Abstract
Homeostatic γC cytokines are essential to support the expansion and function of tumor-specific T cells, but their effects are constrained by suppressor of cytokine signaling (SOCS) proteins as well as phosphoinositide and tyrosine-specific phosphatases. The microRNA miR-155 counteracts these inhibitory hurdles to potentiate intracellular cytokine signaling and T cell antitumor immunity.
Common γ-chain (γC) cytokines such as interleukin-2 (IL-2), IL-7, IL-15, and IL-21 play pivotal roles in regulating T cell homeostasis and inflammatory responses. There is now extensive evidence that these cytokines provide critical signals to sustain engraftment, expansion and effector functions of adoptively transferred tumor-specific T cells.Citation1 A number of intracellular negative regulators of cytokine signaling exist to prevent unrestrained responses to cytokines. For instance, the JAK/STAT pathway, a primary signaling pathway downstream of the γC cytokine-receptor, is attenuated by SOCS family members and protein tyrosine phosphatases.Citation2 In addition, cytokine-induced activation of PI3K/AKT signaling is dampened by phosphoinositide lipid phosphatases.Citation2 While these negative checkpoints are physiologically necessary to balance T cell immune responses, they also drastically impede the effectiveness of adoptively transferred tumor-specific T cells ().
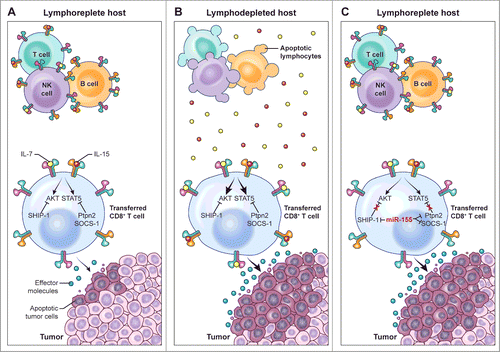
Figure 1. miR-155 augments CD8+ T cell antitumor activity in lymphoreplete hosts by enhancing responsiveness to limited amounts of homeostatic γC cytokines. (A) In the lymphoreplete host, homeostatic cytokine signaling, which is essential to sustain the antitumor function of adoptively transferred tumor-specific CD8+ T cells, is limited by the activity of multiple negative regulators such as SHIP-1, SOCS-1, and Ptpn2. (B) Removal of cytokine “sinks” by lymphodepletion increases the availability of homeostatic γC cytokines, overcoming the inhibitory effects of SHIP-1, SOCS-1, and Ptpn2, thus resulting in enhanced CD8+ T cell antitumor responses. (C) Overexpression of miR-155 in adoptively transferred tumor-specific CD8+ T cells inhibits the expression of SHIP-1, SOCS-1, and Ptpn2, thus resulting in enhanced cytokine signaling and antitumor responses in the presence of limited amounts of homeostatic γC cytokines available in lymphoreplete hosts.
Two main strategies are currently employed in the clinic to tip the balance and promote cytokine signaling. Both approaches aim to increase the availability of homeostatic γC cytokines, by either providing exogenous cytokine support or eliminating endogenous cytokine cellular “sinks” through lymphodepletion ().Citation1,3 These maneuvers, however, are nonspecific and come with high prices in term of toxicities. Lymphodepleting regimens such as high-intensity chemotherapy and total body irradiation have been associated with severe and sometimes lethal side effects.Citation4 Patients can experience prolonged leukopenia increasing the risk of opportunistic infections, such as cytomegalovirus, herpes zoster, respiratory syncitial virus and Pneumocystis carinii. Radiation and chemotherapy are also associated with increased incidence of thromboembolic events, including pulmonary veno-occlusive disease, jugular venous thrombosis and thrombotic microangiopathy.Citation4 Additionally, high-dose chemotherapy regimens incorporating fludarabine might result in cortical blindness. Likewise, exogenous administration of high-dose IL-2 can lead to substantial acute toxicities in multiple organs, including lungs, heart, kidneys, and central nervous system. Most of these toxicities are primarily due to a capillary leak syndrome, which results in a hypovolemic state and fluid accumulation in the tissues manifested as generalized edema, pulmonary congestion, pleural effusions, and ascites.Citation4 Currently, numerous patients are excluded from adoptive T cell-based immunotherapy because of the potential for severe side effects associated with these maneuvers. Furthermore, management of these toxicities necessitates extensive supportive care and long-term inpatient hospitalization causing extraordinary financial pressure on the patients and the healthcare provider.
We have recently described an alternative approach to enhance tumor-specific T cell responsiveness to homeostatic γC cytokines, which is based on releasing the brakes provided by multiple cytokine signaling inhibitors.Citation5 We took advantage of a well-recognized feature of microRNAs, which are capable of regulating a biological process by repressing numerous targets involved in the underlying molecular network. We chose to overexpress the microRNA, miR-155 in tumor-specific T cells because it can inhibit the expression of several negative regulators of cytokine signaling. For example, we and others have demonstrated that miR-155 can target the JAK/STAT inhibitor SOCS-1 as well as the inositol 5-phosphatase SHIP-1, an inhibitor of PI3K/AKT signaling.Citation5-7 Additionally, we have found that miR-155 can also bind to the 3′ UTR region of the protein tyrosine phosphatase Ptpn2 to attenuate its inhibitory effects on STAT5 signaling.Citation5 Consistent with these biological activities, overexpression of miR-155 significantly increased STAT5 and AKT signaling in response to limited amounts of homeostatic γC cytokines available in lymphoreplete hosts, resulting in enhanced T cell survival and profound antitumor responses ().Citation5 Notably, miR-155 overexpression was sufficient in surrogating the therapeutic advantages conveyed by lymphodepletion preconditioning and exogenous cytokine administration (), indicating that this strategy can be effectively used to increase the efficacy of adoptive immunotherapies in a cell-intrinsic manner without the need for life-threatening maneuvers.
A number of therapeutic approaches to augment the efficacy of T cell-based immunotherapies without lymphodepletion preconditioning have been recently proposed, including PD1 blockade in conjunction of IL-2/anti-IL-2 antibody complexes or IL-12 overexpression in tumor-reactive T cells.Citation8,9 However, it is unclear whether such strategies can replace both lymphodepletion and cytokine support because direct comparisons were not performed in these studies. Furthermore, overexpression of IL-12 could not substitute the requirement for lymphodepletion in certain murine models of T-cell therapy.Citation10
Enhancing the activity of both AKT and STAT5 by modulating miR-155 expression provides significant advantages over strategies relying on constitutive activation of these two pathways individually. First, it circumvents the technical challenge of enforcing the expression of two large molecules simultaneously in one cell. Second, it allows fine-tuning of cytokine signaling by targeting multiple molecules without the negative effects that can be associated with constitutive activation of a given downstream pathway. Finally, by cumulatively reducing the expression of several inhibitory components downstream of cytokine signaling, it confers robustness to the regulation of the system.
Overexpression of miR-155 in adoptively transferred tumor-specific T cells not only has the potential to reduce the toxicities associated with current cellular therapies making them available to a broader spectrum of cancer patients, but it also provides the possibility of improving the effectiveness of adoptive immunotherapies. Currently, it is not practical to deliver multiple rounds of T cell infusions because repeated preconditioning treatments are not feasible and the efficacy of T cell therapy is limited in lymphoreplete hosts. The possibility of augmenting the therapeutic efficacy of T cell-based immunotherapies without the need of lymphodepleting preconditioning provides the opportunity to administer highly effective T cell products repeatedly in the short-term to maximize therapeutic outcomes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006; 6:383-93; PMID:16622476; http://dx.doi.org/10.1038/nri1842
- Yasukawa H, Sasaki A, Yoshimura A. Negative regulation of cytokine signaling pathways. Annu Rev Immunol 2000; 18:143-64; PMID:10837055; http://dx.doi.org/10.1146/annurev.immunol.18.1.143
- Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005; 202:907-12; PMID:16203864; http://dx.doi.org/10.1084/jem.20050732
- Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, Restifo NP. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol 2006; 3:668-81; PMID:17139318; http://dx.doi.org/10.1038/ncponc0666
- Ji Y, Wrzesinski C, Yu Z, Hu J, Gautam S, Hawk NV, Telford WG, Palmer DC, Franco Z, Sukumar M et al. miR-155 augments CD8+ T-cell antitumor activity in lymphoreplete hosts by enhancing responsiveness to homeostatic gammac cytokines. Proc Natl Acad Sci U S A 2015; 112:476-81; PMID:25548153; http://dx.doi.org/10.1073/pnas.1422916112
- Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity 2013; 38:742-53; PMID:23601686; http://dx.doi.org/10.1016/j.immuni.2012.12.006
- O'Connell RM, Chaudhuri AA, Rao DS, Baltimore D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci USA 2009; 106:7113-8; PMID:19359473; http://dx.doi.org/10.1073/pnas.0902636106
- Cho HI, Reyes-Vargas E, Delgado JC, Celis E. A potent vaccination strategy that circumvents lymphodepletion for effective antitumor adoptive T-cell therapy. Cancer Res 2012; 72:1986-95; PMID:22367213; http://dx.doi.org/10.1158/0008-5472.CAN-11-3246
- Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012; 119:4133-41; PMID:22354001; http://dx.doi.org/10.1182/blood-2011-12-400044
- Kerkar SP, Muranski P, Kaiser A, Boni A, Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res 2010; 70:6725-34; PMID:20647327; http://dx.doi.org/10.1158/0008-5472.CAN-10-0735
