 Researchers exploiting γδT cell-based immunotherapy have adopted two broad strategies; either stimulating a patient's γδT cells in vivo using systemic administration of zoledronate and IL-2, or using these agents for ex vivo expansion and adoptive transfer. Given the evidence that the cytotoxicity of Vγ9Vδ2+ γδT cells is significantly enhanced by target opsonization, there is a rationale for determining the efficacy of therapeutic combinations of lytic antibodies with agents to activate and expand γδT cells.Citation19 Ch14.18 is a therapeutic anti-GD2 antibody currently in evaluation in a number of clinical trials, and thought to function predominantly by ADCC. It has not been extensively evaluated for killing function in combination with zoledronate and IL-2 in a range of cancer types expressing GD2. Here, we demonstrate that the combination of Vγ9Vδ2+ γδT cells, zoledronate and ch14.18 produced in CHO cells (ch14.18/CHO) leads to significant reductions in tumor growth compared to γδT cells and zoledronate alone, in two GD2-expressing disease models.
Results
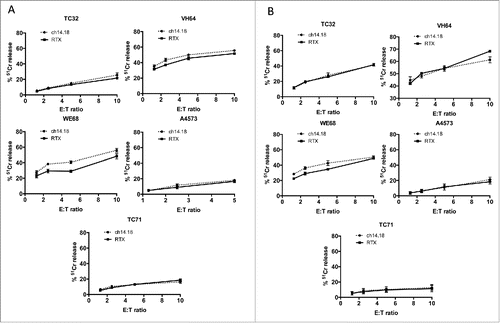
Vδ1+ and Vδ1–/Vδ2– γδT cells kill Ewing's sarcoma cell lines in an antibody-independent manner
Ewing's sarcoma has been reported as expressing GD2, making it a possible target for GD2-directed immunotherapy. We first evaluated the cytotoxic properties, against Ewing's cells, of γδT cells expanded using anti-γδTCR coated artificial antigen presenting cells as we have previously described.Citation5 Vδ1+ () and Vδ1−/Vδ2− () γδT cells killed a range of Ewing's sarcoma cell lines with varying levels of potency (range of killing of lines at 10:1 ET ratio of 15% to 55%, figures represent one of five representative donors). The addition of GD2-opsonizing antibody ch14.18/CHO made no significant difference to the level of cytotoxicity against any of the Ewing's sarcoma cell lines tested. This is consistent with our previous findings against neuroblastomaCitation5 which indicate that Vδ1+ and Vδ1–/Vδ2– γδT cell cytotoxicity is antibody independent.
GD2 on Ewing's sarcoma is an attractive target for Vγ9Vδ2+ γδT cell-mediated ADCC
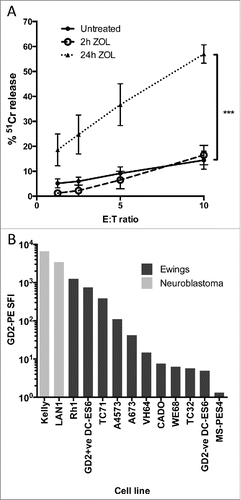
There is currently no established method for specifically expanding Vδ1+ or Vδ1–/Vδ2– γδT cells in vivo, whereas Vγ9Vδ2+ γδT cell numbers can be increased both in blood and ex vivo using the combination of zoledronate and IL-2. Addition of zoledronate to target cells can also increase expression of Vγ9Vδ2+ TCR ligands, and thereby potentially sensitize them to Vγ9Vδ2 TCR-dependent killing. We first evaluated the effect of addition of 5 μM zoledronate to the Ewing's sarcoma line TC71, and observed a marked increase in sensitivity to killing by zoledronate-expanded Vγ9Vδ2+ γδT cells, only after a prolonged 24 h exposure (, n = 7). However, after a standard intravenous dose, zoledronate has a peak plasma concentration of 1.13 μM, falling to <1% of this within 24 hCitation20 suggesting the in vitro effect might not be translatable into clinical relevance. We therefore investigated whether combination treatment with antibody might be a more clinically relevant use of zoledronate-activated γδT cells to kill Ewing's sarcoma. GD2 was found to be heterogeneous for expression on Ewing's cell lines as judged by surface staining and flow cytometric analysis. While lower than that seen in the established GD2bright neuroblastoma lines Kelly and LAN1 ( and ), 9/10 Ewing's sarcoma lines had detectable expression of GD2 compared with isotype control staining. This is in agreement with other published dataCitation14,21 regarding GD2 expression in Ewing's sarcoma, and indicates that the antibody-dependent killing potential of Vδ2+ γδT cells could be exploited in this context. We generated isogenic GD2bright clones of the naturally GD2dim Ewing's sarcoma cell line DC-ES6 to allow us to determine antigen specific effects without confounding factors such as varying expression of other γδT cell stimulating ligands. GD2 expression in these engineered DC-ES6-GD2 cells was almost as bright as that seen on neuroblastoma lines LAN1 and Kelly ().
Figure 2. (A) 24 h treatment with 5 μM zoledronic acid sensitizes the Ewing's sarcoma cell line TC71 to antibody independent lysis by Vδ2+ γδT cells (n = 7, ***p = 0.001). (B) Comparative GD2 staining of Ewing's sarcoma and Neuroblastoma cell lines determined by flow cytometry and expressed as specific fluorescence intensity (SFI). SFI is calculated by dividing the median fluorescence intensity of a sample stained with PE-anti-GD2 with the median fluorescence intensity of a sample of the same cells stained with PE-isotype control.
Vδ2+ γδT cells exhibit cytotoxicity against Ewing's sarcoma cell lines
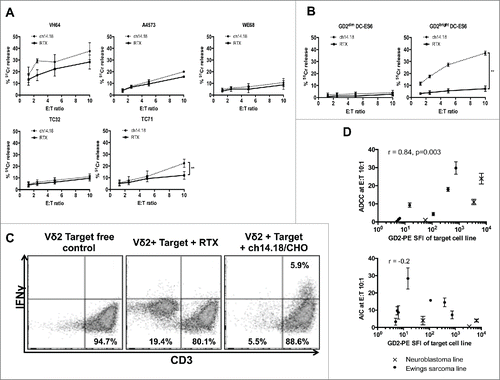
We next evaluated ch14.18/CHO antibody-dependent and -independent killing of Ewing's lines by Vδ2+ γδT cells expanded using zoledronate or isopentenyl-5-pyrophosphate + IL-2. The antibody-independent killing in a 4 h cytotoxicity assay was in the range of 10–20% at E:T ratio of 10:1 (, n = 3–6). Augmentation of cytotoxicity by target opsonization with ch14.18 was only observed in the GD2bright cell line TC71 (p = 0.006, n = 6). Some enhancement of cytotoxicity was seen against Ewing's sarcoma cell lines expressing lower amounts of GD2 but this was not significant. Hence, there appeared to be a threshold level of GD2 below which ADCC was not observed. To confirm that this observation was due to the level of GD2 expression rather than other differences between cell lines, we compared killing of isogenic GD2bright DC-ES6 with wild type GD2dim DC-ES6 cells. Opsonization of GD2bright DC-ES6 significantly enhanced killing by Vδ2+ γδT cells, which was minimal in the absence of ch14.18/CHO antibody (). When Vδ2+ γδT cells were co-cultured overnight with GD2bright DC-ES6, the target cells were eliminated and IFNγ was produced only when ch14.18/CHO was used as an opsonizing antibody (, representative of three donors). To determine the relationship between antigen expression and the efficiency of antibody-dependent killing, we calculated how much cytotoxicity was antibody-dependent (killing with ch14.18/CHO minus killing with Rituximab control antibody) at a consistent effector:target ratio (10:1) in the panel of Ewing's lines and in Kelly and LAN-1 neuroblastoma lines. There was a strong correlation between antigen expression and ADCC (r = 0.87 by Spearman correlation, p = 0.002) but there was no correlation between GD2 expression and antibody-independent killing ().
Figure 3. (A) Killing of wild-type Ewing's sarcoma cell lines by expanded Vδ2+ γδT cells opsonized with ch14.18/CHO anti-GD2 antibody or in the presence of a control antibody (Rituximab) (n = 3–6, **p = 0.0099). (B) Killing of isogenic GD2bright and GD2dim DC-ES6 Ewing's cell lines by Vδ2+ γδT cells in the presence of ch14.18/CHO anti-GD2 opsonizing antibody or Rituximab control antibody (n = 3, ***p = 0.0033). (C) IFNγ expression of Vδ2+ γδT cells in the presence of GD2bright DC-ES6. Elimination of the DC-ES6 population and production of IFNγ is only seen when DC-ES6 is opsonized with ch14.18/CHO. (D) Correlation between the GD2 stain (PE-SFI) of Ewing's sarcoma and neuroblastoma cell lines and the degree to which Vδ2+ γδT cells exert ADCC or AIC against them at effector:target ratio 10:1. R value calculated by Spearman correlation.
Ch14.18/CHO alone does not directly kill Ewing's sarcoma even at supra-therapeutic concentrations
Published studies have demonstrated that binding of anti-GD2 antibodies to GD2+ neuroblastoma, melanoma and small cell lung cancer cell lines leads to increased levels of cell death via caspase-dependent and -independent pathways.Citation15,17,22 This effect may increase the clinical efficacy of anti-GD2 antibodies in a cell-independent manner. We sought to determine whether this was the case against a panel of Ewing's cell lines; neuroblastoma cell lines are sensitive to this direct cytotoxicityCitation17 and were therefore used as a positive control.
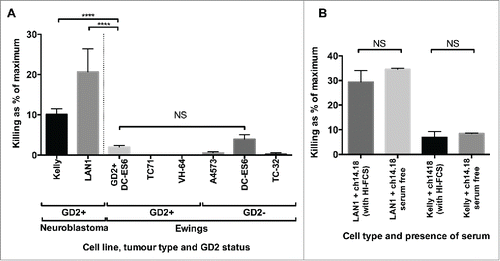
20 μg/mL of ch14.18/CHO was loaded onto a panel of GD2-expressing or non-expressing Ewing's sarcoma cell lines. The level of cell death after 4 h in the absence of immune effector cells was assessed using a 51Cr release assay and was compared to the maximum potential 51Cr release (obtained using Triton-X treatment) or the 51Cr release following coating with control antibody – Rituximab (anti-CD20 mAb) at identical concentration. The amount of cell death due to ch14.18 was determined by subtracting the spontaneous cell death in the Rituximab-treated controls from the cell death in ch14.18/CHO treated cells. There was detectable and reproducible direct cytotoxicity toward GD2 positive neuroblastoma cell lines Kelly and LAN1 by ch14.18/CHO. This effect was not seen in Ewing's sarcoma; ch14.18/CHO had minimal cytotoxicity at the same concentration against Ewing's sarcoma irrespective of GD2 expression; the difference between the killing of Kelly or LAN1 and GD2bright DC-ES6, was highly significant (p =<0.0001). Furthermore, there was no significant difference in direct killing of the paired GD2bright or GD2dim DC-ES6 lines by ch14.18/CHO (). To confirm that the killing of neuroblastoma cell lines was not the result of residual complement in the heat-inactivated fetal calf serum used in culture medium, we repeated the experiment after extensive washing and in the absence of serum. The presence or absence of serum had no significant influence, indicating that the killing of neuroblastoma was a direct effect of the antibody rather than a complement-mediated phenomenon ().
Figure 4. (A) To determine whether the correlation between ADCC and antigen expression was due to cellular stress imparted by the binding of antibody to GD2, we examined the killing of neuroblastoma and Ewing's sarcoma lines opsonized with ch14.18/CHO (20 μg/mL). High levels of spontaneous cell death were only seen in the GD2+ neuroblastoma lines, despite GD2 expression being higher in some of the Ewing's sarcoma lines (n = 9–42, depending on cell line, ****p = <0.0001). (B) To confirm that killing of neuroblastoma cell lines was not simply due to the binding and activation of residual complement in the serum, we compared the effects in the presence or absence of serum, and saw no significant difference (n = 5). The data is expressed as the “% of maximum killing” and represents the 51Cr release from opsonized cells incubated in medium for 4 h as a percentage of the 51Cr release of the same cells treated with Triton X, minus the paired result from cells treated with control antibody.
The combination of VγVδ2+ γδT cells, zoledronate and ch14.18/CHO impairs growth of established neuroblastoma and Ewing's sarcoma in a murine model
Because of fundamental differences between murine and human γδT cells, in vivo modeling of γδT cell function in mice requires the use of immunodeficient animals. We evaluated the effect of adoptive transfer of human zoledronate expanded γδT cells on subcutaneous xenografts of GD2-expressing Ewing's sarcoma cells (TC71) in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice which have no endogenous T or NK cells. Our animal model of neuroblastoma used subcutaneous xenografts of the GD2-expressing cell line Kelly, which we have shown to be susceptible to Vγ9Vδ2+ γδT cell-mediated ADCC in vitro.Citation5 We chose TC71 as an un-manipulated GD2-expressing model of Ewing's sarcoma for comparison. Mice with small established tumors were randomly assigned to treatment groups, this design ensuring that therapeutic benefits of intravenous injections of Vγ9Vδ2+ γδT cells relies not only on the γδT cells being able to kill the tumor cells, but also on their ability to infiltrate the tumor from the bloodstream. Weekly injections of zoledronate (120 μg/kg) +/– ch14.18/CHO (300 μg per mouse) and relatively low doses of Vγ9Vδ2+ γδT cells (1 × 106 cells per week) were given alongside low-dose IL-2 (100 u/mouse i.v.) () and engraftment was evaluated by flow cytometric analysis of blood and spleen taken at the end of the experiment (following three cycles of treatment), confirming successful engraftment of transferred cells in all mice sampled (). In the absence of zoledronate, γδT cells failed to engraft, even when much higher doses (1 × 107 cells per dose) were used. Following three cycles of combination treatment with zoledronate, ch14.18/CHO and Vγ9Vδ2+ γδT cells, tumor growth was significantly impaired when compared to γδT cells + zoledronate only (p = 0.0015) or with ch14.18/CHO + zoledronate (p = 0.04) (, n = 4–6 per group). Interestingly, there was a suggestion of some inhibition of growth from the combination of ch14.18/CHO and zoledronate alone in the absence of any human γδT cells though this was not significantly different from the tumor growth rate seen with γδT cells + zoledronate only (p = 0.19).
Figure 5. (A) Treatment schedule of mice used in in vivo experiments (B) Engraftment of Vγ9Vδ2+ γδT cells in NSG mice bearing TC71 Ewing's sarcoma (representative of six mice sampled) or Kelly neuroblastoma (representative of eight mice sampled) xenografts. Samples taken after culling, following three serial intravenous injections of 1 × 106 γδT cells. (C) Fold change in tumor size of TC71 xenografts in NSG mice receiving Vγ9Vδ2 + zoledronate, Vγ9Vδ2 + zoledronate + ch14.18/CHO or zoledronate + ch14.18/CHO (D) – fold change in tumor size of Kelly xenografts in NSG mice receiving Vγ9Vδ2 + zoledronate, Vγ9Vδ2 + zoledronate + ch14.18/CHO or zoledronate + ch14.18/CHO.
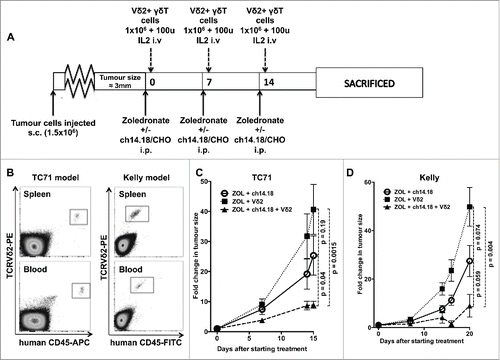
In the neuroblastoma model, the same pattern was observed. In Vγ9Vδ2 + zoledronate treated mice, there was exponential tumor growth; however, when ch14.18/CHO was administered alongside the zoledronate and Vγ9Vδ2+ cells, there was a significant reduction in growth with tumor shrinkage in 2/4 mice. By 20 d following the commencement of treatment (after three cycles of treatment), the difference in growth rate was highly significant between mice receiving zoledronate + Vγ9Vδ2 and those receiving zoledronate + Vγ9Vδ2 + ch14.18/CHO (p = 0.004, n = 4, ). As was observed in the Ewing's model, the addition of ch14.18/CHO + zoledronate in the absence of Vγ9Vδ2+ γδT cells also led to a non-significant reduction in tumor growth compared with the Vδ2 + zoledronate treatment. This minor effect of ch14.18/CHO + zoledronate was consistently observed in repeated experiments in both the Ewing's and neuroblastoma models.
Discussion
We show here that the cytolytic properties of Vγ9Vδ2 γδT cells are preserved in an in vivo setting, making these findings highly translatable to early phase trials. Our new data, combined with results that we have previously published in neuroblastomaCitation5 indicate that maximization of this cytolytic activity has a number of prerequisites – γδTCR engagement and engagement of ADCC via CD16.
Many transformed cells produce increased quantities of mavalonate pathway intermediates such as isopentenyl-5-pyrophosphaste (IPP) which lead to engagement of the Vγ9Vδ2 TCR and stimulate the γδT cells – so called “signal 1.” γδTCR stimulation alone is insufficient to fully activate Vγ9Vδ2+ γδT cells; an additional co-stimulatory signal via receptors such as NKG2D is also required.Citation23 Transformed cells, including Ewing's sarcoma,Citation24 typically express membrane bound NKG2D ligands facilitating this costimulation and sensitizing them to cytotoxic killing; however, some tumors including neuroblastoma have been demonstrated to shed soluble NKG2D ligands which can block NKG2D cross-linking and therefore abrogate this essential second signal.Citation9 This phenomenon may in part explain the low levels of antibody-independent killing exerted by Vγ9Vδ2+ γδT cells against neuroblastoma. Unlike αβT cells, Vδ2+ γδT cells express high levels of the low-affinity Fc Receptor FcγRIII (CD16), the functional role of which in ADCC is most evident in NK cells and macrophages. We previously showed that γδT express only very low levels of the high-affinity Fcγ receptors CD32 and CD64 indicating that ADCC induced by IgG antibodies occurs via CD16 ligation. CD16 provides an alternative route for Vγ9Vδ2+ γδT cell co-stimulation in the presence of an appropriately opsonized target. Naturally, there is variation in the balance of γδTCR and co-stimulatory stimulus between tumor types and also between cell lines of similar tumor origin. This variability presents problems for the therapeutic use of Vγ9Vδ2+ γδT cells as it provides additional avenues of immune escape.
To overcome this variability, the stimulus to either the γδTCR, co-stimulatory receptors or both can be artificially enhanced. Treatment of target cells with zoledronate increases IPP production and thereby increases the availability of γδTCR stimulus,Citation25 sensitizing tumor cells to Vγ9Vδ2+ γδT cell-mediated killing.Citation26,27 The evidence for the clinical efficacy of zoledronate + IL-2 combination treatment in cancer clinical trials is however limited,Citation6 suggesting that approaches for further strengthening of the γδT cell response must be explored.
In NK cells, CD16 engages CD3ζ or FcϵRIγ homo/heterodimers, which bear immunoreceptor tyrosine-based activation motifs (ITAMs), facilitating recruitment of tyrosine kinases ZAP70 and Syk. This triggers a signaling cascade involving SLP-76, the p85 subunit of PI3-kinase and Grb2 leading to an increase in intracellular Ca2+ levels. The breadth of signaling that occurs following CD16 ligation in NK cells is quite different from the relatively small subset of molecules which are implicated in the conventional T cell “signal 2” via co-stimulatory receptors such as CD28. CD28 signaling is viewed as both qualitative (producing a specific signal via a narrowly defined pathway) and quantitative (with effect determined by signal strength).Citation28 Our data demonstrating the correlation of the effect (ADCC) with GD2 staining suggests that the functional effects of CD16 ligation in Vγ9Vδ2+ γδT cells are quantitative – the strength of the signal is directly proportional to the level of binding to the target cell in a context where epitopes are likely to be saturated with antibody. The level of in vitro antibody-dependent killing exerted by γδT cells has previously been shown to correlate with the FcγRIII (CD16) expression of the effectors in question.Citation5,29 Our data support the hypothesis that the number of complete antigen-antibody-CD16 complexes is what influences the strength of the signal.
Translational relevance of Vγ9Vδ2+ γδT cell ADCC
γδT cells are potent effectors of antibody-dependent cytotoxicity that can be expanded in a specific manner without bystander activation of other immune effectors.Citation30 A number of clinical trials have already demonstrated that treating cancer patients with a combination of zoledronate and low dose IL-2 can lead to objective clinical responses in those patients where γδT cell expansion is achieved.Citation6,30–32 Target opsonization with appropriate antibody led to significant increases in Vγ9Vδ2+ cytotoxicity against tumor cells in a number of previous in vitro studiesCitation3,5,19,29,33 but there is a paucity of in vivo evidence supporting the combination of Vγ9Vδ2+ γδT cell activation and tumor-targeting antibody,Citation19,33 and this approach has never been attempted in a clinical setting despite the availability of appropriate agents with known safety profiles in adults and children.
Neuroblastoma is a potential model for this kind of combination treatment. GD2 expression in neuroblastoma is preserved following treatment with anti-GD2 monoclonal antibodies, avoiding problems of antigen loss which are often seen in other antibody-targeted malignancies such as CD20 expressing lymphoma. This makes early phase trials in patients with refractory or relapsed GD2-expressing disease a much more feasible prospect. Here, we have demonstrated that, in the context of neuroblastoma or Ewing's sarcoma expressing high levels of GD2, Vγ9Vδ2+ γδT cells can exert an antibody-dependent suppression of tumor growth, even at low doses. While the clinical utility of targeting GD2 on neuroblastoma has already been validated, it remains to be seen whether this will be of benefit in Ewing's sarcoma due to the heterogeneity of GD2 expression seen in this disease.
The ability of Vγ9Vδ2+ γδT cells to exert in vivo ADCC against solid tumors is highly translatable and may have relevance in the context of other tumor associated antigens against which there are therapeutic antibodies. Furthermore, as there is already extensive phase I safety data on the combination of zoledronate + IL-2 for in vivo γδT cell expansionCitation4,10,30-32 and also on the combination of anti-GD2 antibodies + IL-2,Citation11,12 combining these two approaches would offer a route to studying the benefits of providing an antibody alongside a specifically expanded ADCC competent effector population.
Materials and Methods
Statistical analysis
Statistical analyses were performed using GraphPad Prism Version 6.0e. Error bars, where displayed, indicate the standard error of the mean of data from replicate independent experiments. Significance of difference between samples within figures was confirmed using paired or unpaired t-tests, depending on the experimental setting, with p = <0.05 indicating significance. Correlation between variables was demonstrated by Spearman rank correlation coefficient.
Cell lines
Human neuroblastoma cells lines Kelly and LAN1 and the human Ewing's sarcoma cell lines A673 and Rh1 were originally obtained from the ATCC. The Ewing's sarcoma cell lines TC-71 and Cado were from DSMZ (Braunschweig, Germany). VH-64 and WE-68 cells were gifts from Frans van Valen's laboratory at the Institute of Experimental Orthopedics of University of Muenster, Germany. A-4573 and TC-32 were from the cell line bank at Children's Hospital Los Angeles. MS-PES4 and DC-ES6 were established by our group as described previously.Citation14
Expansion of γδT cells from PBMC
γδT cells were expanded from freshly isolated peripheral blood mononuclear cells (PBMCs) or from isolated pure populations of γδT cells. Cells were obtained from healthy donors following institutional review board approval. Zoledronate-based expansions of Vγ9Vδ2+ γδT cells were performed by culturing freshly isolated PBMC in RPMI-1640 medium containing 10% Fetal calf serum, 1% Penicillin/Streptomycin, 5 μM zoledronate (Zometa – Novartis) and 100 IU/mL IL-2 (PeproTech 200–02), which was refreshed three times weekly. After 14 d of expansion, γδT cell isolation was performed using the Miltenyi γδT cell isolation kit (130-092-892) in accordance with the manufacturer's protocol. Expansion of Vδ1+ or Vδ1–/Vδ2– γδT cells were performed as previously described.Citation5 Cells were incubated at 37°C, 5% CO2.
Generation of GD2+ DC-ES6
Wild-type DC-ES6 were transduced with SFG gammaretrovirus encoding GD2 and GD3 synthase co-expressed via foot and mouth virus 2A self-cleaving peptide sequence, kindly provided by M Pule (UCL). After transduction, single GD2-expressing clones were generated by limiting dilution, and expanded.
Antibodies
The following antibodies were used in this investigation: Treatment grade antibodies Ch14.18/CHO and rituximab were provided by Great Ormond Street Hospital pharmacy. Mouse anti-human CD3-PE/Cy7 (Biolegend 3000316; clone HIT3a), mouse anti-human TCR Vδ1-FITC (Thermo Scientific TCR2730; clone TS8.2), mouse anti-human TCR Vδ2-PE (BioLegend 331408; clone B6), mouse anti-human CD45-APC (Biolegend 304012; clone HI30), mouse anti-human CD45-APC (Biolegend 304006; clone HI30), rat anti-human/mouse CD11b (Biolegend 101228; clone M1/70), mouse anti-human IFNγ-APCCy7 (Biolegend 502530; clone 4S.B3). Live/dead staining was performed using ZombieTM Yellow fixable viability kit (Biolegend 423104), mouse anti-ganglioside GD2-PE (Biolegend 357304, clone 14G2a). Compensation was carried out using single-color controls and eBioScience OneComp eBeads (eBioScience 01-1111). When experiments regarding comparative GD2 expression of neuroblastoma and Ewing's sarcoma cell lines were being carried out, 0.5 × 106 cells were resuspended in 100 μL of buffer and labeled with 3μL of antibody (final concentration 1.5 μg/mL) for 10 min in the fridge before washing twice in PBS. Flow cytometry analysis was carried out on BD LSRII or BD FACSAria flow cytometers and results were analyzed using FlowJo vX.0.7.
Cytotoxicity assays
Expanded and purified γδT cells were used as effector cells. Target cells were coated either with opsonizing antibody or a non-targeting isotype control and labeled with 100 μCi Na251CrO4 in cell culture and tested in a standard chromium release assay as previously described. A range of E:T ratios was used in each case (10:1, 5:1, 2.5:1, 1.25:1) and cells were incubated for 4 h for each assay. Anti-GD2 antibody ch14.18/CHO (clinical grade) was used as a GD2-specific opsonizing antibody, with rituximab anti-CD20 (clinical grade) as a control.
The direct cytotoxicity of ch14.18/CHO was determined by the following formula (background 51Cr release from ch14.18/CHO opsonized/Triton-X induced 51Cr release from ch14.18/CHO opsonized) – (background 51Cr release from Rituximab treated cells/Triton-X-induced 51Cr release from Rituximab-treated cells).
Animal experiments
All experiments and procedures involving animals were conducted in accordance with the relevant laws and institutional procedures, approved by the UK government. Mice were housed in pathogen-free conditions in individually ventilated cages. Animals were originally obtained from Charles River Laboratories (Wilmington, MA) and a breeding colony was subsequently generated and maintained locally.
Adult female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were injected with 1.5 × 106 tumor cells (TC-71 or Kelly) subcutaneously, and tumor growth was monitored using electronic callipers. Once the tumors reached approximately 3 mm in diameter, mice were randomized to treatment groups and groups were coded in a way that had no relation to the treatment received, to aid blinding. Weekly intraperitoneal injections of zoledronate (120 μg/kg) +/- ch14.18/CHO (300 μg per mouse) were administered. In mice who were assigned to the groups receiving γδT cells, 1 × 106 purified Vδ2+ cells were injected intravenously on the day after injection of the zoledronate+ch14.18/CHO. 100 u of human IL-2 were injected intravenously alongside the γδT cells. Fold change in tumor size was calculated based on the size at Day 0 of treatment. Mice were culled when the average tumor diameter reached 15 mm. Blood and spleen samples were taken after culling. Blood samples were treated with ACK lysis buffer (Life Technologies A10492-01) before staining for FACS analysis. Spleen samples were passed through a 70 μM cell strainer before being treated with ACK lysis buffer and being stained for FACS analysis to confirm Vδ2+ γδT cell engraftment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
supplemental_fig._1.pdf
Download PDF (157.5 KB)Acknowledgments
The GD2/GD3 synthase construct was kindly provided by Dr Martin Pule (UCL). Flow cytometry was carried out at the UCL ICH Flow Cytometry Core Facility.
Funding
Funding was received from the Wellcome Trust, Leukaemia and Lymphoma Research, GOSHCC (JA personal support), BRC, Research in Childhood Cancer and SPARKS.
References
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013; 13:88-100; PMID:23348415; http://dx.doi.org/10.1038/nri3384.
- Hayday AC. γδ T Cells and the lymphoid stress-surveillance response. Immunity 2009; 31:184-96; PMID:19699170; http://dx.doi.org/10.1016/j.immuni.2009.08.006.
- Himoudi N, Morgenstern DA, Yan M, Vernay B, Saraiva L, Wu Y, Cohen CJ, Gustafsson K, Anderson J. Human γδT lymphocytes are licensed for professional antigen presentation by interaction with opsonized target cells. J Immunol 2012; 188:1708-16; PMID:22250090; http://dx.doi.org/10.4049/jimmunol.1102654.
- Wilhelm M. T cells for immune therapy of patients with lymphoid malignancies. Blood 2003; 102:200-6; PMID:12623838; http://dx.doi.org/10.1182/blood-2002-12-3665.
- Fisher J, Yan M, Heuijerjans J, Carter L, Abolhassani A, Frosch J, Wallace R, Flutter B, Hubank M, Klein N et al. Neuroblastoma killing properties of V-delta 2 and V-delta2 negative gamma delta T cells following expansion by artificial antigen presenting cells. Clin Cancer Res 2014; 20(22):5720-32; PMID 24893631; http://dx.doi.org/10.1158/1078-0432.CCR-13-3464.
- Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. oncoimmunology 2014; 3:e27572; PMID:24734216; http://dx.doi.org/10.4161/onci.27572.
- Seeger RC. Immunology and immunotherapy of neuroblastoma. Semin Cancer Biol 2011; 21:229-37; PMID:21971567; http://dx.doi.org/10.1016/j.semcancer.2011.09.012.
- Asgharzadeh S, Salo JA, Ji L, Oberthuer A, Fischer M, Berthold F, Hadjidaniel M, Liu CW-Y, Metelitsa LS, Pique-Regi R et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol 2012; 30:3525-32; PMID:22927533; http://dx.doi.org/10.1200/JCO.2011.40.9169.
- Raffaghello L, Prigione I, Airoldi I, Camoriano M, Levreri I, Gambini C, Pende D, Steinle A, Ferrone S, Pistoia V. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia 2004; 6:558; PMID: 15548365; http://dx.doi.org/10.1593/neo.04316.
- Kunzmann V, Smetak M, Kimmel B, Weigang-Koehler K, Goebeler M, Birkmann J, Becker J, Schmidt-Wolf IGH, Einsele H, Wilhelm M. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother 2012; 35:205-13; PMID:22306909; http://dx.doi.org/10.1097/CJI.0b013e318245bb1e.
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010; 363:1324-34; PMID:20879881; http://dx.doi.org/10.1056/NEJMoa0911123.
- Cheung N-KV, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol 2012; 30:3264-70; PMID:22869886; http://dx.doi.org/10.1200/JCO.2011.41.3807.
- Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, Berthold F. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol 2004; 22:3549-57; PMID:15337804; http://dx.doi.org/10.1200/JCO.2004.08.143.
- Kailayangiri S, Altvater B, Meltzer J, Pscherer S, Luecke A, Dierkes C, Titze U, Leuchte K, Landmeier S, Hotfilder M et al. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. Br J Canc 2012; 106:1123-33; PMID:22374462; http://dx.doi.org/10.1038/bjc.2012.57
- Yoshida S, Kawaguchi H, Sato S, Ueda R, Furukawa K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. Jpn J Cancer Res 2002; 93:816-24; PMID:12149148; http://dx.doi.org/10.1111/j.1349-7006.2002.tb01324.x.
- Liu B, Wu Y, Zhou Y, Peng D. Endothelin A receptor antagonism enhances inhibitory effects of anti-ganglioside GD2 monoclonal antibody on invasiveness and viability of human osteosarcoma cells. PLoS ONE 2014; 9:e93576; PMID:24727660; http://dx.doi.org/10.1371/journal.pone.0093576.
- Doronin II, Vishnyakova PA, Kholodenko IV, Ponomarev ED, Ryazantsev DY, Molotkovskaya IM, Kholodenko RV. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer 2014; 14:295; PMID:24773917; http://dx.doi.org/10.1186/1471-2407-14-295.
- Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res 2009; 15:5323-37; PMID:19723653; http://dx.doi.org/10.1158/1078-0432.CCR-09-0737.
- Gertner-Dardenne J, Bonnafous C, Bezombes C, Capietto AH, Scaglione V, Ingoure S, Cendron D, Gross E, Lepage JF, Quillet-Mary A et al. Bromohydrin pyrophosphate enhances antibody-dependent cell-mediated cytotoxicity induced by therapeutic antibodies. Blood 2009; 113:4875-84; PMID:19278954; http://dx.doi.org/10.1182/blood-2008-08-172296.
- Skerjanec A. The pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with varying degrees of renal function. J Clin Pharmacol 2003; 43:154-62; PMID: 12616668; http://dx.doi.org/10.1177/0091270002239824.
- Lipinski M, Braham K, Philip I, Wiels J, Philip T, Dellagi K, Goridis C, Lenoir GM, Tursz T. Phenotypic characterization of Ewing sarcoma cell lines with monoclonal antibodies. J Cell Biochem 1986; 31:289-96; PMID:3760036; http://dx.doi.org/10.1002/jcb.240310406.
- Aixinjueluo W, Furukawa K, Zhang Q, Hamamura K, Tokuda N, Yoshida S, Ueda R, Furukawa K. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: roles of anoikis. J Biol Chem 2005; 280:29828-36; PMID:15923178; http://dx.doi.org/10.1074/jbc.M414041200.
- Nedellec S, Sabourin C, Bonneville M, Scotet E. NKG2D costimulates human V gamma 9V delta 2 T cell antitumor cytotoxicity through protein kinase C theta-dependent modulation of early TCR-induced calcium and transduction signals. J Immunol 2010; 185:55-63; PMID:20511557; http://dx.doi.org/10.4049/jimmunol.1000373.
- Verhoeven DHJ, de Hooge ASK, Mooiman ECK, Santos SJ, Dam ten MM, Gelderblom H, Melief CJM, Hogendoorn PCW, Egeler RM, van Tol MJD et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Mol Immunol 2008; 45:3917-25; PMID:18657862; http://dx.doi.org/10.1016/j.molimm.2008.06.016.
- Sandstrom A, Peigné C-M, Léger A, Crooks JE, Konczak F, Gesnel M-C, Breathnach R, Bonneville M, Scotet E, Adams EJ. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014; 40:490-500; PMID:24703779; http://dx.doi.org/10.1016/j.immuni.2014.03.003.
- Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vγ9Vδ2 T cell cytotoxicity. Cancer Immunol Immunother 2007; 56:1285-97; PMID:17265022; http://dx.doi.org/10.1007/s00262-007-0279-2.
- Nishio N, Fujita M, Tanaka Y, Maki H, Zhang R, Hirosawa T, Demachi-Okamura A, Uemura Y, Taguchi O, Takahashi Y et al. Zoledronate sensitizes neuroblastoma-derived tumor-initiating cells to cytolysis mediated by human γδ T cells. J Immunother 2012; 35:598-606; PMID:22996365; http://dx.doi.org/10.1097/CJI.0b013e31826a745a.
- Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol 2003; 3:939-51; PMID:14647476; http://dx.doi.org/10.1038/nri1248.
- Tokuyama H, Hagi T, Mattarollo SR, Morley J, Wang Q, Fai-So H, Moriyasu F, Nieda M, Nicol AJ. Vγ9Vδ2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs—Rituximab and trastuzumab. Int J Cancer 2008; 122:2526-34; PMID:18307255; http://dx.doi.org/10.1002/ijc.23365.
- Meraviglia S, Eberl M, Vermijlen D, Todaro M, Buccheri S, Cicero G, La Mendola C, Guggino G, D'Asaro M, Orlando V et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol 2010; 16:290-7; PMID:20491785.
- Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D'Asaro M, Gebbia N et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 2007; 67:7450-7; PMID:17671215; http://dx.doi.org/10.1158/0008-5472.CAN-07-0199.
- Lang JM, Kaikobad MR, Wallace M, Staab MJ, Horvath DL, Wilding G, Liu G, Eickhoff JC, McNeel DG, Malkovsky M. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother 2011; 60:1447-60; PMID:21647691; http://dx.doi.org/10.1007/s00262-011-1049-8.
- Capietto AH, Martinet L, Fournie JJ. Stimulated T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol 2011; 187:1031-8; PMID:21670311; http://dx.doi.org/10.4049/jimmunol.1100681.