Abstract
Interleukin-6, a cytokine produced particularly by triple-negative breast cancers, strongly inhibits T cell responses in the tumor microenvironment. Here we tested cryoablation combined with Meriva (a lecithin delivery system of curcumin with improved bioavailability) in mice with metastatic breast cancer (4T1). Cryoablation involves killing of tumor cells through freezing and thawing, resulting in recruitment of tumor-specific T cells, while curcumin stimulates T cells through the reduction of IL-6 in the TME. Cryoablation plus Meriva accumulated and activated CD8+ T cells to multiple tumor-associated antigens such as Mage-b and Survivin (both expressed by 4T1 tumors). This correlated with a nearly complete reduction of 4T1 primary tumors and lung metastases while little effect was observed from saline or Meriva alone (28 d after tumor cell injection). The survival rate in the group of cryoablation plus Meriva was significantly improved compared to all control groups. Using a less aggressive 4T1 model expressing luciferase (4T1.2luc3), we demonstrated that all mice receiving saline or Meriva developed metastases in the lungs and a primary tumor (38 d after tumor cell injection; and died soon after that), but not the mice receiving cryoablation or cryoablation plus Meriva. However, on day 58 the mice receiving cryoablation developed 4T1.2luc3 metastases in the lungs, while mice receiving cryoablation plus Meriva were free of metastases. These results strongly suggest that cryoablation delayed the development of lung metastases on the short-term, but Meriva administered after cryoablation was significantly better in delaying the development of lung metastases and survival on the long-term.
Introduction
Success of cancer vaccination is strongly hampered by immune suppression in the tumor microenvironment (TME). Interleukin (IL)-6 is produced by many types of cancers such as ovarian, lung, and colon cancer, and particularly by metastatic breast cancer.Citation1-3 In a study of patients with metastatic breast cancer it has been shown that 57% (52/90) of the human samples showed IL-6 cytoplasmic immunostaining.Citation4 In another study with 45 breast cancer cases 68.9% of the tumors were positive for IL-6, IL-6 receptor α, and gp130.Citation5 Triple-negative breast cancers (TNBCs) are enriched for stem-like breast cancer cells (CD44+/CD24−/low), which are typically aggressive and highly resistant to current therapies.Citation6-9 These stem-like breast cancer cells produce high levels of IL-6, and have the capacity to metastasize.Citation10 Moreover, IL-6 is capable of converting dormant breast cancer cells into an actively growing tumor.
IL-6 is also a potent regulator of dendritic cell (DC) differentiation in vivo, and is able to turn on the expression of signal transducer and activator of transcription (STAT)3 in DC.Citation11 However, high levels of STAT3 can prevent DC from maturation and subsequent presentation of antigens.Citation12 This in turn may lead to T cell unresponsiveness. In a previous study we found high levels of IL-6 produced by breast cancer cells in an aggressive TNBC mouse model 4T1. This IL-6 strongly reduced T cell responses to Mage-b, but elimination of IL-6 using anti-IL-6 antibodies restored T cell responses to Mage-b in vitro.Citation13
Agents that are able to inhibit IL-6 are of great value for immunotherapies against TNBC and other IL-6-producing cancers. One such agent could be curcumin. Curcumin (diferulolylmethane), a polyphenol derived from the plant Curcuma longa, commonly called turmeric, has a broad anticancer effect through downregulating transcription of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) thereby affecting downstream genes such as c-myc, B-cell lymphoma (Bcl)-2, cyclooxygenase (COX)-2, nitric oxide synthase (NOS), Cyclin D1, tumor necrosis factor (TNF)α and matrix metalloproteinase (MMP)-9.Citation14 Curcumin is also known for reducing immune suppressive cytokines such as IL-6 through the NFκB pathway.Citation15 It has been shown that curcumin improves therapeutic efficacy of doxorubicin or of B16-R lysate against B16-R melanoma in mice, and that curcumin prevents tumor-induced T cell apoptosis in mice.Citation16 We demonstrated that curcumin improved therapeutic efficacy of a Listeria-based vaccine expressing tumor-associated antigen (TAA) Mage-b by reducing the production of IL-6 and increasing the production of IL-12, in correlation with improved T cell responses in blood of the TNBC model 4T1.Citation17 While a strong effect was observed on the lung metastases, little effect was observed on the primary tumors. A disadvantage of this Listeria-Mage-b vaccine is the presence of one TAA in the vaccine. Since most cancers are genetically unstable, more than one TAA in a cancer vaccine is highly recommendable. Cryoablation of tumors is a new approach to create multi-TAA vaccines from patient's own tumors, which can overcome the heterogeneity of the genetically unstable tumors.
Cryoablation has emerged a successful alternative to surgical resection to treat many types of inoperable tumors including kidney, liver, adrenal, lung, and prostate and is therefore clinically feasible.Citation18-21 Cryoablation involves the insertion of a probe into a tumor nodule in order to administer tissue ablative freezing temperatures, which will lead to immediate disruption of the tissue structure and cellular damage.Citation22 The necrosis observed in the ablated lesion is mediated by mechanical disruption from crystallization, osmotic changes, and vascular stasis.Citation23 The necrotic tumor lesion remains within the body, and TAA-specific T cells are recruited through the release of multiple TAAs by the cryo-killed tumor cells and the release of high mobility group box (HMGB)-1 protein [that binds to the receptor for advanced glycation endproducts (RAGE) and toll-like receptors (TLRs) on DCs], resulting in increased TAA presentation by the DCs.Citation24 However, these TAA-specific T cells need help because of the strong immune suppression in the TME. Based on our discussion above, curcumin could be an excellent adjuvant to reduce immune suppression induced by IL-6 and to stimulate TAA-specific T cells in the TME.
In the study presented here we tested the combination of cryoablation and Meriva (the lecithin delivery form of curcumin with improved bioavailability)Citation25 and demonstrated a nearly complete elimination of the lung metastases and tumor in breast cancer model 4T1. This correlated with significant reduction in the production of IL-6 in the primary tumors and a significant improvement of CD8+ T cell responses to multiple TAAs of host's own tumor and lung metastases. Most importantly, we found that cryoablation and Meriva significantly improved the survival rate compared to all control groups, with minimal side effects. In summary, our results suggest that cryoablation and Meriva could be a safe and successful alternative for surgery followed by radiation and/or chemotherapy in patients with metastatic breast cancer.
Results
The effect of Meriva on IL-6, tumor and lung metastases in 4T1 model
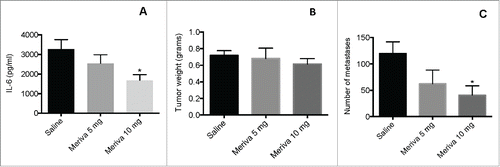
In a previous study we have shown that curcumin reduced IL-6 in primary tumors and that this correlated with improved T cell responses to TAA in mice with TNBC (4T1 model).Citation17 One of the disadvantages of curcumin is the poor bioavailability in vivo. In the current study we used Meriva, which is a lecithin delivery system of curcumin, endowed with enhanced bioavailability.Citation25 Various doses of Meriva (5, 10, and 25 mg per mouse) were tested [starting when tumors were 1–1.5 cm (largest diameter)] for its ability to reduce IL-6 production, and as shown in , 10 mg per mouse was the most successful dose (25 mg per mouse was less effective than 10 mg; data not shown). While Meriva alone had little effect on the primary tumors (), a significant decrease in the number of lung metastases () was observed at a dose of 10 mg/mouse.
Figure 1. Meriva reduced IL-6 production in 4T1 breast tumors. BALB/c mice were injected with 105 4T1 tumor cells in the mammary fat pad, and treated with various doses of Meriva (0, 5, 10, and 25 mg/mouse) orally (every 3 d for 14 d starting when tumors are 1–1.5 cm in diameter), and 2 d after the last treatment mice were euthanized and their tumors were homogenized and analyzed for the presence of IL-6 by ELISA (A). In addition the effect of Meriva was measured on tumor weight (B) and lung metastases (C). Representative of two experiments, n = 3 mice per group. The error bars represent the SEM. All groups were compared to the Saline group. Mann-Whitney test. *P < 0.05. Values P < 0.05 were considered statistically significant.
Effect of cryoablation and Meriva on MDSC in 4T1 model
Since cryoablation eliminates primary tumors we hypothesized that it may reduce the number of myeloid-derived suppressor cells (MDSC) because tumors attract MDSC. Here, we analyzed the effect of cryoablation and Meriva on MDSC. Mice were injected with 4T1 tumor cells in the mammary fat pad, and on day 14 (when tumors were 1–1.5 cm) treated with cryoablation. Three days after cryoablation, mice were orally fed with Meriva for 14 d at 10 mg per dose per mouse once every 3 d. All mice were euthanized at the end of the Meriva treatment (day 30 after tumor cell injection) and showed a significant reduction in the percentage of MDSC by cryoablation compared to the saline group (81.2% ± 6.1% to 41.7% ± 18.4%) (). A similar decrease was observed in the percentage of MDSC in the group of cryoablation plus Meriva compared to the saline group (81.2% ± 6.1% to 37.8% ± 18.5%), while Meriva did not affect the percentage of MDSC.
The effect of cryoablation plus Meriva on T cell responses to TAA in 4T1 model
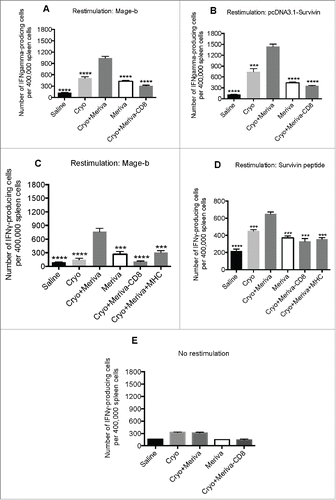
After showing that curcumin reduces the production of IL-6 in the primary tumors and cryoablation reduced the MDSC population, which are both strongly involved in T cell inhibition, we tested whether cryoablation plus Meriva could generate and stimulate CD8+ T cell responses to various TAA expressed by the 4T1 tumors and lung metastases. Mice were injected with 4T1 tumor cells in the mammary fat pad, and fed orally fed with Meriva as described above. All mice were euthanized at the end of the Meriva treatment and analyzed for CD8+ T cell responses to various TAAs such as Mage-b and Survivin in the spleen. Both TAAs are highly expressed in the lung metastases and tumor of the 4T1 model.Citation26 As shown in , the production of IFNγ by spleen cells restimulated with both TAAs Mage-b and Survivin of mice receiving cryoablation and Meriva, was significantly higher compared to all control groups. In addition, depletion of CD8+ T cells in the spleen cultures of mice receiving cryoablation and Meriva resulted in a significant reduction in the number of IFNγ-producing spleen cells compared to non-CD8+-depleted spleen cells of the same mice, indicating strong CD8+ T cell responses to TAA Mage-b and Survivin. To confirm that the CD8+ T cell responses were directed to TAA the experiment was repeated but now in the presence and absence of major histocompatibility complex (MHC) class I antibodies. The significant inhibition of the CD8+ T cell responses to Mage-b and Survivin confirmed that the CD8+ T cell responses were directed to both TAA (). No significant immune responses (production of IFNγ) were observed in cultures with CD8+-depleted and non-depleted spleen cells without any re-stimulation (), confirming the specificity of the re-stimulation with Mage-b and Survivin.
Figure 2. Cryoablation but not Meriva reduced the MDSC population in blood of 4T1 mice. BALB/c mice were injected with 105 4T1 tumor cells in the mammary fat pad and treated with cryoablation (20%) 14 d later. Meriva was administered (10 mg/300 μL Saline; orally), every 3 d for 14 d, starting on day 3 after cryoablation and then euthanized for analysis 2 d after the last treatment (day 30 after tumor cell injection). Myeloid-derived suppressor cells (MDSC)(CD11b+Gr1+) were analyzed in blood by flow cytometry and shown in flow cytometry profile (A) or averaged (B). The percentage of MDSC was determined in the total leucocyte population in blood. Representative of two experiments with n = 5 mice per group. The error bars represent the SEM. All groups were compared to cryoablation plus Meriva group. Mann-Whitney test. *P < 0.05. Values P < 0.05 were considered statistically significant.
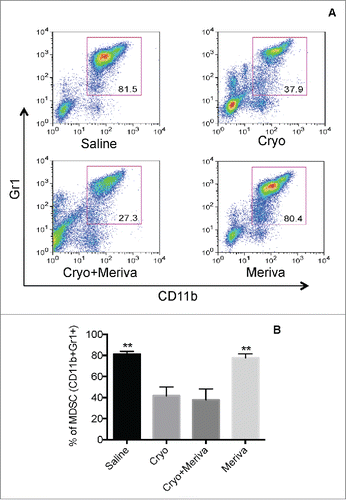
Figure 3. TAA-specific T cell responses generated through cryoablation were significantly improved by Meriva in 4T1 model. BALB/c mice were challenged with 4T1 tumor cells and treated with cryoablation plus Meriva as described in . Two days after the last treatment all mice were euthanized and analyzed for CD8+ T cell responses to TAA in vitro in the spleen by ELISPOT. CD8+ T cell responses to Mage-b (transfection of spleens of treated and control mice with pcDNA3.1-Mage plus pCMV-GM-CSF) (A), or to Survivin (transfection of spleens of treated and control mice with pcDNA3.1-Survivin plus pCMV-GM-CSF) (B) were analyzed, and a similar experiment was performed with or without anti-MHC Class I antibodies added to the wells as indicated in the figure and restimulated with pc-DNA3.1-Mage-b (C) or Survivin peptides (D). No restimulation was used as negative control (E). CD8+ T cells were depleted using magnetic beads technique. The results of two experiments were averaged with n = 5 mice per group. The error bars represent the SEM. All groups were compared to the cryoablation plus Meriva group. Mann-Whitney test. *P < 0.05, ** P < 0.01, ****P < 0.0001. Values P < 0.05 were considered statistically significant.
The effect of cryoablation plus Meriva on tumor and lung metastases in 4T1 model
After showing the strong CD8+ T cell responses to Mage-b and Survivin, we analyzed the effect of cryoablation and Meriva on lung metastases and tumor. Mice were injected with 4T1 tumor cells in the mammary fat pad, and orally fed with Meriva as described above. All mice were euthanized at the end of the Meriva treatment and analyzed for tumor weight and number of metastases in the lungs. We expected based on earlier studies that at this time point lung metastases already have spread. A significant decrease in the number of lung metastases (90%) and tumor growth (85%) was observed in the group of mice that received cryoablation plus Meriva compared to the groups that received Meriva or Saline alone, but not compared to the group that received cryoablation alone (28 d after tumor cell injection) (). We also analyzed tumor size (in mm2) during treatment and found (28 d after tumor cell injection) that the average tumor size in the mice that received cryoablation plus Meriva was significantly smaller compared to the group of saline or Meriva alone but not compared to cryoablation alone (Fig. S1).
Effect of cryoablation and Meriva on survival
As shown in , cryoablation plus Meriva strongly reduced tumor growth and the number of lung metastases. Here, we analyzed the effect of cryoablation and Meriva on the survival time of the tumor-bearing mice in the different treatment and control groups. Of note is that after cryoablation we continued to give Meriva throughout the experiment (day 58). The survival rate of the mice that received cryoablation plus Meriva was not only significantly better compared to the mice that received saline but also compared to the mice that received cryoablation or Meriva alone (). In a separate pilot study, we injected 4T1.2 tumor cells expressing luciferase (4T1.2Luc3) to monitor the effect of treatment on tumor and lung metastases live in vivo. The 4T1.2Luc3 tumors and metastases grew slower than the 4T1 tumors and lung metastases. As shown in , on day 38, the mice that received saline or Meriva had big tumors and multiple metastases in the lungs, while the mice that received cryoablation alone or cryoablation plus Meriva were free of tumor and lung metastases. Noteworthy, on day 58 when all mice in the saline or Meriva group were already dead, was that metastases started appearing in the mice that received cryoablation alone but not in the mice that received cryoablation plus Meriva (). This correlated with the significantly longer survival of the mice that received cryoablation plus Meriva compared to the mice that received saline, cryoablation or Meriva ().
Figure 4. Cryoablation and Meriva significantly reduced metastatic breast cancer in 4T1 model. BALB/c mice were injected with 105 4T1 tumor cells in the mammary fat pad and treated with cryoablation (20% protocol) and Meriva as described in . All mice were euthanized 2 d after the last treatment and analyzed for tumor weight (A) and the number of metastases in the lung was counted using a preparation microscope (B). A representative example of a primary tumor and metastases in the lungs (perfused with Indian Ink) is depicted in C. The error bars represent the SEM. All groups were compared to the cryoablation plus Meriva group. Mann-Whitney test. *P < 0.05, *** P < 0.001. Values P < 0.05 were considered statistically significant.
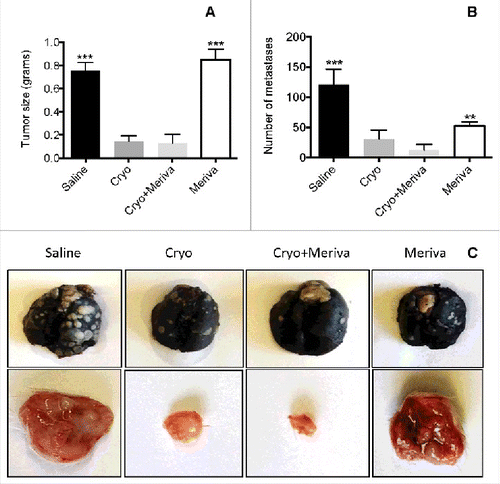
Figure 5. Cryoablation followed by Meriva significantly improved the survival rate and reduced the number of lung metastases. BALB/c mice were injected with 105 4T1 tumor cells in the mammary fat pad and treated with cryoablation (20% protocol) and Meriva as described in , but now maintained until they succumbed spontaneously (A). The only difference with is that in this survival and IVIS study Meriva was administered until the mice succumbed, instead of for 14 d only. Four groups were included – I: Saline; II: cryoablation; III: cryoablation and Meriva; and IV: Meriva. In this experiment n = 9 mice per group. Results were analyzed by Log-rank Mantel-Cox test. P < 0.05 is significant. All groups were compared to the cryoablation plus Meriva group. M = Meriva; Cryo = cryoablation. Median survival times: saline 37 d, cryoablation 41 d, cryoablation plus Meriva 51 d, and Meriva 41 d. In parallel, mice were injected with 105 4T1.2luc3 cells (same groups as in A), to analyze the effect of treatment live by the IVIS system (B). 4T1.2luc3 tumor cells grew more slowly then 4T1 cells. Therefore, Cryoablation was given on day 21 after tumor cell injection instead of day 14, and Meriva was administered 3 d after cryoablation. On day 38 all mice were monitored by IVIS. n = 3 mice per group. On day 58, when all mice in the saline group and Meriva group were dead the mice in the cryoablation group and in the cyroablation and Meriva group were still alive and monitored by in vivo imaging system (IVIS) (C). The total luciferase signal (tumor plus metastases) for each mouse was quantified on days 38 and 58 as shown in Supplemental Table 1.
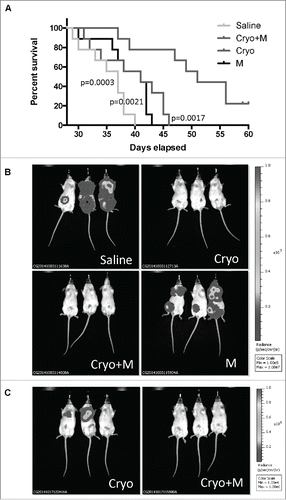
Interestingly, the luciferase signal in the metastases was stronger than in the primary tumor. The primary tumors in the 4T1 model are often necrotic. This may have caused reduced blood flow and thus less luciferase signal in the tumors compared to the lung metastases.
Discussion
This study demonstrates that the combination of cryoablation and Meriva nearly completely eliminated metastatic breast cancer in the highly aggressive TNBC model 4T1, significantly extended the survival rate, and significantly improved CD8+ T cell responses to TAA expressed by the 4T1 lung metastases and tumors. Our results strongly suggest that Meriva improved CD8+ T cell responses through the reduction of IL-6 in the primary tumors. Most importantly, we demonstrated that Meriva improved CD8+ T cell responses (significant increase in the production of IFNγ), generated by cryoablation (through immunogenic tumor cell death) to multiple TAA expressed by the 4T1 tumors and metastases. The CD8+ T cell responses to the TAA were inhibited with anti-MHC class I antibodies, supporting our conclusion that the CD8+ T cell responses were directed to TAA.
Cryoablation induces or reduces cytokines that may alter immune responses to the tumor and metastases. In our study, cryoablation induced the production of IFNγ in the spleen cells. This may have delayed the development of lung metastases on the short term but not on the long term. More detailed analysis of the production of IFNγ at various time points after cryoablation (3 h, days 1, 5, and 9) by ELISA showed that level of IFNγ in the spleen cells was highest on day 9 after cryoablation (Fig. S2), while IL-6 was not affected by cryoablation, and IL-10 and IL-12 were not detected (data not shown). None of these cytokines were detected in the serum after cryoablation. However, in another breast cancer model MT-901 (murine cell line derived from dimethylbenzantracen), IFNγ and IL-12 significantly increased in the serum, while IL-10 was not affected by cryoablation.Citation27 So far, cytokine levels in serum of patients with breast cancer after cryoablation has not been reported, but in patients with prostate cancer it was found that IFNγ and TNFα significantly increased after cryoablation in the serum,Citation28 while in patients with hepatic cancer TNFα and IL-10 increased significantly in the serum after cryoablation.Citation29 In summary, these results indicate that the production of cytokines for each cancer may be different and should be analyzed for each individual patient to develop a personalized combination therapy. Based on patient's cytokine profile, an optimal adjuvant could be selected for combining with cryoablation. For instance, patients with high IL-6 levels may benefit from cryoablation plus Meriva, while patients with low levels of IL-12 may benefit from cryoablation with cyclic di-guanylate (c-di-GMP)Citation30 or CpG.Citation31
During the early stage of cancer development (day 28 after tumor development), we did not find a significant difference between cryoablation plus Meriva or cryoablation alone in our efficacy studies. This may not be surprising because cryoablation itself induced high levels of IFNγ, although less compared to cryoablation plus Meriva. However, in the survival study mice that received cryoablation plus Meriva lived significantly longer than the mice in all other groups. It is of note that in the survival study Meriva (in both groups, cryoablation plus Meriva or Meriva alone) was administered until death, while in the efficacy studies Meriva was administered for 14 d only. In parallel with this survival study, a pilot experiment was performed with less aggressive 4T1.2luc3 tumor cells that express luciferase in order to monitor the effect of treatment on the development of lung metastases and tumor live using the in vivo imaging system (IVIS) for small animals. In the early stage (day 38 after tumor cell injection), all mice in the saline group or Meriva groups exhibited a primary tumor and metastases in the lungs, while no tumors or metastases were detected yet in the mice that received cryoablation plus Meriva or cryoablation alone. However, in a later stage (day 58 after tumor cell injection), when all mice in the saline and Meriva group were dead, two out of the three mice that received cryoablation alone showed metastases in the lung but no tumors, while the mice that received cryoablation and Meriva were free of metastases and tumors. These results suggest that cryoablation has delayed the development of lung metastases in the early stage of cancer, while cryoablation and Meriva delayed the development of lung metastases also in a more advanced stage of cancer. Post-mortem analysis of the 4T1.2luc3 mice that received cryoablation plus Meriva showed that all the mice eventually died of metastases in the lungs, because the primary tumors were barely detectable or absent while their lungs were filled with metastases. However, Meriva delayed the development of metastases in the mice that received cryoablation, compared to all other control groups. Post-mortem analysis was also performed on 4T1 mice in the survival study. At the time of death, their lungs were filled with metastases, even in the mice that received cryoablation plus Meriva, but development of the metastases (small tumors were visible at time of death) in the mice that received cryoablation plus Meriva also here seems delayed compared to all control groups. Of note is that no toxicities were visible in any of these mice. The discrepancy between the results in (post-mortem mice), i.e. significant differences in the number of metastases in 5A between the groups that received cryoablation plus Meriva compared to all control groups which was not observed in 4B, is most likely due to time differences of analyses in correlation with a more aggressive 4T1 model compared to the 4T1.2.luc3.
Meriva clearly could not prevent the development of metastases completely in the mice with cryoablation. This might be partly due to the fact that Meriva reduced about 50% of the IL-6 production and that the remaining IL-6 may have been sufficient to allow the metastases to grow but delayed compared to cryoablation without Meriva. However, it is also possible that the metastases became non-responsive to Meriva in the long term. If so, more detailed analysis to potential mechanisms would be interesting.
The results obtained in this study strongly suggest that the combination therapy with cryoablation and Meriva involves several mechanisms that ultimately lead to improved T cell responses. We have shown that cryoablation reduces the MDSC population () and that MDSC isolated from the 4T1 model in this study strongly inhibits T cell activation (Fig. S3). We also have shown that eliminating MDSC results in improved T cell responses in the 4T1 model,Citation32,33 which support the improved T cell responses in correlation with reduced MDSC population in the current study (). In addition, Meriva reduces IL-6 (). We have shown earlier that curcumin improves T cell responses through reduction of IL-6 in the 4T1 model.Citation34 We have also shown that high levels of IL-6 inhibits T cell responses to TAA Mage-b, and that these T cell responses could be restored using anti-IL-6 antibodies.Citation35 Finally, cryoablation strongly reduces tumor size, which will reduce tumor-induced immune suppression, and cryoablation also leads to the production of IFNγ. In summary, it is most likely that improved T cell responses generated by Meriva have played a role in the dramatic effect on tumor and metastases and on the survival of the 4T1-treated mice but as discussed here other mechanisms were involved as well. A mechanistic overview of the potential mechanisms involved in T cell activation by cryoablation and Meriva is shown in Fig. S4. To directly link survival data with improved CD8+ T cell responses, new studies will be required, i.e., analyzing the effect of CD8+ T cell depletions in vivo on the survival of 4T1 mice treated with cryoablation in the presence and absence of Meriva, as well as analyzing cytokine profiles and T cell responses in metastases and tumors at different time points before, during and after treatment.
Cryoablation is also effective against other cancers. Waitz et al. showed that cryoablation combined with anti-CTLA4 antibodies, induced potent CD8+ T cell responses to TAA SPAS-1 in correlation with a significant reduction in the growth of the primary tumor and improved survival of a prostate cancer model TRAMP C2.Citation36 Machlenkin et al. showed that cryoablation with immature DC injected intratumorally before cryoablation prolonged survival, induced tumor-specific CD8+ T cell responses, and strongly reduced the number of metastases in the Lewis Lung model and prevented growth of B16 melanoma tumors upon rechallenge.Citation37 In summary, cryoablation combined with adjuvants is a new highly promising treatment strategy for various types of cancer.
Cryoablation has also been tested in early-stage breast cancer patients and showed to be highly effective on the primary tumor.Citation18,38,39 Their results indicate that cryoablation may prevent surgery, and is less invasive with lower side effects than surgery, chemotherapy or radiation. Our preclinical results strongly suggest that cryoablation combined with Meriva might be also effective in a later stage when lung metastases have already been spread, and deserves further analysis in humans. If successful, this would be a real breakthrough in the management of metastatic breast cancer. It is important to note that Meriva is affordable to everybody, and that cryoablation could be improved at practically no cost.
Materials and Methods
Mice
Normal female BALB/c mice aged 3 months were obtained from Charles River Laboratories and maintained in the animal facility of Albert Einstein College of Medicine according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and according to the guidelines of the Albert Einstein Institute for Animal Studies.
Cells, cell culture, plasmids, and peptides, Meriva
The TNBC 4T1 cell line, derived from a spontaneous mammary carcinoma in a BALB/c mouseCitation40 was cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1 mM mixed non-essential amino acids, 2 mM L-glutamine, insulin (0.5 USP units/mL), penicillin (100 units/mL) and streptomycin (100 μg/mL). The 4T1.2luc3 tumor cell line was developed in the laboratory of Dr. Cheryl Jorcyk,Citation41 and was cultured in the same medium as the 4T1 cell line.
The plasmids pcDNA3.1-Mage-b and pcDNA3.1-Survivin were developed in our laboratory as described previously,Citation42 pCMV-GM-CSF was kindly provided by Steve Johnson, University of Texas-Southwestern Medical center Dallas,Citation43 TX. Survivin peptides were purchased from the Proteomics Resource Center, Rockefeller University, NY.
Meriva was purchased from Thorne Research (Dover, ID). The Meriva contained more than 20% curcuminoids complexed with soy lecithin (Phytosome®) in a 1:2 ratio.Citation44
Tumor challenge, cryoablation and immunotherapy regimens
Mice were injected with 105 4T1 cells in 50 μL of DMEM into the mammary fat pad, resulting in a primary tumor in the mammary fat pad palpable after 7 d and metastases in lungs only within 2–4 weeks after injection.
Various cryoablation protocols have been tested, i.e. 10%, 20%, 50%, 100%. Cryoablation involves the insertion of a probe into a tumor nodule in order to administer tissue ablative freezing temperatures, which will lead to immediate disruption of the tissue structure and cellular damage. The necrosis observed in the ablated lesion is mediated by mechanical disruption from crystallization, osmotic changes, and vascular stasis. The best effect (strongest effect on tumors and lowest side effects) was observed with the 20% protocol (freezing for 30 sec at −50°C). In the 20% cryoablation experiments the freezing rate was set at 20% of the maximum (the maximum is 100%). There are two reasons for using this setting. The first reason is that mice tumors are smaller than human tumors, and therefore freeze more rapidly than tumors in humans. Consequently, at 100% rate there would not be sufficient time for interstitial ice to form and the resulting tumor cell kill would be due to other mechanisms then described above. The lower freeze settings provide enough time for the full cryotherapy-mediated tumor cell killing to occur. Also, at higher freezing rates there is potential for the ice ball to become large and injure underlying healthy tissue, which is largely avoided at lower freezing rates. The 20% protocol has been used for all experiment in this manuscript. Fourteen days after injection of the tumor cells, when tumors reached 1–1.5 cm (largest diameter), mice were randomized for experiments. Cryoablation was applied under a light anesthesia (mixture of isoflurane and oxygen), and by inserting a probe (PERC-17R: 15 mm; HealthTronics; Austin, TX) using the 20% protocol of freezing and thawing of the tumor cells with argon and helium gas at the needle hub, respectively (stick program)(EndoCare; Cryo-28–298, Irvine, CA). After the treatment, mice were monitored for bleeding or any other complication.
Three days after the cryoablation, oral administration of Meriva 10 mg/300 μL saline per mouse (Thorne Research; Dover, ID) was started every 3 d and continued for 14 d. In the survival study, Meriva administration was continued until death. [Human Equivalent Dose (HED in mg/kg) = Animal Dose (mg/kg) × Animal Km ÷ Human Km = (500 mg/kgx3)/37 = 40 mg/kg].Citation45 Control groups included mice receiving saline, Meriva, or cryoablation only. All mice were euthanized 28 d after tumor cell injection and analyzed for tumor weight and number of metastases in the lungs. The number of metastases was determined by perfusion of the lungs with Indian Black Ink (American MastersTech Scientific Inc., Lodi, CA). Metastases were counted as white nodules per mouse using a preparation microscope (Accu-Scoop 3075-LED-GEM, Hicksville, NY).
Detection of 4T1.2luc3 tumor and metastases by IVIS
Mice were injected with 105 4T1.2lu3 cells (in 50 μL DMEM) into the mammary fat pad as described above for 4T1, resulting in a primary tumor in the mammary fat pad and metastases in the lung. The growth of the 4T1.2luc3 was slower than 4T1 tumor cells, i.e., tumors were palpable on day 14 instead of day 7. Therefore, cryoablation was applied on day 21 after tumor cell injection and Meriva treatment 3 d later. The luciferase activity was measured using an intensified charged-coupled device video camera of the in vivo imaging system (IVIS 100; Xenogen). The 4T1.2luc3 tumor-bearing mice were injected through retro-orbital injections with 200 μL of D-luciferin sodium salt (Synchem OHG; catalog number BC218) dissolved in phosphate-buffered saline (100 mg/kg of body weight). Luciferine distributed systemically for 3 min while the animals were anesthetized (mixture of isoflurane and oxygen). Anesthetized animals were placed in the camera chamber, and a bioluminescent signal was acquired for 5 min. Bioluminescence measurements produced by the IVIS 100 system are expressed as a pseudo-color on a gray background, with red denoting the highest intensity and blue the lowest. To quantify the luminescence, we outlined a region of interest and analyzed it by use of the Living Image (version 2.11; Xenogen) and Igor Pro (version 4.02A; WaveMetrics) software.
ELISPOT
Spleen cells were isolated from treated and control mice bearing 4T1 tumors and lung metastases and analyzed for T cell responses to Mage-b and Survivin by ELISPOT as described previously.Citation46 Briefly, 4 × 105 spleen cells of mice that received cryoablation and Meriva or of control mice were transfected with plasmids pcDNA3.1-Mage-b or pcDNA3.1-Survivin and pCMV-GM-CSF (1 μg of each plasmid per 5 × 106 spleen cells) using Lipofectamine 2000. After 72 h, the frequency of IFNγ-producing cells was determined by ELISPOT according to standard protocols (BD Biosciences), using an ELISPOT reader (CTL Immunospot S4 analyzer, Cellular Technology Ltd, Cleveland, OH). To determine the CD8+ T cell component of the responses, spleen cells were depleted of CD8+ T cells using anti-CD8+α (Clone 53-6.7)-conjugated magnetic beads, according to the manufacturer's instructions (Miltenyi, Auburn, CA).
In a separate experiment, the same ELISPOT assay was performed using Mage-b or Survivin for restimulation in the presence or absence of anti-MHC class I antibodies (final concentration 1 μg/mL).
ELISA
To test the effect of Meriva on IL-6 production in vivo, 3 mm3 from each tumor tissue of mice that received 0, 5, 10 or 25 mg of Meriva in 300 μL of water orally, was homogenized in an Eppendorf tube with glass beads and 1 mL of PBS containing 0.1% Triton-X100, 2 mM EDTA, and protease inhibitors by a Wig-L-Bug for 30 sec as described previously,Citation47 and serial dilutions (undiluted, 10x, 100x) were made of the tumor cell lysate after homogenization, and then analyzed for the concentration of IL-6 by ELISA according to manufacturer's instructions (BD PharMingen). Briefly, the plates were incubated with capture antibody (anti-IL-6), then incubated with the serial dilutions of tumor cell lysate, followed by detection antibody (biotinylated anti-mouse IL-6) plus avidin-horseredish peroxidase conjugate, and then finally incubated with substrate (tetramethylbenzidine and hydrogen peroxide). The reaction was stopped with 2 Normal sulfuric acid, and results were analyzed by an ELISA reader at 450 nm. The IL-6 levels were normalized for protein concentrations. IL-6 levels were calculated from three points of the linear portion of the standard curve, and subjected to statistical analysis, using ANOVA. For the quantitative ELISA, five mice were used in each group.
Cytokine levels were also analyzed in the serum at various time points after cryoablation (3 h, 1, 5, and 9 d). Briefly, serial 2-fold dilutions were made of the serum samples and analyzed for the production of IL-6, IL-10, TNFα, IFNγ, and IL-12 as described above. Lower detection limits of the cytokines tested are: IL-6: 15.6 pg/mL; IL-12: 15.6 pg/mL; TNFα: 15.5 pg/mL; IFNγ: 3.1 pg/mL; IL-10: 31.3 pg/mL; IL-1β: 15.6 pg/mL. All ELISA kits were purchased from BD Biosciences.
Flow cytometry analysis
Immune cells from blood of treated and control mice were isolated as described previously.Citation13,46 To identify MDSC, anti-CD11b-Alexa488/PerCP-cy5.5 and anti-Gr1-PerCP-cy5.5 (clone RB6-8C5) antibodies were used. Depending on the sample size, 100,000–300,000 cells were acquired by scanning using a Fluorescence Activated Cell Sorter (flow cytometry) (Beckton and Dickinson; FACScalibur), and analyzed using FlowJo 7.6 software. Cell debris and dead cells were excluded from the analysis based on scatter signals and use of Fixable Blue or Green Live/Dead Cell Stain Kit (Invitrogen). All antibodies were purchased from BD PharMingen or eBiosciences.
Statistical analysis
To statistically analyze the effects of cryoablation and Meriva on the growth of lung metastases and tumors and immune responses in the mouse models, the unpaired t test and the Mann-Whitney were used. For the survival data, the Log-rank Mantel-Cox test was used. Values P < 0.05 were considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the pub-lisher's website.
1049802_supplemental_files.zip
Download Zip (626.8 KB)Acknowledgments
We would like to thank Dr. Govindarajan Srimathveeravalli (Radiology, Memorial Sloan Kettering Cancer Center, New York) for the fruitful discussions about the various techniques for minimally invasive image-guided interventional therapy of cancer and for initiating the cryoablation in our laboratory. We would also like to thank HealthTronics for generously providing the cryoablation machine and great service for free throughout the whole study. We would also like to thank Dr. Antonella Riva and Dr. Giovanna Petrangolini (Indena SpA, Milan, Italy) for their great scientific support on the Meriva, and editorial support. We also thank Dr. Li-Min Ting, Department of Medicine, for her excellent assistance with imaging the 4T1.2luc mice through IVIS.
Funding
This work was supported by NCI grant R21 AI090652-01, the Anticancer Fund, and The Paul F Glenn Center for the Biology of Human Aging Research 34118A.
References
- Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest 2007; 117:3660-3; PMID:18060028
- Waldner MJ, Foersch S, Neurath MF. Interleukin-6–a key regulator of colorectal cancer development. Int J Biol Sci 2012; 8:1248-53; PMID:23136553; http://dx.doi.org/10.7150/ijbs.4614
- Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA, Thompson R, Schioppa T, Nemeth J, Vermeulen J, et al. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res 2011; 17:6083-96; PMID:21795409
- Sotiriou C, Lacroix M, Lespagnard L, Larsimont D, Paesmans M, Body JJ. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett 2001; 169:87-95; PMID:11410329
- Kuang Y, Zhang Z, Zhang X. [Interleukin-6 and its soluble receptors in human breast cancer]. Zhonghua Zhong Liu Za Zhi 1998; 20:305-7; PMID:10920992
- Idowu MO, Kmieciak M, Dumur C, Burton RS, Grimes MM, Powers CN, Manjili MH. CD44(+)/CD24(−/low) cancer stem/progenitor cells are more abundant in triple-negative invasive breast carcinoma phenotype and are associated with poor outcome. Hum Pathol 2012; 43:364-73; PMID:21835433; http://dx.doi.org/10.1016/j.humpath.2011.05.005
- Liu S, Wicha MS. Targeting breast cancer stem cells. J Clin Oncol 2010; 28:4006-12; PMID:20498387; http://dx.doi.org/10.1200/JCO.2009.27.5388
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science 2009; 324:1670-3; PMID:19556499; http://dx.doi.org/10.1126/science.1171837
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest 2010; 120:41-50; PMID:20051635
- Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ, Choudhury SA, Maruyama R, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(−) stem cell-like breast cancer cells in human tumors. J Clin Invest 2011; 121:2723-35; PMID:21633165
- Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, et al. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 2004; 173:3844-54; PMID:15356132
- Xie J, Qian J, Wang S, Freeman ME, 3rd, Epstein J, Yi Q. Novel and detrimental effects of lipopolysaccharide on in vitro generation of immature dendritic cells: involvement of mitogen-activated protein kinase p38. J Immunol 2003; 171:4792-800; PMID:14568957
- Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br J Cancer 2008; 99:741-9; PMID:18728665; http://dx.doi.org/10.1038/sj.bjc.6604526
- Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 2011; 10:12; PMID:21299897; http://dx.doi.org/10.1186/1476-4598-10-12
- Aggarwal BB, Shishodia S, Takada Y, Banerjee S, Newman RA, Bueso-Ramos CE, Price JE. Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB pathway in breast cancer cells and inhibits lung metastasis of human breast cancer in nude mice. Clin Cancer Res 2005; 11:7490-8; PMID:16243823; http://dx.doi.org/10.1158/1078-0432.CCR-05-1192
- Odot J, Albert P, Carlier A, Tarpin M, Devy J, Madoulet C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int J Cancer 2004; 111:381-7; PMID:15221965; http://dx.doi.org/10.1002/ijc.20160
- Singh M, Ramos I, Asafu-Adjei D, Quispe-Tintaya W, Chandra D, Jahangir A, Zang X, Aggarwal BB, Gravekamp C. Curcumin improves the therapeutic efficacy of Listeria(at)-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med 2013; 2:571-82; PMID:24156030; http://dx.doi.org/10.1002/cam4.94
- Vlastos G, Verkooijen HM. Minimally invasive approaches for diagnosis and treatment of early-stage breast cancer. Oncologist 2007; 12:1-10; PMID:17227896; http://dx.doi.org/10.1634/theoncologist.12-1-1
- Lippa N, Sargos P, Italiano A, Kind M, Dallaudiere B, Hauger O, Cornelis F. Standardization of selection criteria for percutaneous image-guided cryoablation of recurrent soft-tissue sarcomas. Diag Interv Imaging 2014; 95:1071-7; PMID:24637209; http://dx.doi.org/10.1016/j.diii.2014.02.008
- Cornelis F, Buy X, Andre M, Oyen R, Bouffard-Vercelli J, Blandino A, Auriol J, Correas JM, Pluvinage A, Freeman S, et al. De novo renal tumors arising in kidney transplants: midterm outcome after percutaneous thermal ablation. Radiology 2011; 260:900-7; PMID:21771957; http://dx.doi.org/10.1148/radiol.11110122
- Havez M, Lippa N, Al-Ammari S, Kind M, Stoeckle E, Italiano A, Gangi A, Hauger O, Cornelis F. Percutaneous image-guided cryoablation in inoperable extra-abdominal desmoid tumors: a study of tolerability and efficacy. Cardiovasc Int Radiol 2014; 37:1500-6; PMID:24402645; http://dx.doi.org/10.1007/s00270-013-0830-9
- Weld KJ, Landman J. Comparison of cryoablation, radiofrequency ablation and high-intensity focused ultrasound for treating small renal tumours. BJU Int 2005; 96:1224-9; PMID:16287435; http://dx.doi.org/10.1111/j.1464-410X.2005.05848.x
- Baust JG, Gage AA, Bjerklund Johansen TE, Baust JM. Mechanisms of cryoablation: clinical consequences on malignant tumors. Cryobiology 2014; 68:1-11; PMID:24239684; http://dx.doi.org/10.1016/j.cryobiol.2013.11.001
- Kono K, Mimura K. Immunogenic tumor cell death induced by chemoradiotherapy in a clinical setting. Oncoimmunology 2013; 2:e22197; PMID:23482346; http://dx.doi.org/10.4161/onci.22197
- Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol 2007; 60:171-7; PMID:17051370; http://dx.doi.org/10.1007/s00280-006-0355-x
- Gomez-Cabrero A, Wrasidlo W, Reisfeld RA. IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PLoS One 2013; 8:e73607; PMID:24014113; http://dx.doi.org/10.1371/journal.pone.0073607
- Sabel MS, Nehs MA, Su G, Lowler KP, Ferrara JL, Chang AE. Immunologic response to cryoablation of breast cancer. Breast Cancer Res Treat 2005; 90:97-104; PMID:15770533; http://dx.doi.org/10.1007/s10549-004-3289-1
- Si T, Guo Z, Hao X. Immunologic response to primary cryoablation of high-risk prostate cancer. Cryobiology 2008; 57:66-71; PMID:18593573; http://dx.doi.org/10.1016/j.cryobiol.2008.06.003
- Osada S, Imai H, Tomita H, Tokuyama Y, Okumura N, Matsuhashi N, Sakashita F, Nonaka K. Serum cytokine levels in response to hepatic cryoablation. J Surg Oncol 2007; 95:491-8; PMID:17219394; http://dx.doi.org/10.1002/jso.20712
- Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2014; 2:901-10; PMID:24913717; http://dx.doi.org/10.1158/2326-6066.CIR-13-0123
- Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of interleukin-12 and tumor necrosis factor-alpha. Cell Immunol 1996; 167:72-8; PMID:8548847; http://dx.doi.org/10.1006/cimm.1996.0009
- Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer 2013; 108:2281-90; PMID:23640395; http://dx.doi.org/10.1038/bjc.2013.206
- Chandra D, Gravekamp C. Myeloid-derived suppressor cells: Cellular missiles to target tumors. Oncoimmunology 2013; 2:e26967; PMID:24427545; http://dx.doi.org/10.4161/onci.26967
- Singh M, Ramos I, Asafu-Adjei D, Quispe-Tintaya W, Chandra D, Jahangir A, Zang X, Aggarwal BB, Gravekamp C. Curcumin improves the therapeutic efficacy of Listeria(at)-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med 2013; 2:571-82; PMID:24156030; http://dx.doi.org/10.1002/cam4.94
- Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br J Cancer 2008; 99:741-9; PMID:18728665; http://dx.doi.org/10.1038/sj.bjc.6604526
- Waitz R, Solomon SB, Petre EN, Trumble AE, Fasso M, Norton L, Allison JP. Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy. Cancer Res 2012; 72:430-9; PMID:22108823; http://dx.doi.org/10.1158/0008-5472.CAN-11-1782
- Machlenkin A, Goldberger O, Tirosh B, Paz A, Volovitz I, Bar-Haim E, Lee SH, Vadai E, Tzehoval E, Eisenbach L. Combined dendritic cell cryotherapy of tumor induces systemic antimetastatic immunity. Clin Cancer Res 2005; 11:4955-61; PMID:16000595; http://dx.doi.org/10.1158/1078-0432.CCR-04-2422
- Morin J, Traore A, Dionne G, Dumont M, Fouquette B, Dufour M, Cloutier S, Moisan C. Magnetic resonance-guided percutaneous cryosurgery of breast carcinoma: technique and early clinical results. Cana J Surg 2004; 47:347-51; PMID:15540687
- Roubidoux MA, Sabel MS, Bailey JE, Kleer CG, Klein KA, Helvie MA. Small (< 2.0-cm) breast cancers: mammographic and US findings at US-guided cryoablation–initial experience. Radiology 2004; 233:857-67; PMID:15567802; http://dx.doi.org/10.1148/radiol.2333031734
- Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res 1992; 52:1399-405; PMID:1540948
- Bolin C, Sutherland C, Tawara K, Moselhy J, Jorcyk CL. Novel mouse mammary cell lines for in vivo bioluminescence imaging (BLI) of bone metastasis. Biol Proced Online 2012; 14:6; PMID:22510147; http://dx.doi.org/10.1186/1480-9222-14-6
- Sypniewska RK, Hoflack L, Tarango M, Gauntt S, Leal BZ, Reddick RL, Gravekamp C. Prevention of metastases with a Mage-b DNA vaccine in a mouse breast tumor model: potential for breast cancer therapy. Breast Cancer Res Treat 2005; 91:19-28; PMID:15868428; http://dx.doi.org/10.1007/s10549-004-6454-7
- Chambers RS, Johnston SA. High-level generation of polyclonal antibodies by genetic immunization. Nat Biotechnol 2003; 21:1088-92; PMID:12910245; http://dx.doi.org/10.1038/nbt858
- Cuomo J, Appendino G, Dern AS, Schneider E, McKinnon TP, Brown MJ, Togni S, Dixon BM. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J Nat Prod 2011; 74:664-9; PMID:21413691; http://dx.doi.org/10.1021/np1007262
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J 2008; 22:659-61; PMID:17942826; http://dx.doi.org/10.1096/fj.07-9574LSF
- Castro F, Leal B, Denny A, Bahar R, Lampkin S, Reddick R, Lu S, Gravekamp C. Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br J Cancer 2009; 101:1329-37; PMID:19826426; http://dx.doi.org/10.1038/sj.bjc.6605329
- Gravekamp C, Leal B, Denny A, Bahar R, Lampkin S, Castro F, Kim SH, Moore D, Reddick R. In vivo responses to vaccination with Mage-b, GM-CSF and thioglycollate in a highly metastatic mouse breast tumor model, 4T1. Cancer Immunol Immunother 2008; 57:1067-77; PMID:18094967; http://dx.doi.org/10.1007/s00262-007-0438-5
