Abstract
The role of inflammation in cancer is controversial as both tumor-promoting and tumor-suppressive aspects of inflammation have been reported. In particular, it has been shown that pro-inflammatory cytokines, like interleukin-1α (IL-1α), IL-1β, IL-6, and tumor necrosis factor α (TNFα), may either promote or suppress cancer. However, the cellular and molecular basis underlying these opposing outcomes remains enigmatic. Using mouse models for myeloma and lymphoma, we have recently reported that inflammation driven by tumor-specific T helper 1 (Th1) cells conferred protection against B-cell cancer and that interferon-γ (IFN-γ) was essential for this process. Here, we have investigated the contribution of several inflammatory mediators. Myeloma eradication by Th1 cells was not affected by inhibition of TNF-α, TNF-related weak inducer of apoptosis (TWEAK), or TNF-related apoptosis-inducing ligand (TRAIL). In contrast, cancer elimination by tumor-specific Th1 cells was severely impaired by the in vivo neutralization of both IL-1α and IL-1β (collectively named IL-1) with IL-1 receptor antagonist (IL-1Ra). The antitumor functions of tumor-specific Th1 cells and tumor-infiltrating macrophages were both affected by IL-1 neutralization. Secretion of the Th1-derived cytokines IL-2 and IFN-γ at the incipient tumor site was severely reduced by IL-1 blockade. Moreover, IL-1 was shown to synergize with IFN-γ for induction of tumoricidal activity in tumor-infiltrating macrophages. This synergy between IL-1 and IFN-γ may explain how inflammation, when driven by tumor-specific Th1 cells, represses rather than promotes cancer. Collectively, the data reveal a central role of inflammation, and more specifically of the canonical pro-inflammatory cytokine IL-1, in enhancing Th1-mediated immunity against cancer.
Abbreviations
| APC | = | antigen-presenting cell |
| ATCC | = | American Type Culture Collection |
| FGF-b | = | fibroblast growth factor-b |
| G-CSF | = | granulocyte colony-stimulating factor |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| IFN-γ | = | interferon-γ |
| Ig | = | immunoglobulin |
| IL | = | interleukin |
| IL-1Ra | = | IL-1 receptor antagonist |
| i.p. | = | intraperitoneally |
| LIF | = | leukemia inhibitory factor |
| LN | = | lymph node |
| LPS | = | lipopolysaccharide |
| mAb | = | monoclonal antibody |
| M-CSF | = | monocyte colony-stimulating factor |
| MHC | = | major histocompatibility complex |
| PBS | = | phosphate-buffered saline |
| PDGF-bb | = | platelet-derived growth factor-bb |
| s.c. | = | subcutaneously |
| SCID | = | severe combined immunodeficiency |
| TCR | = | T cell receptor |
| Tg | = | transgenic |
| TGF-β | = | transforming growth factor-β; Th, T helper |
| TNFα | = | tumor necrosis factor α |
| TRAIL | = | TNF-related apoptosis-inducing ligand |
| TWEAK | = | TNF-related weak inducer of apoptosis. |
Introduction
There is an ongoing debate concerning the role of inflammation in cancer because both tumor-promoting and tumor-suppressive aspects of inflammation have been reported. Tumor-specific Th1 cells, which produce the cytokine interferon-γ (IFN-γ), seem to be particularly efficient at fighting cancer.Citation1-6 Accordingly, we and others have proposed that inflammation driven by tumor-specific Th1 cells, with resultant IFN-γ-mediated activation of tumor-infiltrating macrophages, may suppress malignancies.Citation7-9 In contrast, Th2 cells, alternatively activated macrophages (often called “M2 macrophages”), and chronic inflammation may instead promote tumor growth and metastasis.Citation8-13 Thus, the opposing effects of inflammation in various cancer situations may reflect the existence of various types of inflammations.Citation9 However, it remains largely unclear how the same inflammatory mediators, such as cytokines, may either promote or suppress cancer in various contexts.
To characterize a protective inflammatory immune response mediated by tumor-specific Th1 cells, we have used myeloma-specific T cell receptor transgenic (TCR-Tg) mice. In this transgenic system, CD4+ T cells recognize a bona fide tumor-specific antigen (idiotype) derived from the L-chain variable region of the immunoglobulin A (IgA) that is secreted by the MOPC315 murine myeloma cell line.Citation14,15 Because, MOPC315 cells lack major histocompatibility complex (MHC) class II molecules, recognition of MOPC315 by CD4+ T cells occurs via indirect presentation of secreted antigens on tumor-infiltrating antigen-presenting cells (APCs), predominantly macrophages.Citation1 Idiotype-specific TCR-Tg mice were made homozygous for the severe combined immunodeficiency (SCID) mutation, which prevents rearrangement of endogenous TCR chains and thereby ensures the unique specificity of the T cells.Citation16 The high frequency of tumor-specific CD4+ T cells in TCR-Tg SCID mice renders the mice resistant to subcutaneously (s.c.) injected syngeneic MOPC315 myeloma cells, while non-transgenic mice rapidly develop tumors. In this model system, cancer prevention by the immune system (i.e. cancer immunosurveillance) is antigen-specific, depends on antigen secretion by the cancer cells, is mediated by CD4+ T cells, and does not require the presence of B cells, γδ T cells, CD8+ T cells, or natural killer (NK) cells.Citation15-18
In a series of reports, we have described the main cellular events leading to MOPC315 myeloma clearance in idiotype-specific TCR-Tg SCID mice.Citation1,17-20 Starting at around day +3 after the s.c. injection of MOPC315 cells, naive tumor-specific CD4+ T cells become activated in the lymph node (LN) that drains the incipient tumor site.Citation1 In this draining LN, tumor-specific CD4+ T cells acquire a Th1 phenotype characterized by the production of IFNγ.Citation1,20 Effector tumor-specific Th1 cells migrate from the draining LN to the incipient tumor site, where they collaborate with macrophages in order to recognize and eliminate myeloma cells.Citation1,19,20 Upon recognition of tumor-derived idiotypic peptides presented on MHC class II molecules by macrophages, tumor-specific Th1 cells secrete IFNγ. IFNγ is required for successful myeloma eradication and a dual role of IFNγ was identified. First, IFNγ renders macrophages cytotoxic to myeloma cells. Second, IFNγ induces macrophages to secrete the angiostatic chemokines CXCL9 and CXCL10, which may halt tumor progression by inhibiting angiogenesis.Citation1,18 The peak of the antitumor immune response is at around day +8 after MOPC315 injection, when the strongest activation of CD4+ T cells and macrophages is observed.Citation1,20 Most myeloma cells are eradicated by day + 15 after s.c. injection into idiotype-specific TCR-Tg SCID mice.Citation1 We have recently reported that myeloma eradication by idiotype-specific TCR-Tg SCID mice was associated with increased levels, at the incipient tumor site, of several canonical pro-inflammatory cytokines including interleukin (IL)-1α, IL-1β, IL-6, and tumor necrosis factor α (TNFα).Citation18 We concluded that inflammation driven by tumor-specific Th1 cells protects against B-cell cancer.Citation18 However, the importance of the inflammatory reaction itself for cancer eradication was not investigated.
In the present study, the importance of inflammation for cancer eradication by tumor-specific Th1 cells was evaluated by selectively blocking several inflammatory mediators. We report that successful myeloma rejection by TCR-Tg SCID mice required neither TNFα, TWEAK, nor TRAIL. In contrast, tumor elimination was severely impaired by in vivo neutralization of both IL-1α and IL-1β (collectively named IL-1) with IL-1 receptor antagonist (IL-1Ra). IL-1 was shown to be essential for the antitumor functions of both tumor-specific Th1 cells and tumor-infiltrating macrophages. Collectively, the data reveal the central role of inflammation, and more specifically of the canonical pro-inflammatory cytokine IL-1, in supporting Th1-mediated adaptive immunity against cancer.
Results
Myeloma eradication by Th1 cells is associated with sustained macrophage activation and local release of numerous inflammatory cytokines
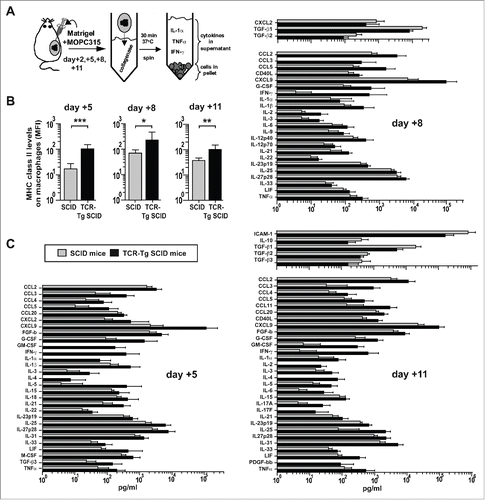
In order to monitor macrophage activation and cytokine secretion at the incipient tumor site during the course of myeloma eradication by tumor-specific Th1 cells, we used the Matrigel cytokine assay. This technique consists of injecting mice s.c. with myeloma cells embedded in a collagen gel named Matrigel.Citation18 At various time points after injection, the Matrigel plugs may be excised, allowing the analysis of infiltrating immune cells and locally secreted cytokines (). For comparison, two groups of mice were injected: tumor-resistant TCR-Tg SCID mice and tumor-prone non-transgenic SCID mice. In both types of mice, we have previously reported that MOPC315-containing Matrigel plugs were rapidly infiltrated by a large population of CD11b+ cells, which consisted almost exclusively of macrophages with possibly a few CD11b+ CD11c+ dendritic cells.Citation1 In TCR-Tg SCID mice, we have also shown that Th1-derived IFN-γ induced both upregulation of surface MHC class II molecules and tumoricidal activity in Matrigel-infiltrating macrophages.Citation1,18 Therefore, we measured surface MHC class II levels on Matrigel-infiltrating CD11b+ cells in order to assess macrophage activation by Th1 cells at various time points after s.c. injection of MOPC315 cells. In parallel, the levels of 46 cytokines in the Matrigel extracellular matrix were quantified. On day +2 after injection, no difference was observed between the groups, concerning macrophage activation and cytokine levels (data not shown). At day +5, +8, and +11, surface MHC class II levels on Matrigel-infiltrating CD11b+ macrophages were found to be significantly upregulated in tumor-resistant TCR-Tg SCID mice, compared to tumor-prone SCID mice (). Moreover, myeloma eradication was accompanied by a significant increase in the local concentration of 29 cytokines at day +5, 23 cytokines at day +8, and 30 cytokines at day +11 (). In contrast, a few cytokines with known anti-inflammatory properties, like transforming growth factor (TGF)-β1, TGF-β2, TGF-β3, and IL-10, had lower levels in tumor-resistant TCR-Tg SCID compared to tumor-prone SCID mice at day +8 and day +11 (). Thus, successful myeloma eradication mediated by tumor-specific Th1 cells was associated with sustained IFN-γ-mediated activation of macrophages and with local release of numerous inflammatory mediators including IL-1α, IL-1β, IL-6, IL-23, and TNFα.
Figure 1. Cytokine profile of myeloma eradication mediated by tumor-specific Th1 cells. (A) Experimental design. MOPC315 myeloma cells were mixed with cold liquid Matrigel before s.c. injection into idiotype-specific TCR-Tg SCID or control non-transgenic SCID mice. At body temperature, the Matrigel solution forms a gel plug that contains the myeloma cells, infiltrating immune cells, and locally secreted cytokines. At indicated time points, the Matrigel plugs were excised, dissolved with collagenase, and centrifuged. Cells in the pellet were analyzed by flow cytometry. Extracellular cytokines in the Matrigel were quantified by Luminex technology. (B) MHC class II levels on Matrigel-infiltrating CD11b+ macrophages in TCR-Tg SCID or control SCID mice at indicated time points (mean ±S.D, n = 8−12 mice per group). MFI, mean fluorescence intensity. *p = 0.02, **p = 0.001, *** p < 0.0001 (Mann-Whitney test). (C) The concentration of 46 cytokines in Matrigel supernatant was measured for control SCID (gray bars) or TCR-Tg SCID (black bars) mice (n = 8−12). Only cytokines with significantly higher levels (p < 0.05, Mann-Whitney test) in one group compared with the other are included in the bar graphs (mean ± SD). Data are representative for two independent experiments.
IL-1 is required for myeloma elimination by tumor-specific Th1 cells
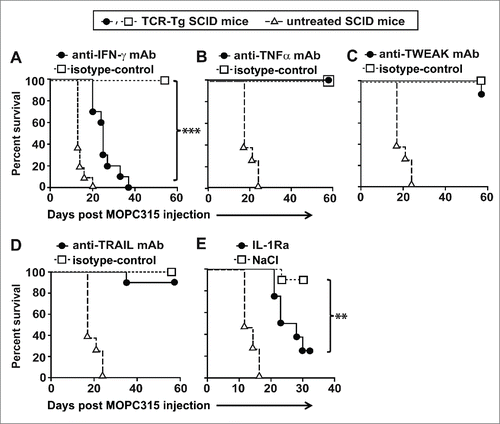
To clarify the mechanism of myeloma eradication, we performed a series of tumor challenge experiments, using established protocols to selectively block candidate effector molecules. As previously reported,Citation1 tumor rejection by TCR-Tg SCID mice was abrogated by blocking IFN-γ in vivo with a monoclonal antibody (mAb) (). In contrast, no effect was observed when the mice were treated with blocking mAb to neutralize TNFα, TWEAK, or TRAIL, suggesting that these molecules were not essential for myeloma elimination by Th1 cells (). Strikingly, neutralization of the common receptor for both IL-1α and IL-1β with IL-1Ra severely impaired tumor protection in TCR-Tg mice (). Moreover, when using serum concentration of the myeloma protein (the monoclonal IgA secreted by MOPC315) as a surrogate marker of tumor burden, mice treated with IL-1Ra had significantly higher levels of myeloma protein as compared to vehicle-treated mice at day +17 (Supplementary Fig. S1). Thus, protective antitumor immunity in TCR-Tg SCID mice is dependent on both IFN-γ and IL-1.
Figure 2. Both IFN-γ and IL-1 are required for myeloma elimination by tumor-specific Th1 cells. Idiotype-specific TCR-Tg SCID (n = 6−10 mice per group) or SCID mice (n = 4−6) were injected s.c. with MOPC315 myeloma cells in PBS. Tumor growth was recorded over time. Mice with a tumor diameter ≥10 mm were euthanized. (A–D) TCR-Tg SCID mice were treated i.p. with blocking mAb against (A) IFN-γ, (B) TNFα, (C) TWEAK, or (D) TRAIL or with isotype-matched control mAb. (E) TCR-Tg SCID mice were implanted s.c. with osmotic pumps releasing IL-1Ra or vehicle (NaCl).
IL-1 is required for the antitumor functions of both tumor-specific Th1 cells and tumor-infiltrating macrophages
The role of IL-1 for myeloma eradication by Th1 cells was investigated by treating tumor-specific TCR-Tg SCID mice with IL-1Ra (or vehicle) before analyzing the immune response at day +8 (). Neutralization of both IL-1α and IL-1β with IL-1Ra had no clear effect on the activation of naïve tumor-specific CD4+ T cells in the LN draining the incipient tumor site, as measured by expression of the early activation marker CD69 (). Moreover, intracellular staining of IFN-γ and T-bet indicated that IL-1 blockade did not influence the Th1 polarization of the tumor-specific CD4+ T cells in LN and Matrigel plugs (). However, quantification of 46 locally secreted cytokines revealed a significant downregulation of 11 cytokines (soluble CD40L, G-CSF, GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-17A, and IL-25) and a significant upregulation of two molecules (soluble ICAM-1 and TGF-β1) in the IL-1Ra-treated group (). The significant reduction of two critical Th1-derived cytokines, namely IFN-γ and IL-2, in the IL-1Ra-treated group strongly suggests that IL-1 is regulating the function (i.e., cytokine secretion) of tumor-specific Th1 cells at the incipient tumor site.
Figure 3. Roles of IL-1 for myeloma eradication mediated by tumor-specific Th1 cells. (A) Experimental design. Idiotype-specific TCR-Tg SCID mice were injected s.c. with MOC315 in Matrigel and treated s.c. daily with IL-1Ra or vehicle (NaCl). Draining LN and Matrigel plugs were analyzed at day +8. (B) Tumor-specific CD4+ T cells in pooled draining LN and Matrigel plugs from IL-1Ra or NaCl−treated mice (n = 8−10 mice per group) were analyzed by flow cytometry. Tumor-specific T cells were gated using the GB113 mAb specific for the transgenic TCR. Levels of surface CD69 or intracellular T-bet and IFN-γ were recorded (solid lines). Dotted lines indicate isotype-matched control mAb. (C) The concentration of 46 cytokines in the extracellular matrix of the Matrigel plugs was quantified for IL-1Ra-treated (black bars) and NaCl−treated (gray bars) TCR-Tg SCID mice (n = 11, mean ± SD). Only cytokines which showed significantly (p < 0.05, Mann–Whitney test) higher levels in one group compared with the other are included in the bar graphs. (D) MHC class II levels on Matrigel-infiltrating CD11b+ cells in NaCl or IL-1Ra-treated TCR-Tg SCID mice (n = 8−10, mean ± SD). **p = 0.003 (Mann–Whitney test). (E) Growth inhibition assay. Matrigel-infiltrating CD11b+ macrophages were isolated from IL-1Ra (black bars) or NaCl (gray bars)-treated TCR-Tg SCID mice (n = 10 mice per group), pooled for each group and tested, at various effector to target ratios, for their ability to suppress the in vitro proliferation of MOPC315 cells (mean of quadruplicates ±S.D). MΦ, macrophages.
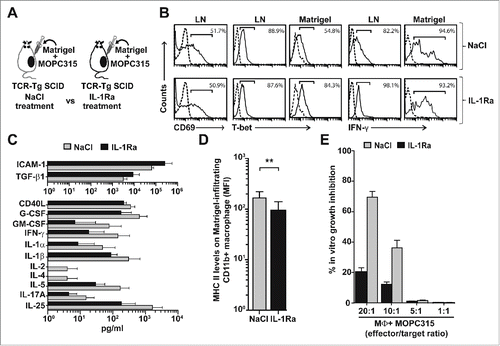
The effects of IL-1 neutralization on tumor-infiltrating macrophages were also investigated. Matrigel-infiltrating CD11b+ macrophages were significantly less activated as measured by surface MHC class II levels in mice that had received IL-1Ra when compared to controls (). In the next experiment, we tested whether this reduction in macrophage activation upon IL-1Ra treatment was associated with lower tumoricidal activity. Matrigel-infiltrating CD11b+ macrophages from the two experimental groups were tested for their ability to inhibit MOPC315 myeloma growth in vitro. In accordance with previous reports,Citation1,17,18 sorted Matrigel-infiltrating macrophages from control NaCl−treated TCR-Tg SCID mice strongly suppressed the in vitro growth of MOPC315 myeloma cells (, ˜70 percent myeloma growth inhibition at 20:1 effector to target ratio). In contrast, macrophages isolated from IL-1Ra-treated mice were essentially unable to suppress MOPC315 in vitro growth, even at the highest (20:1) effector to target ratio (, only 20% residual growth inhibition). Collectively, the data reveal that IL-1 has a central role in the antitumor effector functions of both tumor-specific Th1 cells (cytokine secretion) and tumor-infiltrating macrophages (activation and tumoricidal activity).
IFN-γ and IL-1β synergize to induce tumoricidal activity in macrophages
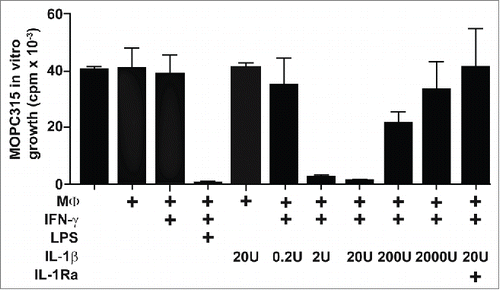
The effect of IL-1 on macrophage activation was further investigated in vitro using non-activated macrophages isolated from non-transgenic SCID mice. In accordance with a previous report,Citation1 Matrigel-infiltrating macrophages purified from SCID mice were unable to inhibit MOPC315 in vitro growth (). Addition of IFN-γ into the culture medium was not sufficient by itself to induce tumoricidal activity in macrophages. In contrast, when macrophages were treated with both IFN-γ and lipopolysaccharide (LPS), which is a well-known potent combination to render macrophages cytotoxic toward cancer cells,Citation21 MOPC315 in vitro growth was blocked (). IL-1β could not by itself induce tumoricidal activity of macrophages and was not directly toxic to MOPC315 cells (, S2 and S3). However, a combination of IFN-γ and an appropriate dose of IL-1β rendered macrophages able to efficiently inhibit the growth of cancer cells (). The synergy was abrogated by the addition of IL-1Ra, confirming that the observed effect was not the result of LPS contamination in the reagents used (). Thus, the combination of optimal doses of IFN-γ and IL-1β represents a potent means of shifting tumor-associated macrophages toward a tumoricidal phenotype. Together with our previous findings, these in vitro data strongly suggest that a main function of IL-1 in vivo in the immune response against cancer is to synergize with IFN-γ to induce tumoricidal activity in macrophages.
Figure 4. IFN-γ and IL-1β synergize to induce tumoricidal activity in macrophages. SCID mice (n = 12) were injected with MOPC315 in Matrigel. At day +8, Matrigel-infiltrating CD11b+ macrophages were purified using mAb-conjugated magnetic beads, pooled, treated as indicated (with IFN-γ, LPS, various concentrations of IL-1β, and IL-1Ra), and tested for their ability to suppress the in vitro proliferation of MOPC315 (effector to target ratio 10:1). MΦ, macrophages. Data are presented as mean of triplicates ±SD and representative for two independent experiments.
Discussion
To investigate the mechanism of myeloma eradication by idiotype-specific Th1 cells, we performed a series of tumor challenge experiments with TCR-Tg SCID mice using protocols to selectively block candidate effector molecules. The key function of IFN-γ for antitumor immunity has previously been demonstrated and was here confirmed Citation1,18,22,23 It has also been reported that TNFα, TRAIL, and TWEAK molecules, all members of the TNF superfamily, may be used by activated macrophages to kill cancer cells in vitro and in vivo.Citation24-27 However, when we neutralized TNFα, TRAIL, or TWEAK in TCR-Tg SCID mice, tumor prevention was not affected, suggesting that these molecules were not required for myeloma eradication. In contrast, experiments with IL-1Ra, which blocks both IL-1α and IL-1β, revealed that IL-1 was essential for tumor elimination by Th1 cells. The partial observed effect for IL-1Ra is presumably due to incomplete receptor blockade because of the very short half-life (<2 h in serum) that has been reported for IL-1Ra in vivo.Citation28 Thus, both IFN-γ and IL-1 are required for successful antitumor immunity mediated by tumor-specific Th1 cells. Our study reveals a central role of inflammation, and more specifically of the canonical pro-inflammatory cytokine IL-1, in enhancing Th1-mediated immunity against cancer.
To our knowledge, our data represent the first direct demonstration of the central role of IL-1 for successful antitumor immunity. There are, however, a few previous reports in mice suggesting that IL-1 may be used therapeutically to treat cancer. Intratumoral injection of IL-1α successfully cured mice from MethA sarcoma and B16 melanoma.Citation29 Moreover, IL-1α injected intramuscularly was effective in reducing the number of lung metastases in mice with Lewis lung carcinoma.Citation29 Intraperitoneal injection of IL-1β on days +6−8 of tumor growth caused complete regression of subcutaneous SA1 sarcoma and L5178Y lymphoma in mice.Citation30 Experiments with T-cell deficient mice and cell transfer suggested that the therapeutic effect of IL-1β consisted in stimulating the antitumor immunity mediated by CD4+ T cells.Citation30 Activated invasive RO1 T-lymphoma cells that displayed short-term IL-1α expression manifested reduced tumorigenicity and could be used to treat mice with lymphoma.Citation31 Similarly, fibrosarcoma cells transfected with IL-1α became strongly immunogenic and failed to generate tumors in mice.Citation32 It was proposed that IL-1α was activating the antitumor immune response mediated by CD8+ T cells and NK cells.Citation32 In summary, our data represent the first demonstration that IL-1 is required for successful antitumor immunity. However, the antitumor therapeutic potential of IL-1 has been suggested by several mouse studies and a role for CD4+ T cells, CD8+ T cells, and NK cells has been proposed.
We performed a series of experiments to clarify the role if IL-1 in antitumor immunity. IL-1 neutralization was shown to affect the antitumor functions of both tumor-specific Th1 cells and tumor-infiltrating macrophages. IL-1 had a central role in promoting a cancer-suppressive pro-inflammatory cytokine milieu at the incipient tumor site. In particular, secretion of the Th1-derived cytokines IL-2 and IFN-γ at the incipient tumor site was severely reduced by IL-1 blockade. Our study revealing the central role of IL-1 for T-cell mediated antitumor immunity are in accordance with published data suggesting a key function of IL-1 for T-cell biology in general, in particular for Th1 cells. For example, IL-1α has been reported to promote Th1 differentiation allowing successful immunity against the parasite Leishmania major.Citation33 Experiments with model antigens such as pigeon cytochrome C and ovalbumin revealed the importance of IL-1 for CD4+ T-cell expansion and survival.Citation34 An early study by Ralph Steinman and coworkers revealed that IL-1 could enhance T-cell dependent immunity by amplifying the function of dendritic cells.Citation35 More recently, IL-1 was shown to enhance the expansion and effector functions of CD8+ T cells that were specific for various antigens such as ovalbumin or pathogen-derived peptides.Citation36 Collectively, available data support a key role of the pro-inflammatory cytokines IL-1α and IL-β for T-cell mediated immunity against both pathogens and cancer.
We here report that in vivo blockade of IL-1α and IL-1β with IL-1Ra impaired the activation and tumoricidal activity of tumor-infiltrating macrophages. Moreover, IL-1 was shown in vitro to synergize with IFN-γ for induction of tumoricidal activity in macrophages. Previous in vitro studies revealed that two signals are required to render macrophages cytotoxic to cancer cells. IFN-γ was the first cytokine clearly identified to activate macrophages to become tumoricidal, when given in combination with dead bacteria or bacterial products such as LPS.Citation7,37 It is now established that LPS activation of macrophages is mediated through toll-like receptor-4 (TLR4) ligation and signaling.Citation38 As the IL-1R and TLR-4 share a common intracellular signaling pathway,Citation39 it is not unexpected that IL-1 may replace LPS for rendering macrophages tumoricidal. In fact, an in vitro study by Hori et al. showed that combined use of IFN-γ and IL-1 could render macrophages tumoricidal against TNFα-insensitive P815 murine mastocytoma cells, in a concentration dependent manner.Citation40 This is consistent with our findings, showing that tumor-infiltrating macrophages that do not demonstrate an antitumor activity can become tumoricidal when treated with a combination of IFN-γ and optimal concentrations of IL-1β. The observation that high concentrations of IL-1β failed to activate macrophages, even in the presence of IFN-γ, indicates a negative feedback loop in the induction of macrophages tumoricidal activity. Niinobu and colleagues reported that high-levels of nitric oxide released from cytokine-activated macrophages (IFN-γ, TNF-α,, and IL-1β) may sensitize macrophages themselves to Fas-mediated apoptosis.Citation41 They further hypothesized that this mechanism may be a negative feedback loop serving to promote resolution of inflammation by accelerating deletion of macrophages by apoptosis.Citation41 Thus, our data on the induction of macrophage tumoricidal activity by optimal concentrations of IL-1 in conjunction with IFN-γ confirm previous in vitro observations and validate these findings in vivo using a mouse model for cancer eradication mediated by Th1-activated macrophages.
In a previous study with the same model system,Citation18 we measured 33 cytokines at the incipient tumor site at one single time point, namely day +8, which represents the peak of the antitumor immune response. We have now expanded this work by including several additional time points (day +2, +5, and +11) and 14 new cytokines (CCL11, CCL20, soluble CD40L, soluble ICAM-1, IL-17F, IL-21, IL-22, IL-23p19, IL-25, IL-27p28, IL-31, IL33, TGF-β2, TGF-β3). The kinetics data revealed that the increased levels of inflammatory cytokines (such as IL-1, IL-6, and TNF-α) and the activation of tumor-infiltrating macrophages were not restricted to day +8 but were observed during the whole process of myeloma rejection, from day +5 until day +11. All the new cytokines investigated were found to be significantly either upregulated (CCL11, CCL20, soluble CD40L, IL-17F, IL-21, IL-22, IL-23p19, IL-25, IL-27p28, IL-31, and IL33) or downregulated (soluble ICAM-1, TGF-β2, and TGF-β3) at one or several time points. Several of these cytokines associated with cancer rejection should deserve further investigations. In particular, IL-21, IL-27, and IL33, that were all upregulated at three time points investigated (day +5, +8, and +11) may be particularly promising candidates for cancer immunotherapy. In fact, IL-21 has already been used to treat patients with metastatic melanoma and renal cell carcinoma in phase I/II trials and promising antitumor activities were reported.Citation42-44 IL-27 was shown to be able to inhibit the growth of human multiple myeloma and acute myeloid leukemia cells injected into immunodeficient mice.Citation45-47 Moreover, the alarmin IL-33 has been reported, in mouse models, to enhance antitumor immune responses mediated by Th1 cells, CD8+ T cells, and NK cells.Citation48-50
The role of inflammation and inflammatory cytokines in cancer is controversial. On one hand, inflammation is widely considered to be detrimental in terms of cancer occurrence, growth and metastasis.Citation10,12,51-53 For instance, it is well documented that chronic inflammatory diseases such as ulcerative colitis, gastritis, and rheumatoid arthritis lead to increased risk of developing colorectal cancer, gastric cancer, and lymphoma, respectively.Citation54-56 High IL-6 serum levels were reported to be associated with shorter survival of patients with B-cell malignancies or lung cancer.Citation57-59 Studies in mice have documented tumor-promoting effects of IL-1α and IL-1β, in particular for cancer metastasis and tumor angiogenesis.Citation60,61 Furthermore, epidemiological studies indicated that daily intake of aspirin, a non-steroidal anti-inflammatory drug, was associated with reduced cancer risk.Citation62-64 On the basis of these observations, dampening inflammation has been suggested as a novel strategy to fight cancer.Citation10,51-53 Our mouse data suggest that IL-1 blockade may weaken T-cell mediated antitumor immunity and thereby may potentially have a detrimental effect on patients with cancer.
Cancer-suppressive aspects of inflammation are also well documented (reviewed in).Citation9 For example, high densities of tumor-infiltrating immune cells, which is a typical sign of inflammation, have been shown to be associated with longer patient survival for several malignancies including ovarian, colorectal, and breast cancers.Citation2,6,65,65 Certain types of cancer chemotherapies appear to stimulate antitumor immunity and mouse studies revealed a key role of IL-1β in this process.Citation67 Therefore, considering inflammation as a cancer-promoting process only is likely to be a misleading oversimplification.Citation9 To solve the controversy, we have proposed that the opposing effects of inflammation in various cancer situations may reflect the existence of various types of inflammations.Citation9 On the basis of our mouse studies with the MOPC315 myeloma, we have previously reported that successful cancer immunosurveillance mediated by tumor-specific CD4+ T cells was consistently associated with elevated local levels of both pro-inflammatory (IL-1α, IL-1β, and IL-6) and Th1-associated cytokines (IFNγ, IL-2 and IL-12).Citation18 Cancer eradication was achieved by collaboration between tumor-specific Th1 cells and tumor-infiltrating, antigen-presenting macrophages. Th1 cells induced secretion of IL-1β and IL-6 by macrophages. Th1-derived IFNγ was shown to render macrophages directly cytotoxic to cancer cells, and to induce macrophages to secrete angiostatic chemokines. From this previous work, we concluded that inflammation, when driven by tumor-specific Th1 cells, was protective against cancer.Citation9,18 Our present study provides direct pieces of evidence for a beneficial function of certain types of inflammations against cancer by revealing the central role of IL-1 in enhancing Th1-mediated antitumor immunity.
Materials and Methods
Mice and injection of tumor cells
Heterozygous idiotype (λ2315)-specific TCR-Tg SCID miceCitation16 or SCID littermates on BALB/c background were bred and housed at the Department of Comparative Medicine, Oslo University Hospital Rikshospitalet, Oslo, Norway. MOPC315 is a transplantable BALB/c plasmacytoma obtained from the American Type Culture Collection (ATCC) and propagated as in vitro growing cells. Adult mice were injected s.c., in the interscapular region or in the flank, with 1–1.6 × 105 MOPC315 cells suspended either in 100 μL phosphate-buffered saline (PBS, Gibco) or 250 μL Growth Factor-Reduced Matrigel (BD Biosciences). Tumor growth was followed over time by palpation. Mice with a tumor diameter ≥10 mm were euthanized. The study was approved by the National Committee for Animal Experiments (Oslo, Norway).
Matrigel cytokine assay
A detailed protocol for the Matrigel cytokine assay Citation18 is available at www.nature.com/protocolexchange/protocols/2130. Briefly, MOPC315 cells were mixed with ice-cold Matrigel and 250 μL of the mixture was carefully injected s.c. into each mouse under anesthesia. Matrigel, which is liquid at + 4°C, gelifies at body temperature and forms a gel plug containing the cancer cells. At various time points after injection, mice were sacrificed and the s.c. Matrigel plugs were excised and treated with collagenase. Dissolved Matrigel solution was centrifuged and the cell pellet was analyzed by flow cytometry. Cytokine levels in Matrigel supernatants were measured by Luminex technology using single-plex or multiplex bead assays (Bio-Plex, Bio-Rad Laboratories). Samples were analyzed as singlets or duplicates, and standards in duplicates, using a Luminex-100 instrument with Bio-Plex Manager 6.0 software (Bio-Rad Laboratories). The following 46 cytokines were measured: CCL2, CCL3, CCL4, CCL5, CCL11, CCL20, soluble CD40L, CXCL1, CXCL2, CXCL9, FGF-basic, G-CSF, GM-CSF, soluble ICAM-1, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17A, IL-17F, IL-18, IL-21, IL-22, IL-23p19, IL-25, IL-27p28, IL-31, IL-33, LIF, M-CSF, PDGF-bb, TFG-β, TFG-β, TFG-β, TNFα, and VEGF.
Analysis of cells by flow cytometry
Single-cell suspensions from draining axillary LN and Matrigel plugs were obtained by use of a stainless steel sieve (Sigma). Non-specific antibody binding was blocked by incubation with PBS containing 30% heat-inactivated (56°C, 30 min) normal rat serum and 100 μg/mL anti-FcγRII/III mAb (clone 2.4G2, ATCC). Cells were stained for 15 min on ice with specific mAb in PBS supplemented with 0.5% bovine serum albumin (Biotest). The following commercially available mAb were used, conjugated with fluorescein, phycoerythrin, allophycocyanin or biotin: CD4+ (GK1.5), CD11b (M1/70), IFNγ (XMG1.2), MHC class II I-A/I-E (M5/114.15.2), TCR β chain (H57-597) (BD Biosciences); CD11b (3A33), CD69 (H1.2F3) (Southern Biotechnology); T-bet (4B10) (eBioscience). GB113 is a clonotype-specific mAb which recognizes the λ2315-specific TCR expressed by the TCR-Tg SCID mice.Citation68 GB113 and 2.4G2 (anti-FcγRII/III) mAb were affinity-purified, and GB113 was further biotinylated in our laboratory. Biotinylated mAb were detected with streptavidin conjugated to peridinin chlorophyll protein (BD Biosciences). For intracellular cytokine detection, cells were stimulated with phorbol myristate acetate and ionomycin (both from Sigma) in the presence of monensin (GolgiStop, BD Biosciences) in vitro for 4 h before staining with Cytofix/Cytoperm Plus reagents (BD Biosciences) and specific mAb. Quadruple-stained cells were analyzed on a FACScalibur instrument with CellquestPro (BD Biosciences) and FlowJo version 10 (FlowJo) softwares.
In vivo blockade of IFN-γ, TNFα, TWEAK, and TRAIL
In accordance with previously established protocols,Citation1,69-72 mice were injected intraperitoneally (i.p.) three times a week with 250 μg blocking mAb against IFN-γ (XMG2.1, ATCC), TNFα (μP6-XT22), TWEAK (MTW-1), or TRAIL (N2B2), or with isotype-matched control rat IgG1 (Y13-259, ATCC) or rat IgG2a (Y13-238, ATCC) mAb.
Neutralization of the common receptor for IL-1α and IL-1β with IL-1Ra
For tumor challenge experiments (), Alzet osmotic pumps (model 2004; 0.25 μL/h, 28 d) were filled with 200 μL of 6.7 mg/mL IL-1Ra (Anakinra/Kineret, Biovitrum) or vehicle (9 mg/mL NaCl) and implanted s.c. on the flank of anesthetized mice. MOPC315 myeloma cells (1.6 × 105) suspended in 100 μl PBS were injected s.c. where the delivery tip of the osmotic pump was situated. For short-term in vivo experiments (), mice were treated s.c. daily with 100 μl of 400 μg/mL IL-1Ra or vehicle (9 mg/mL NaCl).
Growth inhibition assay with in vivo activated macrophages
TCR-Tg SCID mice (n = 10) were injected s.c. with MOC315 in Matrigel and treated s.c. daily with IL-1Ra or vehicle as described above. Matrigel-infiltrating CD11b+ cells were isolated by FACSAria (BD Biosciences) at day +8. Sorted CD11b+ cells (≥95% pure) were pooled for each group, irradiated (2,000 rad) and added at various effector/target ratios to MOPC315 cell cultures (104 tumor cells per well) in quadruplicates. Cultures were pulsed with [3H]thymidine (Hartmann Analytic) after 48 h and harvested 12 h later. Inc. [3H]thymidine was measured on a 1450 MicroBeta Trilux microplate scintillation counter (Perkin Elmer).
Growth inhibition assay with in vitro activated macrophages
SCID mice (n = 12) were injected with MOPC315 in Matrigel. At day +8, CD11b+ cells were isolated using mAb-conjugated magnetic beads (MACS, Miltenyi Biotec). Isolated cells were counted and seeded (105 cells per well) in triplicates in 96-well flat-bottom cell culture plates (Costar). After 2 h incubation at 37°C in 5% CO2, the cells were washed three times with fresh media (RPMI1640, Gibco), before addition of IFN-γ (4U, PeproTech), IL-1β (PeproTech), LPS from Escherichia coli (25 ng, Sigma), [3H]thymidine, and 104 MOPC315 cells. The plates were incubated at 37°C in 5% CO2 and harvested after 18 h. Inc. [3H]thymidine was measured on a 1450 MicroBeta Trilux microplate scintillation counter.
Statistical analysis
For tumor challenge experiments, differences in survival were analyzed utilizing the log-rank test. Flow cytometry data and cytokine levels were analyzed using the Mann–Whitney test. Statistical analysis was performed using GraphPad Prism 5 software (GraphPad Software).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Author Contributions
O.A.W.H., A.C., and K.B.L. performed the experiments, collected the data, and prepared the figures. H.Y. provided key reagents (mAbs). All authors analyzed and discussed the data. O.A.W.H. and A.C. designed the study and wrote the manuscript. K.B.L., H.Y., and B.B. contributed in writing the manuscript.
1039763_Figure_S3.ai
Download (1.1 MB)1039763_Figure_S2.ai
Download (1.2 MB)1039763_Figure_S1.ai
Download (1.2 MB)Acknowledgments
We thank Inger Øynebråten, Anders Aune Tveita, and Peter O. Hofgaard for critical reading of the manuscript.
Funding
This work was supported by grants from the Southern and Eastern Norway Regional Health Authority, the Research Council of Norway, Anders Jahres fond til vitenskapens fremme, Legatet til Henrik Homans Minde, and Legatet til mine om H. G. og Andrine Berg og deres sønn Hans Gysler Berg.
References
- Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity 2005; 22:371-83; PMID:15780993; http://dx.doi.org/10.1016/j.immuni.2005.02.003
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960-4; PMID:17008531; http://dx.doi.org/10.1126/science.1129139
- Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, Sorlie T, Warnberg F, Haakensen VD, Helland A et al. Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci U S A 2012; 109:2802-7; PMID:21908711; http://dx.doi.org/10.1073/pnas.1108781108
- Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344:641-5; PMID:24812403; http://dx.doi.org/10.1126/science.1251102
- Haabeth OA, Tveita AA, Fauskanger M, Schjesvold F, Lorvik KB, Hofgaard PO, Omholt H, Munthe LA, Dembic Z, Corthay A et al. How Do CD4(+) T Cells Detect and Eliminate Tumor Cells That Either Lack or Express MHC Class II Molecules? Front Immunol 2014; 5:174; PMID:24782871; http://dx.doi.org/10.3389/fimmu.2014.00174
- Corthay A. Does the Immune System Naturally Protect Against Cancer? Front Immunol 2014; 5:197; PMID:24860567; http://dx.doi.org/10.3389/fimmu.2014.00197
- Schreiber RD, Pace JL, Russell SW, Altman A, Katz DH. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. Journal of immunology 1983; 131:826-32; PMID:NOT_FOUND
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002; 23:549-55; PMID:12401408; http://dx.doi.org/10.1016/S1471-4906(02)02302-5
- Haabeth OA, Bogen B, Corthay A. A model for cancer-suppressive inflammation. Oncoimmunology 2012; 1:1146-55; PMID:23170261; http://dx.doi.org/10.4161/onci.21542
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008; 454:436-44; PMID:18650914; http://dx.doi.org/10.1038/nature07205
- Heusinkveld M, de Vos van Steenwijk PJ, Goedemans R, Ramwadhdoebe TH, Gorter A, Welters MJ, van Hall T, van der Burg SH. M2 macrophages induced by prostaglandin E2 and IL-6 from cervical carcinoma are switched to activated M1 macrophages by CD4+ Th1 cells. J Immunol 2011; 187:1157-65; PMID:21709158; http://dx.doi.org/10.4049/jimmunol.1100889
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 2013; 339:286-91; PMID:23329041; http://dx.doi.org/10.1126/science.1232227
- DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009; 16:91-102; PMID:19647220; http://dx.doi.org/10.1016/j.ccr.2009.06.018
- Bogen B, Gleditsch L, Weiss S, Dembic Z. Weak positive selection of transgenic T cell receptor-bearing thymocytes: importance of major histocompatibility complex class II, T cell receptor and CD4 surface molecule densities. Eur J Immunol 1992; 22:703-9; PMID:1547816; http://dx.doi.org/10.1002/eji.1830220313
- Lauritzsen GF, Weiss S, Dembic Z, Bogen B. Naive idiotype-specific CD4+ T cells and immunosurveillance of B-cell tumors. Proc Natl Acad Sci U S A 1994; 91:5700-4; PMID:7911244; http://dx.doi.org/10.1073/pnas.91.12.5700
- Bogen B, Munthe L, Sollien A, Hofgaard P, Omholt H, Dagnaes F, Dembic Z, Lauritzsen GF. Naive CD4+ T cells confer idiotype-specific tumor resistance in the absence of antibodies. Eur J Immunol 1995; 25:3079-86; PMID:7489746; http://dx.doi.org/10.1002/eji.1830251114
- Corthay A, Lundin KU, Lorvik KB, Hofgaard PO, Bogen B. Secretion of tumor-specific antigen by myeloma cells is required for cancer immunosurveillance by CD4+ T cells. Cancer research 2009; 69:5901-7; PMID:19567679; http://dx.doi.org/10.1158/0008-5472.CAN-08-4816
- Haabeth OA, Lorvik KB, Hammarstrom C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun 2011; 2:240; PMID:21407206; http://dx.doi.org/10.1038/ncomms1239
- Lorvik KB, Bogen B, Corthay A. Fingolimod blocks immunosurveillance of myeloma and B-cell lymphoma resulting in cancer development in mice. Blood 2012; 119:2176-7; PMID:22383793; http://dx.doi.org/10.1182/blood-2011-10-388892
- Lorvik KB, Haabeth OAW, Clancy T, Bogen B, Corthay A. Molecular profiling of tumor-specific Th1 cells activated in vivo. OncoImmunology 2013; 2:e24383; PMID:23762808; http://dx.doi.org/10.4161/onci.24383
- Peri G, Polentarutti N, Sessa C, Mangioni C, Mantovani A. Tumoricidal activity of macrophages isolated from human ascitic and solid ovarian carcinomas: augmentation by interferon, lymphokines and endotoxin. Int J Cancer 1981; 28:143-52; PMID:6172388; http://dx.doi.org/10.1002/ijc.2910280206
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001; 410:1107-11; PMID:11323675; http://dx.doi.org/10.1038/35074122
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 1998; 95:7556-61; PMID:9636188; http://dx.doi.org/10.1073/pnas.95.13.7556
- Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A 1975; 72:3666-70; PMID:1103152; http://dx.doi.org/10.1073/pnas.72.9.3666
- Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med 1999; 189:1343-54; PMID:10209050; http://dx.doi.org/10.1084/jem.189.8.1343
- Nakayama M, Kayagaki N, Yamaguchi N, Okumura K, Yagita H. Involvement of TWEAK in interferon gamma-stimulated monocyte cytotoxicity. J Exp Med 2000; 192:1373-80; PMID:11067885; http://dx.doi.org/10.1084/jem.192.9.1373
- Halaas O, Vik R, Ashkenazi A, Espevik T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand J Immunol 2000; 51:244-50; PMID:10736093; http://dx.doi.org/10.1046/j.1365-3083.2000.00671.x
- Granowitz EV, Porat R, Mier JW, Pribble JP, Stiles DM, Bloedow DC, Catalano MA, Wolff SM, Dinarello CA. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin-1 receptor antagonist in healthy humans. Cytokine 1992; 4:353-60; PMID:1420996; http://dx.doi.org/10.1016/1043-4666(92)90078-6
- Nakamura S, Nakata K, Kashimoto S, Yoshida H, Yamada M. Antitumor effect of recombinant human interleukin 1 alpha against murine syngeneic tumors. Jpn J Cancer Res 1986; 77:767-73; PMID:3093425
- North RJ, Neubauer RH, Huang JJ, Newton RC, Loveless SE. Interleukin 1-induced, T cell-mediated regression of immunogenic murine tumors. Requirement for an adequate level of already acquired host concomitant immunity. J Exp Med 1988; 168:2031-43; PMID:3143799; http://dx.doi.org/10.1084/jem.168.6.2031
- Voronov E, Weinstein Y, Benharroch D, Cagnano E, Ofir R, Dobkin M, White RM, Zoller M, Barak V, Segal S et al. Antitumor and immunotherapeutic effects of activated invasive T lymphoma cells that display short-term interleukin 1alpha expression. Cancer Res 1999; 59:1029-35; PMID:10070959
- Song X, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, Shendler Y, Bjorkdahl O, Segal S, Dinarello CA et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol 2003; 171:6448-56; PMID:14662844; http://dx.doi.org/10.4049/jimmunol.171.12.6448
- Von Stebut E, Ehrchen JM, Belkaid Y, Kostka SL, Molle K, Knop J, Sunderkotter C, Udey MC. Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med 2003; 198:191-9; PMID:12860932; http://dx.doi.org/10.1084/jem.20030159
- Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A 2009; 106:7119-24; PMID:19359475; http://dx.doi.org/10.1073/pnas.0902745106
- Koide SL, Inaba K, Steinman RM. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med 1987; 165:515-30; PMID:2950198; http://dx.doi.org/10.1084/jem.165.2.515
- Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med 2013; 210:491-502; PMID:23460726; http://dx.doi.org/10.1084/jem.20122006
- Schultz RM, Kleinschmidt WJ. Functional identity between murine gamma interferon and macrophage activating factor. Nature 1983; 305:239-40; PMID:6412144; http://dx.doi.org/10.1038/305239a0
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282:2085-8; PMID:9851930; http://dx.doi.org/10.1126/science.282.5396.2085
- Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A 1998; 95:588-93; PMID:9435236; http://dx.doi.org/10.1073/pnas.95.2.588
- Hori K, Mihich E, Ehrke MJ. Role of tumor necrosis factor and interleukin 1 in gamma-interferon-promoted activation of mouse tumoricidal macrophages. Cancer Res 1989; 49:2606-14; PMID:2496917
- Niinobu T, Fukuo K, Yasuda O, Tsubakimoto M, Mogi M, Nishimaki H, Morimoto S, Ogihara T. Negative feedback regulation of activated macrophages via Fas-mediated apoptosis. American journal of physiology Cell physiology 2000; 279:C504-9; PMID:10913017
- Petrella TM, Tozer R, Belanger K, Savage KJ, Wong R, Smylie M, Kamel-Reid S, Tron V, Chen BE, Hunder NN et al. Interleukin-21 has activity in patients with metastatic melanoma: a phase II study. J Clin Oncol 2012; 30:3396-401; PMID:22915661; http://dx.doi.org/10.1200/JCO.2011.40.0655
- Santegoets SJ, Turksma AW, Powell Jr DJ, Hooijberg E, de Gruijl TD. IL-21 in cancer immunotherapy: At the right place at the right time. Oncoimmunology 2013; 2:e24522; PMID:23894713; http://dx.doi.org/10.4161/onci.24522
- Bhatia S, Curti B, Ernstoff MS, Gordon M, Heath EI, Miller WH, Jr., Puzanov I, Quinn DI, Flaig TW, VanVeldhuizen P et al. Recombinant interleukin-21 plus sorafenib for metastatic renal cell carcinoma: a phase 1/2 study. J Immunother Cancer 2014; 2:2; PMID:24829759; http://dx.doi.org/10.1186/2051-1426-2-2
- Cocco C, Giuliani N, Di Carlo E, Ognio E, Storti P, Abeltino M, Sorrentino C, Ponzoni M, Ribatti D, Airoldi I. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin Cancer Res 2010; 16:4188-97; PMID:20587591; http://dx.doi.org/10.1158/1078-0432.CCR-10-0173
- Zorzoli A, Di Carlo E, Cocco C, Ognio E, Ribatti D, Ferretti E, Dufour C, Locatelli F, Montagna D, Airoldi I. Interleukin-27 inhibits the growth of pediatric acute myeloid leukemia in NOD/SCID/Il2rg−/− mice. Clin Cancer Res 2012; 18:1630-40; PMID:22383738; http://dx.doi.org/10.1158/1078-0432.CCR-11-2432
- Swarbrick A, Junankar SR, Batten M. Could the properties of IL-27 make it an ideal adjuvant for anticancer immunotherapy? Oncoimmunology 2013; 2:e25409; PMID:24083081; http://dx.doi.org/10.4161/onci.25409
- Haraldsen G, Balogh J, Pollheimer J, Sponheim J, Kuchler AM. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol 2009; 30:227-33; PMID:19359217; http://dx.doi.org/10.1016/j.it.2009.03.003
- Villarreal DO, Wise MC, Walters JN, Reuschel EL, Choi MJ, Obeng-Adjei N, Yan J, Morrow MP, Weiner DB. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res 2014; 74:1789-800; PMID:24448242; http://dx.doi.org/10.1158/0008-5472.CAN-13-2729
- Gao X, Wang X, Yang Q, Zhao X, Wen W, Li G, Lu J, Qin W, Qi Y, Xie F et al. Tumoral Expression of IL-33 Inhibits Tumor Growth and Modifies the Tumor Microenvironment through CD8+ T and NK Cells. J Immunol 2015; 194:438-45; PMID:25429071; http://dx.doi.org/10.4049/jimmunol.1401344
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539-45; PMID:11229684; http://dx.doi.org/10.1016/S0140-6736(00)04046-0
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860-7; PMID:12490959; http://dx.doi.org/10.1038/nature01322
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/10.1016/j.cell.2010.01.025
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001; 48:526-35; PMID:11247898; http://dx.doi.org/10.1136/gut.48.4.526
- Polk DB, Peek RM, Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer 2010; 10:403-14; PMID:20495574; http://dx.doi.org/10.1038/nrc2857
- Ekstrom K, Hjalgrim H, Brandt L, Baecklund E, Klareskog L, Ekbom A, Askling J. Risk of malignant lymphomas in patients with rheumatoid arthritis and in their first-degree relatives. Arthritis Rheum 2003; 48:963-70; PMID:12687538; http://dx.doi.org/10.1002/art.10939
- Pelliniemi TT, Irjala K, Mattila K, Pulkki K, Rajamaki A, Tienhaara A, Laakso M, Lahtinen R. Immunoreactive interleukin-6 and acute phase proteins as prognostic factors in multiple myeloma. Finnish Leukemia Group. Blood 1995; 85:765-71; PMID:7530507
- Preti HA, Cabanillas F, Talpaz M, Tucker SL, Seymour JF, Kurzrock R. Prognostic value of serum interleukin-6 in diffuse large-cell lymphoma. Ann Intern Med 1997; 127:186-94; PMID:9245223; http://dx.doi.org/10.7326/0003-4819-127-3-199708010-00002
- Martin F, Santolaria F, Batista N, Milena A, Gonzalez-Reimers E, Brito MJ, Oramas J. Cytokine levels (IL-6 and IFN-gamma), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 1999; 11:80-6; PMID:10080883; http://dx.doi.org/10.1006/cyto.1998.0398
- Bani MR, Garofalo A, Scanziani E, Giavazzi R. Effect of interleukin-1-beta on metastasis formation in different tumor systems. J Natl Cancer Inst 1991; 83:119-23; PMID:1988686; http://dx.doi.org/10.1093/jnci/83.2.119
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci U S A 2003; 100:2645-50; PMID:12598651; http://dx.doi.org/10.1073/pnas.0437939100
- Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012; 379(9826):1591-601; PMID:22440947
- Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, Lee R, Belch JF, Wilson M, Mehta Z et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet 2012; 379(9826):1602-12; PMID:22440946
- Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol 2012; 13(5):518-27; PMID:22440112
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:12529460; http://dx.doi.org/10.1056/NEJMoa020177
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 2011; 29:1949-55; PMID:21483002; http://dx.doi.org/10.1200/JCO.2010.30.5037
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009; 15:1170-8; PMID:19767732; http://dx.doi.org/10.1038/nm.2028
- Bogen B, Lauritzsen GF, Weiss S. A stimulatory monoclonal antibody detecting T cell receptor diversity among idiotype-specific, major histocompatibility complex-restricted T cell clones. Eur J Immunol 1990; 20:2359-62; PMID:1700755; http://dx.doi.org/10.1002/eji.1830201030
- Inoue A, Koh CS, Yahikozawa H, Yanagisawa N, Yagita H, Ishihara Y, Kim BS. The level of tumor necrosis factor-alpha producing cells in the spinal cord correlates with the degree of Theiler's murine encephalomyelitis virus-induced demyelinating disease. Int Immunol 1996; 8:1001-8; PMID:8757945; http://dx.doi.org/10.1093/intimm/8.7.1001
- Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res 2000; 60:5862-9; PMID:11059784
- Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7:94-100; PMID:11135622; http://dx.doi.org/10.1038/83416
- Yoriki R, Akashi S, Sho M, Nomi T, Yamato I, Hotta K, Takayama T, Matsumoto S, Wakatsuki K, Migita K et al. Therapeutic potential of the TWEAK/Fn14 pathway in intractable gastrointestinal cancer. Exp Ther Med 2011; 2:103-8; PMID:22977477
